Abstract
Background
Baseline high neuromuscular drive is present in chronic obstructive pulmonary disease (COPD). In moderate-to-very severe COPD patients, both static and/or dynamic pulmonary hyperinflation have been demonstrated at rest.
Aim
To assess the influence of dynamic hyperinflation on neuromuscular drive at rest.
Methods
We recruited 22 patients with severe-to-very severe COPD showing resting dynamic pulmonary hyperinflation, as assessed by the baseline reduction of inspiratory capacity (IC) (<80% of predicted). IC, occlusion pressure (P0.1), maximal inspiratory pressure (MIP), and their ratio were measured at end-expiratory lung volume (EELV) before and after acute inhalation of 400 mcg of albuterol (metered-dose inhaler plus spacer). In these patients the bronchodilator response was assessed also as lung volume changes.
Results
Only in COPD patients with a marked increase in IC (>12% of baseline and at least 200 mL) after bronchodilator, resting P0.1 showed a clinically significant decrease, despite the EELV diminution (P < 0.001). MIP was augmented following EELV reduction and therefore the P0.1/MIP ratio was markedly decreased (P < 0.001). In contrast, no changes in these indices were found after bronchodilator in COPD patients with insignificant variations of IC. Breathing pattern parameters did not vary in both sub-groups after albuterol.
Conclusion
Following bronchodilator, significant P0.1 decrease, MIP increase, and reduction of the P0.1/MIP ratio were found only in COPD patients with a marked IC increase and these changes were closely related. These findings suggest that bronchodilators, by decreasing dynamic hyperinflation, may control exertional and/or chronic dyspnea partly through a reduction of central neuromuscular drive.
Introduction
In patients with moderate-to-severe chronic obstructive pulmonary disease (COPD), parameters reflecting static and dynamic pulmonary hyperinflation (DH) such as end-expiratory lung volume (EELV) or inspiratory capacity (IC) correlate better than forced expiratory volume in 1 second (FEV1) with chronic dyspnea,Citation1 and progressive DH is thought to be the main limiting factor of their exercise capacity because of related intolerable breathlessness.Citation2,Citation3 In COPD patients, the occurrence of DH, either at rest or during exercise, is thought to induce dyspnea mainly by causing neuromechanical (or neuroventilatory) dissociation.Citation3 However, a high inspiratory drive that is widely documented in severe-to-very severe COPDCitation5 might also contribute to increased dyspnea in these patients.
By increasing the expiratory flow reserve at low lung volumes, bronchodilators can reduce chronic and exertional dyspnea essentially by decreasing baseline EELV (or increasing IC) in moderate-to-severe COPD patientsCitation6–Citation8 with DH, and better neuromechanical coupling is believed to be responsible to a large extent for such improvement.Citation9
The aim of the study was to assess whether the acute reduction of resting DH possibly obtained by bronchodilator administration could influence the neuromuscular inspiratory drive and its ratio with the inspiratory muscles’ strength in stable COPD patients with marked airflow obstruction.
Methods
We prospectively evaluated a cohort of stable severe to very severe COPD outpatients with baseline IC values less than 80% of their predicted values, consecutively enrolled at the Respiratory Rehabilitation Unit, Hospital Domus Salutis, Brescia, Italy. The diagnosis of COPD was made according to the following criteria: (1) smoking history of more than 20 pack-years and/or the presence of other known risk factors for COPD; (2) baseline FEV1/vital capacity ratio less than the 5th percentile of normal limits;Citation10 (3) increase of FEV1 less than 10% of the predicted value and less than 200 mL in absolute value after 400 mcg of inhaled albuterol (metered-dose inhaler plus spacer); (4) no history or evidence of other diseases with chronic airflow obstruction such as chronic asthma, bronchiectasis, constrictive bronchiolitis, tuberculosis, and cystic fibrosis.
At least 24 hours after withdrawal of long-acting beta-2 agonists, short and long-acting anti-cholinergics, and slow-release theophylline, in the absence of exacerbation in the preceding 12 weeks, the patients underwent both in baseline condition and 30 minutes after the inhalation of albuterol (400 mcg by metered-dose inhaler plus spacer) pulmonary function tests (spirometry, maximal flow/volume curve, lung volumes by N2-multibreath wash-out test) (System 1070, Medical Graphics, St Paul, MN, USA), determination of mouth pressure 100 milliseconds after the beginning of quiet inspiration during airways occlusion (P0.1) performed at EELV, and measurements of maximal inspiratory pressure (MIP) at EELV during a Muller maneuver (Resp Mech module, Medical Graphics, St Paul, MN, USA). Both P0.1 and MIP were obtained in triplicate with adequate time intervals among the different measurements and the values used for analysis were the average of the two highest ones. Subsequently, the patients were classified, according to the IC changes after acutely inhaled albuterol, in volume non-responders (increase of IC < 12% and 200 mL of baseline: group 1) and volume responders (increase of IC ≥ 12% and 200 mL of baseline: group 2).
All spirometric parameters were analyzed as percent of predicted values.Citation10 The IC predicted values were those proposed by Tantucci et al.Citation11 Predicted values of IC for those patients aged less than 65 were obtained by back-extrapolating the reference equations. The patients were recruited and tested if able to correctly perform the pulmonary function tests according to the American Thoracic Society guidelines.Citation12 The study was approved by the Ethics Committee of the Hospital “Spedali Civili” of Brescia and each patient signed an informed consent for collection and treatment of data.
Statistical analysis
Differences between groups were assessed according to an unpaired nonparametric test (Mann-Whitney test) while comparisons of functional parameters before and after albuterol within groups were performed by a paired nonparametric test (Wilcoxon test). The Pearson’s linear correlations were used to establish association between the variables of interest and the determination coefficients were also given. A P-value less than 0.05 was considered as statistically significant. The calculations were made using the SPSS 14.0 statistical package (IBM Corporation, Armonk, NY, USA). Data were expressed as mean ± standard deviation.
Results
Twenty-two COPD patients (18 male) with a mean age of 72 ± 6 years and FEV1 equal to 0.78 ± 0.26 L (33% ± 11% predicted) were studied. Their anthropometric and functional characteristics are shown in . At baseline, a severe reduction of FEV1 with a marked increase of residual volume and functional residual capacity and reduction of IC were observed in these patients who exhibited as expected high values of P0.1.
Table 1 Anthropometric and functional characteristics observed in all patients and two groups of them, divided according to the absence (n = 7) or presence (n = 15) of significant change of IC (>12% from baseline and 200 mL) after acute bronchodilator at rest
No significant differences, however, were found at rest for spirometric parameters, lung volumes, neuromuscular drive, and maximal isometric force of inspiratory muscles between volume non-responders (group 1: increase of IC < 12% and 200 mL of baseline) and volume responders (group 2: increase of IC ≥ 12% and 200 mL of baseline) ().
Following inhalation of albuterol, FEV1 increased by 50 ± 50 mL (from 0.87 ± 0.25 L to 0.92 ± 0.22 L) in group 1 and by 130 ± 70 mL (from 0.74 ± 0.27 L to 0.87 ± 0.28 L) in group 2.
P0.1, MIP, and their ratio (P0.1/MIP%) are displayed in , before and after albuterol for all patients and those with and without significant increase of IC (as a percentage of baseline).
Table 2 Resting values of IC and P0.1, MIP, and their ratio before (Pre-Br) and after (Post-Br) acute administration of bronchodilator
Despite the reduction of EELV as reflected by the increase of IC, P0.1 was significantly reduced in volume responder COPD patients (P < 0.001). Since MIP values increased with decreasing EELV, P0.1/MIP% was markedly decreased in volume responder COPD patients after albuterol. In contrast, marginal changes in P0.1 and no changes in MIP and P0.1/MIP% were observed in volume non-responder COPD patients after bronchodilator.
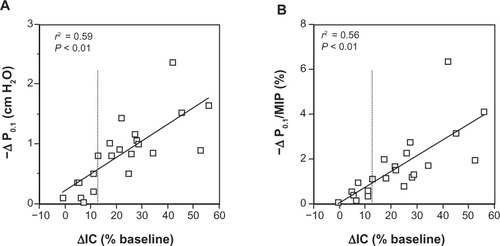
Changes in IC (as a percentage of baseline) and P0.1 following acute administration of albuterol are plotted in , panel A, showing a close direct correlation between reduction of DH (as reflected by the IC increase) and decreased neuromuscular drive at rest (r2 = 0.59; P < 0.01). A similar relationship was found between changes in IC (as a percentage of baseline) and P0.1/MIP% ratio, as shown in , panel B (r2 = 0.56; P < 0.01). No variations of the breathing pattern para meters such as tidal volume (Vt), respiratory rate (RR), inspiratory (Ti) and expiratory time (Te), mean inspiratory flow (VT/Ti), and duty cycle (Ti/Ttot) were observed before and after albuterol administration in both groups of COPD patients.
Figure 1 Relationship between changes in IC and P0.1 (A) and P0.1/MIP% (B), following bronchodilator in groups of stable severe-to-very severe COPD patients at rest.
Abbreviations: IC, inspiratory capacity; P0.1, mouth pressure 100 millseconds after the beginning of quiet inspiration during airways occlusion; MIP, maximal inspiratory pressure; COPD, chronic obstructive pulmonary disease.
Discussion
The results of this study indicate that in stable COPD patients at rest the neuromuscular output to inspiratory muscles is related to the degree of pulmonary hyperinflation and the reduction of DH possibly achieved by bronchodilator induces a significant decrease of inspiratory drive. Since bronchodilators have been shown to limit exertional and chronic dyspnea in COPD mainly by decreasing DH, our findings suggest that this may occur partly because of reduction on central motor output to inspiratory muscles.
It has been demonstrated that COPD patients have a high neural drive even at rest, as reflected by the increased baseline P0.1.Citation5 Many factors have been implicated to explain this elevated motor command to inspiratory muscles. Included among them are increased airflow resistance, abnormal gas exchange, weak respiratory muscles, and high ventilatory requirements.Citation13
Much evidence has been collected showing that distressing breathlessness in moderate-to-severe COPD patients is mostly linked to the occurrence of DH.Citation3 The imbalance between the volume displacement and the muscular effort required to achieve it, known as neuromechanical or neuroventilatory dissociation, is thought to be the main mechanism by which DH causes chronic and exertional dyspnea in COPD.Citation9 Other mechanisms, however, have been invoked in COPD patients as able to generate dyspnea and particularly an increased sense of work/effort following stimuli such as increased ventilatory requirements, elevated EELV, and a related increase in elastic inspiratory threshold load due to intrinsic positive end-expiratory pressure.Citation3,Citation9,Citation14 A high inspiratory neural drive, especially in the presence of functionally or intrinsically weakened inspiratory muscles is in fact associated with a greater respiratory effort.Citation15
The neural pathways underlying the sense of work/effort include corollary discharge from motor cortical and bulbar centersCitation16 and possibly multiple afferences from mechanical and metabolic receptors of respiratory and skeletal muscles to the sensory cortex that are believed to contribute to the dyspnea sensation.Citation13
In our work we showed a clear link between neuromuscular output level and severity of DH at rest in stable COPD patients and the possibility of significantly reducing it when an effective desufflation is achievable, in this case after acute bronchodilator inhalation, as indicated by a marked IC increase.
The effect on the reduction of respiratory drive observed in COPD patients who significantly increase IC after bronchodilator is likely even greater than we have shown, given the corresponding reduction in EELV that per se should increase, not decrease P0.1 because of better force-length relationship of the inspiratory muscles. In fact, although we did not measure directly EELV, with the reasonable assumption that total lung capacity remains unchanged after acute inhalation of albuterol, the IC variation specularly reflects the EELV change.
Therefore, the decrease of neuromuscular output at rest in COPD patients after bronchodilating drugs may reflect an effective desufflation in the absence of important FEV1 change also when the lung volumes and IC measurements cannot be adequately performed.
Drugs or non-pharmacological interventions that are effective in decreasing DH may diminish both the degree of neuromuscular uncoupling and the amount of neuromuscular drive. It is conceivable that either mechanism can contribute to reduce chronic and exertional dyspnea in COPD.
Finally, our results could be useful to explain the wide range of resting values of P0.1 observed in COPD patients with apparently similar severity of airflow obstruction, as measured by spirometry, taking into account the possible effect of different degrees of pulmonary hyperinflation.
Some limits of the study need to be addressed. The amount of neural drive is indirectly assessed by the P0.1 measurement at the mouth. The dynamically hyperinflated COPD patients have some intrinsic positive end-expiratory pressure. Thus, changes of esophageal P0.1 (that truly reflect the neuromuscular output) occur before those of mouth P0.1 and the two measurements correspond only when the initial pressure decay is linear, as it usually is. The inspiratory muscles in COPD can be intrinsically weak (myopathy, sarcopenia, etc) and P0.1 could be influenced by the force developed at the beginning of inspiration, without carefully reflecting the central neural drive. However, this seems unlikely because early (the first 100 milliseconds) contraction of inspiratory muscles is not impaired under these circumstances, as found in several neuromuscular diseases.Citation17,Citation18
Although baseline parameters were not significantly different between volume and non-volume responders, the first group tends to be younger with more females, showing slightly higher P0.1 and lower MIP. Since the sample size is small, we cannot exclude that these differences could be relevant when larger cohorts are examined.
In conclusion, a large P0.1 decrease, MIP increase, and reduction of the P0.1/MIP ratio were found after bronchodilator only in COPD patients with a marked IC increase. More interestingly, the improvement of DH and the decrease in neuromuscular drive were closely related. These findings indicate that decreasing DH by bronchodilators is associated with a reduction of the central neuromuscular drive and effort/work related sensation, suggesting that corollary discharge linked to an augmented central inspiratory output is an adjunctive mechanism promoting dyspnea in COPD patients with dynamic hyperinflation.
Disclosure
The authors report no conflicts of interest in this work.
References
- EltayaraLBecklakeMRVoltaCAMilic-EmiliJRelationship between chronic dyspnea and expiratory flow limitation in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19961546 Pt 1172617348970362
- O’DonnellDEBreathlessness in patients with chronic airflow limitation. Mechanisms and managementChest199410639049128082376
- O’DonnellDERevillSMWebbKADynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001164577077711549531
- DiazOVillafrancaCGhezzoHRole of inspiratory capacity on exercise tolerance in COPD patients with and without expiratory flow limitation at restEur Respir J200016226927510968502
- SorliJGrassinoALorangeGMilic-EmiliJControl of breathing in patients with chronic obstructive lung diseaseClin Sci Mol Med1978543295304630805
- TantucciCDuguetASimilowkiTZelterMDerenneJPMilic EmiliJEffect of salbutamol on dynamic hyperinflation in chronic obstructive pulmonary disease patientsEur Respir J19981247998049817148
- BoniECordaLFranchiniDVolume effectand exertional dyspnea after bronchodilator in patients with COPD with and without expiratory flow limitation at restThorax200257652853212037229
- O’DonnellDEVoducNFitzpatrickMWebbKAEffect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary diseaseEur Respir J2004241869415293609
- O’DonnellDEWebbKAExertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflationAm Rev Respir Dis19931485135113578239175
- [No authors listed]Standardized lung function testing. Report working partyBull Eur Physiopathol Respir198319Suppl 51956616097
- TantucciCPinelliVCossiSReference values and repeatability of inspiratory capacity for men and women aged 65–85Resp Med20061005871877
- [No authors listed]Lung function testing: selection of reference values and interpretative strategies. American Thoracic SocietyAm Rev Respir Dis19911445120212181952453
- ManningHLSchwartzsteinRMPathophysiology of dyspneaNew Engl J Med1995333154715537477171
- YanSSensation of inspiratory difficulty during inspiratory threshold and hyperinflationary loadings. Effect of inspiratory muscle strengthAm J Respir Crit Care Med19991605 Pt 11544154910556118
- O’ConnellJMCampbellAHRespiratory mechanics in airways obstruction associated with inspiratory dyspneaThorax19763166696771013938
- McCloskeyDICorollary discharges: motor commands and perceptionBrookhartJMMountcastleVBHandbook of Physiology Section 1 the Nervous System Volume I Cellular Biology of Neurons, Part 2BethesdaAmerican Physiology Society198114151417
- BéginRBureauMALupienLLumieuxBControl of breathing in Duchenne’s muscular dystrophyAm J Med19806922272346773416
- TantucciCMassucciMPipernoRBettiLGrassiVSorbiniCAControl of breathing and respiratory muscle strength in patients with multiple sclerosisChest19941054116311708162744