Abstract
Accumulating evidence suggests that cell injury in lung tissues is closely connected to disease progression in chronic obstructive pulmonary disease (COPD). Microparticles (MPs) are shed membrane vesicles that are released from platelets, leukocytes, red blood cells, and endothelial cells when these cells are activated or undergo apoptosis under inflammatory conditions. Based on increasing evidence that endothelial injury in the pulmonary capillary vasculature leads to lung destruction, and because cardiovascular diseases are the main cause of death among individuals with COPD, endothelial MPs (EMPs) are now receiving attention as potential biomarkers for COPD. There are eight types of EMPs which are defined by the presence of different endothelial markers on the cell membrane: vascular endothelial-cadherin; platelet endothelial cell adhesion molecule; melanoma cell adhesion molecule; E-selectin; CD51; CD105; von Willebrand factor; and CD143 EMPs. Vascular endothelial-cadherin, platelet endothelial cell adhesion molecule, and E-selectin EMPs are increased in patients with stable COPD and are further increased in patients with exacerbated COPD compared to non-COPD patients. In addition, the levels of these three EMPs in patients with stable COPD are significantly correlated with lung destruction and airflow limitation. These results indicate that endothelial injury is closely connected to the pathophysiology of COPD. Interestingly, the variations in the levels of the eight EMP subtypes were not identical with changes in patient condition. Although the clinical significance of the differences in these eight EMP subtypes remains unclear, evaluating the expression pattern of endothelial antigens on circulating MPs might predict the presence and degree of endothelial injury in COPD patients. In addition, circulating MPs are proposed to have several physiological functions in vivo, such as intercellular crosstalk; the increase in EMPs in COPD seems to play a role in the pathophysiology of this disease.
Introduction
Chronic obstructive pulmonary disease (COPD) is a lung disease characterized by nearly irreversible lung destruction which results in airflow limitation.Citation1 The severity of the disease is determined according to the degree of airflow limitation, which is measured using forced expiratory volume in one second (FEV1). However, there are several limitations in the use of FEV1 to evaluate the daily condition and disease progression of COPD patients. First, the sensitivity of FEV1 in evaluating daily condition is not high. Second, although both frequent exacerbationCitation2–Citation5 and bronchial hyperresponsivenessCitation6 are associated with rapid FEV1 decline, FEV1 does not reflect these conditions. Third, clinical manifestations and radiological observations are variable among COPD patients even when the degree of airflow limitation is the same.Citation7 For these reasons, new biomarkers for COPD are being sought.Citation8
Accumulating evidence indicates that injured cells in the lung tissue are closely involved in the pathophysiology of COPD.Citation9,Citation10 In animal models, the administration of a vascular endothelial growth factor receptor inhibitor induced apoptosis of pulmonary capillary endothelial cells, leading to emphysematous changes.Citation11 The number of apoptotic epithelial and endothelial cells is increased in emphysematous lungs compared with normal lungs.Citation12 In addition, senescence of alveolar epithelial and endothelial cells is accelerated in patients with emphysema.Citation13 Greater numbers of apoptotic lung cells are observed in lung tissues from COPD patients than in those from smokers without COPD.Citation9,Citation14,Citation15 Furthermore, morphological and biochemical markers of autophagy are increased in the lungs of patients with COPD compared with normal lung tissue.Citation16 These results indicate the importance of injured cells in the pathophysiology of lung destruction and COPD.
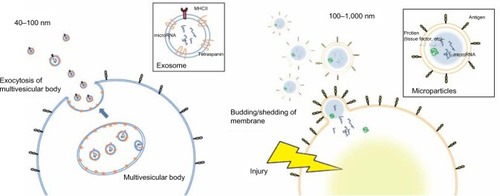
Activated or injured cells release chemical mediators, such as alarmins and microvesicles, into the circulation, and these mediators can potently modulate systemic immune responses.Citation17–Citation20 There are three major types of microvesicles, which are primarily distinguished by size: exosomes (40–100 nm), microparticles (MPs) (0.1–1.0 μm), and apoptotic bodies (1–3 μm). In this review, we focus on endothelium-derived MPs, which can be detected by their endothelial-specific antigens using fluorescence-activated cell sorting (FACS),Citation18–Citation23 and discuss their potential as biomarkers for COPD.
MPs
MPs are membrane vesicles that are released by budding or shedding from the plasma membrane of activated or injured cells in response to inflammation ().Citation18–Citation23 MP release from platelets,Citation24 red blood cells,Citation25 endothelial cells, tumor cells,Citation26,Citation27 and leucocytesCitation28 has been reported. Because MPs are shed membrane vesicles, the antigens expressed on the original cells are expressed on the membrane of the MPs. For example, CD144, CD31, and CD62E are expressed on endothelial cell-derived MPs, whereas CD31 and CD41 are expressed on platelet-derived MPs. Various stimuli, such as increased intracellular Ca2+29 and the activation of protein kinase C (PKC)Citation30 and purinergic receptors of adenosine triphosphate (ATP) such as the P2X7 and P2Y receptors,Citation31,Citation32 increase the rate of MP release. The contents of MPs are also variable and can include nucleic acids, particularly messenger ribonucleic acid (mRNA) and microRNA,Citation22 proteolytic enzymes such as metalloproteinases,Citation33 tissue factors,Citation34 and alarmins.Citation35 Released MPs interact with their target cells by binding to the surface of the target cell via specific receptors, followed by direct fusion of the shedding vesicle with the plasma membrane of the target cell, endocytic uptake of the vesicle, and horizontal transfer of molecules and RNA to the target cell.Citation20 MPs play many roles, including modulating the biological functions of cells.Citation36 For example, during leukocyte activation and arrest, activated mast cells and platelets deliver chemoattractants to endothelial cells through circulating MPs.Citation37 MPs also display proinflammatory and prothrombotic activities through the activation of Toll-like receptors and other signaling pathways.Citation17,Citation21 MPs are released from cells in response to various conditions or stimuli and may be heterogeneous.Citation38
Figure 1 Comparison of the size and release mechanism of exosomes and MPs.
Abbreviations: MP, microparticle; MHC II, major histocompatibility class II.
MPs and smoking
Smoking is the most important risk factor for COPD. Cigarette smoke increases autophagyCitation16,Citation39 and induces apoptosis and necrosis in lung cells.Citation40–Citation42 Smoking increases the levels of circulating MPs. In vitro studies have demonstrated that cigarette smoke extract induces the release of tissue factor-positive MPs as well as MPs with proteolytic activity attributed to matrix metalloproteinase-14 (MMP-14) from human macrophages in vitro.Citation43,Citation44 Incubating pulmonary microvascular endothelial cells or aortic endothelial cells with cigarette smoke extract induces the release of CD31+/CD41− MPs and CD146+ MPs in vitro.Citation40 Secondhand smoke exposure induces the rapid release of circulating CD144+ endothelial microparticles (EMPs), within a few hours.Citation45 Other groups have reported that CD31+/CD42b− EMPs are increased in healthy active smokers and further increased in active smokers with emphysema compared with healthy non-smokers.Citation46
MPs and oxidative stress
Oxidative stress induced by cigarette smoke exposure or activated neutrophils and/or macrophages in response to infection is also involved in the pathophysiology of COPD.Citation47,Citation48 Oxidative stress induces MP release. H2O2 induces apoptosis in endothelial cells and the release of CD31+/CD41− or CD146+ MPs from aortic and pulmonary microvascular endothelial cells in vitro.Citation40 Plasma levels of glutathione peroxidase, a marker of oxidative stress, are correlated with increased levels of platelet-, erythrocyte-, and endothelial-derived MPs independent of other factors involved in endothelial injury in patients with metabolic disease.Citation49
MPs and infection
Viral and/or bacterial infection was reported to be detected in up to 60% of COPD exacerbation and has been found to be associated with a rapid decline in lung function.Citation50,Citation51 Several articles reported the impact of infections on circulating MPs,Citation52–Citation55 and the number of circulating MPs is significantly increased by infection. Therefore, the presence of airway infection may induce MP release in patients with COPD exacerbation.
EMPs
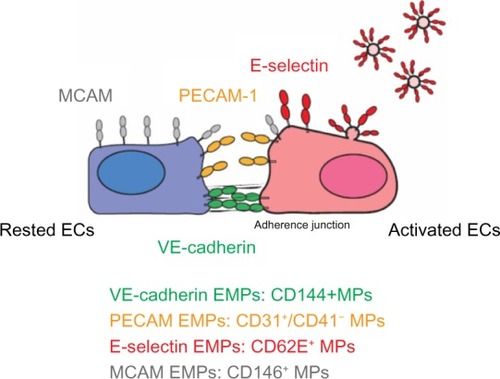
EMPs are released from injured endothelial cells during apoptosis and cellular activation.Citation19,Citation56,Citation57 EMPs are defined by the surface expression of endothelial antigens, such as CD144 (vascular endothelial [VE]-cadherin), CD31 (platelet endothelial cell adhesion molecule [PECAM]-1), CD62e (E-selectin), CD146 (melanoma cell adhesion molecule [MCAM]), and CD51 (vitronectin) ( and ). Circulating EMP numbers are increased in patients with vascular disorders such as cardiovascular diseases,Citation58–Citation60 vasculitis,Citation61,Citation62 thrombosis,Citation63,Citation64 renal failure,Citation65–Citation67 hyperlipidemia,Citation68 and metabolic syndrome.Citation69,Citation70 The increase in circulating EMPs reflects the degree of endothelial injury in such conditions ().
Table 1 Endothelial markers used for detecting EMPs
Table 2 Numbers of circulating EMPs in various diseases
Figure 2 Location of four EMP markers on ECs.
Abbreviations: EMPs, endothelial microparticles; ECs, endothelial cells; PECAM, platelet endothelial cell adhesion molecule; MCAM, melanoma cell adhesion molecule; VE, vascular endothelial; MPs, microparticles.
In vitro studies have demonstrated that different stimuli, such as tumor necrosis factor α, H2O2, and cigarette smoke extract, induce the release of different types of EMPs from cultured endothelial cells. The pattern of EMP release in response to a specific stimulus differs depending on the type of endothelial cell.Citation40 These observations suggest that different types of EMPs are released depending on the site of inflammation and the type of stimulus. Some clinical studies have compared two or more subtypes of EMPs in patients with specific diseases and observed differences in the types of released EMPs (). Although the clinical significance of these differences in EMP release remains unclear, the pattern of increased EMPs may reflect differences in inflammatory stimuli and the activated site in vivo.
Table 3 Articles comparing two or more EMP subtypes in subjects with pathological conditions
VE-cadherin EMPs
VE-cadherin EMPs are defined as CD144+ MPs. VE-cadherin is expressed only on endothelial cells and is therefore the most specific marker for endothelial cells.Citation71 In contrast to PECAM-1 and MCAM, VE-cadherin is specifically located at adherence junctions.Citation72 VE-cadherin has been proposed to function as a gatekeeper of endothelial junctions.Citation37,Citation73 VE-cadherin does not support leukocyte migration but instead might function as an obstacle to migrating cells. Changes in the localization of VE-cadherin are associated with neutrophil migration and increased vascular permeability.Citation74 The release of VE-cadherin EMPs may reflect the structural destruction of the endothelium rather than the inflammatory condition of the lung.
PECAM EMPs
PECAM-1 (CD31) is concentrated at endothelial junctions and is also expressed on the surfaces of platelets, neutrophils, and subsets of lymphocytes. In contrast to VE-cadherin, PECAM-1 is located outside of the adherence junctions on endothelial cells.Citation72,Citation75 Platelet-specific antigens such as CD41 or CD42b are used to distinguish PECAM EMPs from platelet-derived MPs; PECAM EMPs are defined as CD31+/CD41− or CD42b− MPs. PECAM-1 is not used to detect leukocyte-derived MPs because its expression on leukocytes is too low.Citation76 PECAM-1 is a signaling molecule that plays diverse roles in vascular biology, including the regulation of platelet function, angiogenesis, T-cell and B-cell activation, endothelial cell permeability, and transmigration across the endothelium.Citation37,Citation77–Citation81 Our in vitro study indicated that PECAM EMPs are released from pulmonary microvascular endothelial cells mainly in response to apoptosis induced by stimulation by H2O2 or cigarette smoke extract.Citation40 In addition, a clinical study indicated that approximately 90% of PECAM EMPs also express annexin V in COPDCitation82 and 60% of PECAM EMPs express annexin V in active smokers.Citation46 Therefore, the released EMPs likely reflect the apoptosis of injured endothelial cells.
MCAM EMPs
MCAM EMPs are defined as CD146+ MPs. MCAM is an adhesion molecule found on endothelial cells that is involved in processes such as endothelial permeability, signaling transduction, cell migration, angiogenesis, and the immune response.Citation83 MCAM is located outside of the adherence junctions; however, MCAM expression is not restricted to cell junctions and is detected on the apical side of cultured endothelial cells.Citation72 MCAM has been detected not only on endothelial cells but also on other cell types such as melanoma cells,Citation84 a subset of T and B lymphocytes and natural killer (NK) cells under pathological conditions,Citation85 and pericytes.Citation86 Thus, CD146+ MPs can be released from other cell types as well as endothelial cells. In addition, the expression of MCAM, another marker of endothelial injury, is also used to detect circulating endothelial cells.Citation85,Citation87 Thus, circulating endothelial cells can be confounded with MCAM EMPs in FACS analysis.Citation88
E-selectin EMPs
E-selectin is expressed only on endothelial cells and E-selectin EMPs are defined as CD62e+ MPs. E-selectin is rapidly induced on activated endothelial cells a few hours after inflammatory stimulation, whereas VE-cadherin, PECAM-1, and MCAM are constitutively expressed on endothelial cells.Citation89 E-selectin plays an important role in recruiting leukocytes to the site of injury during inflammation.Citation37,Citation77–Citation81 In vitro studies have indicated that tumor necrosis factor α upregulates E-selectin expression on endothelial cells within a few hours, resulting in the increased release of E-selectin EMPs.Citation40,Citation90 Thus, the presence of E-selectin EMPs might reflect the degree of ongoing endothelial inflammation.
CD51 EMPs
CD51 is the integrin α chain and is also known as the vitronectin receptor α chain. CD51 forms a heterodimer with an integrin β3 chain, such as glycoprotein IIIa (GPIIIa) or the CD61 molecule, and binds to vitronectin, von Willebrand factor (vWF), and fibronectin.Citation91 CD51 is found on endothelial cells, B lymphocytes, monocytes, macrophages, and platelets.Citation92 CD51 plays important roles in leukocyte homing and rolling and angiogenesis.Citation37,Citation93 Although the specificity of endothelial origin is less than the other four EMP subtypes, CD51 MPs are defined as EMPs in some articles.Citation94–Citation96
COPD and EMPs
We examined the levels of VE-cadherin, PECAM, MCAM, and E-selectin EMPs in 80 patients with stable COPD and 27 patients with exacerbated COPD.Citation97 The MESA group examined the numbers of PECAM, E-selectin, and CD51 EMPs in 104 patients with stable COPD and 76 non-COPD patients.Citation96 These are the only two reported studies of EMP numbers in COPD patients.
Circulating EMPs in patients with stable COPD
Recent studies have indicated that the main causes of death in COPD are cardiovascular events such as ischemic heart diseases and stroke, not respiratory events.Citation98 Vascular abnormalities in the endothelium in both the pulmonaryCitation99 and systemic vasculaturesCitation100 have been reported in COPD patients. Impaired endothelial function as assessed by flow-mediated dilation of the brachial artery is associated with low FEV1 in COPD.Citation101 Subclinical arteriosclerosis, as evaluated by carotid intima–media thickness and focal athermanous plaque, is exaggerated early in the disease process of COPD.Citation102 Reduced white matter integrity throughout the brain has been detected by magnetic resonance imaging in COPD patients, suggesting cerebral small vessel injury caused by COPD-associated conditions.Citation103,Citation104 The repair capacity of endothelial progenitor cells is also significantly impaired in the early stage of COPD.Citation97,Citation105 Furthermore, some animal studies have demonstrated apoptosis of pulmonary capillary endothelial cells due to emphysematous changes.Citation11 These reports indicate that endothelial injury is closely connected to the pathophysiology of COPD.
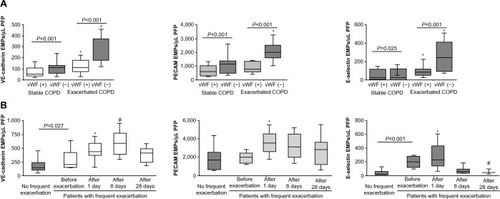
We reported that VE-cadherin EMPs, PECAM EMPs, and E-selectin EMPs were significantly increased in patients with stable COPD compared to ex-smoker controls independent of smoking history, sex, body mass index, and age.Citation82 This increase in PECAM EMPs was confirmed in a larger study performed by the MESA group.Citation96 However, when adjusted for other factors, there were no significant differences in the levels of CD51 EMPs and E-selectin EMPs, although both EMPs tended to increase in COPD in the MESA group’s study. According to the Global initiative for chronic Obstructive Lung Disease (GOLD) stage progression, VE-cadherin EMPs, PECAM EMPs, and E-selectin EMPs were significantly increased in our study, although we could not analyze differences between each stage due to the small sample sizes. The MESA group reported that PECAM EMPs were significantly increased in mild and moderate COPD compared to control subjects in analyses adjusted for other factors; in contrast CD51 EMPs were not significantly elevated and E-selectin EMPs were only elevated in severe COPD. These results indicate that PECAM, VE-cadherin, and E-selectin EMPs are increased in patients with stable COPD compared to healthy controls independent of other factors associated with endothelial injury.
These two studies also examined the relationships between the degree of lung destruction and the numbers of EMPs. PECAM EMPs, E-selectin EMPs, and VE-cadherin EMPs were inversely correlated with parameters of lung functions such as the percent predicted FEV1. The MESA group also reported that PECAM EMPs were significantly associated with parameters of lung destruction such as percent emphysema (low attenuation area; LAA), pulmonary microvascular perfusion as assessed by magnetic resonance imaging, and diffusing capacity of the lung for carbon monoxide (DLCO). However, there was no significant correlation between E-selectin EMP levels and these parameters. By contrast, E-selectin EMPs were significantly related to hyperinflation characterized by both increased RV and a higher RV/TLC ratio; no such relationship was observed for PECAM EMPs. These results indicate that endothelial injury combined with impaired mobilization capacity of endothelial progenitor cellsCitation97,Citation105 might be involved in the pathogenesis of emphysema and COPD.
Endothelial injury occurs in both the pulmonary and systemic vasculatures in COPD. Thus, EMPs can be released from both the pulmonary vasculatures and systemic vasculatures. To distinguish between EMPs originating from pulmonary and systemic circulation, the absence of vWFCitation82 and the presence of angiotensin-converting enzyme (ACE; CD143)Citation46 on EMPs were examined. vWF is a marker of endothelial cells that is not expressed on pulmonary capillary cells.Citation106,Citation107 Thus, vWF-negative EMPs are of pulmonary capillary origin. The pulmonary capillary endothelium contains abundant ACE. Thus, ACE-positive EMPs are of pulmonary capillary origin. In active smokers with normal spirometry and low DLCO, approximately 75% of PECAM EMPs were ACE positive, indicating pulmonary capillary origin.Citation46 In stage II COPD patients, approximately 60% of the increased EMPs were vWF negative (). In addition, the numbers of EMPs in stage IV COPD patients with severe emphysema were lower than those with mild emphysema, reflecting the reduction of pulmonary capillary vascular beds in emphysema. These results confirmed two facts. First, the increased EMPs in COPD mainly originate from the pulmonary capillary vasculature. Second, the numbers of vWF-negative or ACE-positive EMPs are sensitive markers for the detection of endothelial injury in the pulmonary capillary vasculature.
Figure 3 EMP levels in patients with exacerbated COPD.
Abbreviations: EMPs, endothelial microparticles; COPD, chronic obstructive pulmonary disease; VE, vascular endothelial; PECAM, platelet endothelial cell adhesion molecule; PFP, platelet free plasma; vWF, von Willebrand factor.
Multivariable analysis revealed that E-selectin levels were higher in patients with stable COPD with a history of frequent exacerbation (two or more episodes of exacerbation each year) than in COPD patients without frequent exacerbation.Citation82 Unfortunately, the relationship between the number of EMPs and the rate of exacerbation was not analyzed in the MESA study.Citation96 E-selectin EMP levels may reflect ongoing endothelial inflammation. Because exacerbations are associated with accelerated loss of lung function,Citation2–Citation5 we hypothesize that high E-selectin EMP levels led to a rapid loss of lung function in patients with stable COPD with a history of frequent exacerbation.
Circulating EMPs in patients with exacerbated COPD
Recent studies have indicated that an exacerbation episode is connected to the progression of COPD. A history of frequent exacerbation and/or numbers of exacerbation episodes is associated with future rapid FEV1 decline, increased morbidity, readmission rates, and mortality.Citation108–Citation112 Exacerbation episodes are also associated with emphysema progression, as evaluated by chest computed tomography scans in patients with COPD.Citation113 Although the importance of exacerbation in COPD management is becoming clearer, the diagnosis and evaluation of exacerbation are mainly based on clinical symptoms.
Accumulating evidence suggests that further endothelial injury occurs during exacerbation. For example, flow and nitroglycerin-mediated peripheral vascular dilation are impaired during acute exacerbation.Citation114 In addition, inflammatory responses, particularly platelet activation, are upregulated during exacerbation. Platelet aggregation is exaggerated,Citation115 mean platelet volume is increased,Citation116 and plasma vWF and fibrinogen levels are increased during exacerbation.Citation117 To clarify the influence of COPD exacerbation on the endothelium, we examined the numbers of EMPs during exacerbation.
VE-cadherin, PECAM, and E-selectin EMPs were significantly higher in patients during COPD exacerbation than in stable patients. Interestingly, the majority of the EMPs that were increased during COPD exacerbation were vWF negative (), indicating that pulmonary capillary endothelial cells were the main targets of injury during exacerbation. In addition, exacerbation is associated with the progression of emphysema,Citation113 which is connected to endothelial injury in the pulmonary vasculature.Citation11 These reports confirmed that endothelial injury, primarily in pulmonary capillary endothelial cells, occurred during exacerbation.
The trends in the levels of circulating VE-cadherin EMPs, PECAM EMPs, and E-selectin EMPs differed after exacerbation (). All EMP levels increased significantly one day after the onset of exacerbation, but after 28 days, the levels of VE-cadherin EMPs and PECAM EMPs remained high, while the level of E-selectin EMPs declined to less than the baseline level. The persistent high VE-cadherin and PECAM EMP levels after 28 days indicate that endothelial injury induced by exacerbation continues even after clinical symptoms disappear. Similar to E-selectin EMP levels, plasma fibrinogen levels also increase during exacerbation and decrease significantly in 4–6 weeks.Citation118–Citation120 Although there are no data to indicate a significant correlation between E-selectin EMP levels and plasma fibrinogen levels, these reports provide additional evidence that E-selectin EMP levels reflect ongoing endothelial inflammation and that VE-cadherin and PECAM EMP levels reflect endothelial injury as a consequence of endothelial inflammation. The decrease in the high E-selectin EMP levels during the early phase of exacerbation to levels lower than the baseline after treatment indicates that inflammation is present on the endothelium even during the stable phase before the onset of exacerbation. Most of the patients who underwent exacerbation received a systemic corticosteroid therapy, however, the role of steroid in the EMP levels is not defined yet. Further analyses are needed to clarify the impact of the COPD drugs on the kinetics of EMPs. Furthermore, drug therapies that ameliorate increased EMP levels may have an effect on prognosis of COPD patients.
Effects of increased EMPs on COPD pathophysiology
Increased numbers of EMPs are observed in patients with stable COPD. The release of EMPs increases further during exacerbation. In addition, COPD patients with a history of frequent exacerbation exhibit high levels of circulating EMPs not only during exacerbation but also in the stable phase. However, the effects of this increase in EMPs on COPD pathophysiology remain unclear. In patients with severe systemic inflammatory syndrome, EMPs are produced and actively bind to leukocytes.Citation121 MPs from human atherosclerotic plaques promote transendothelial migration of monocytes.Citation122 Circulating MPs in patients with myocardial infarction induce endothelial dysfunction.Citation123 Based on these reports, the increased EMPs released from injured pulmonary endothelium might induce further endothelial injury in both the pulmonary vasculature and distant systemic vasculature; this injury might be involved in further lung destruction as well as the increased incidence of cardiovascular diseases in COPD patients in both the stable phase and after exacerbation.Citation108 Because the MPs released in response to various stimuli are heterogeneous, future studies should examine the effects of MPs on the endothelium using MPs isolated from COPD patients. Prospective studies are also needed to clarify the relationship between increased EMP levels and the incidence of cardiovascular diseases.
Technical difficulties with measuring EMPs
There are several technical difficulties reported in the measurement of MPs. For example, there is no clear definition of MPs. The differences between exosomes and MPs remain unclear. Thus, there is no standard protocol for isolating and detecting circulating MPs from the plasma, and the results of microparticle studies are often inconsistent.Citation124 Differences in flow cytometers influence the sensitivity of MP detection. In addition, differences in centrifugation protocols influence the number of MPs. Clear definitions and standardization of protocols are essential.
Other remaining questions
Although much evidence for a link between COPD and circulating EMPs has been documented, there are several issues to clarify in the future.
Firstly, the effects of increased EMPs on the pathophysiological condition or progression of exacerbation are not clear. MPs are not the passive parameter induced from activated or injured cells but rather active modulators that promote both pro-inflammatory and anti-inflammatory signals.Citation125 MPs contain proteins and microRNAs and have a capacity to deliver those components to distant endothelial cells.Citation22 Therefore, increased EMPs may influence vascular function and systemic inflammation under COPD exacerbation.
Secondly, the release of MPs originating from other cell types is not clearly evaluated in COPD patients. Other MPs, such as platelet-derived and leukocyte-derived MPs, play different roles in endothelial phenotypes; in particular, platelet-derived MPs are known to increase in cardiovascular diseases, including myocardial infarction.Citation126 Therefore, the role of other MPs in the comorbidity and the prognosis of COPD would be a great interest.
Lastly, epithelial-derived MPs in airways are not elucidated in COPD patients. Tissue factor-bearing MPs, which demonstrate procoagulant activity, are elevated in the pulmonary edema fluid of acute respiratory distress syndrome patients;Citation127 these MPs appear to be derived from alveolar epithelial cells. Similarly, epithelial injury present in COPD airways may produce the epithelial-derived MPs.
Conclusion
In this review, we proposed the potential use of circulating MPs, particularly EMPs, as novel biomarkers for COPD (). In addition, by comparing subtypes of MPs that are increased in COPD patients, we may be able to reclassify heterogeneous COPD. Furthermore, the relationship between COPD and other MPs, such as platelet-derived MPs, could be an intriguing area of investigation in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- RabeKFHurdSAnzuetoAGlobal Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- VestboJEdwardsLDScanlonPDECLIPSE InvestigatorsChanges in forced expiratory volume in 1 second over time in COPDN Engl J Med2011365131184119221991892
- KannerREAnthonisenNRConnettJELung Health Study Research GroupLower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health studyAm J Respir Crit Care Med2001164335836411500333
- CalverleyPMAndersonJACelliBTORCH investigatorsSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- BrutscheMHDownsSHSchindlerCSAPALDIA TeamBronchial hyperresponsiveness and the development of asthma and COPD in asymptomatic individuals: SAPALDIA cohort studyThorax200661867167716670173
- AgustiACalverleyPMCelliBEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigatorsCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- VestboJRennardSChronic obstructive pulmonary disease biomarker(s) for disease activity needed – urgentlyAm J Respir Crit Care Med2010182786386420884938
- HensonPMVandivierRWDouglasISCell death, remodeling, and repair in chronic obstructive pulmonary disease?Proc Am Thorac Soc20063871371717065379
- SchmidtEPTuderRMRole of Apoptosis in Amplifying Inflammatory Responses in Lung DiseasesJ Cell Death201020103415322081757
- KasaharaYTuderRMTaraseviciene-StewartLInhibition of VEGF receptors causes lung cell apoptosis and emphysemaJ Clin Invest2000106111311131911104784
- KasaharaYTuderRMCoolCDLynchDAFloresSCVoelkelNFEndothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysemaAm J Respir Crit Care Med20011633 Pt 173774411254533
- TsujiTAoshibaKNagaiAAlveolar cell senescence in patients with pulmonary emphysemaAm J Respir Crit Care Med2006174888689316888288
- HodgeSHodgeGHolmesMReynoldsPNIncreased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessationEur Respir J200525344745415738287
- VandivierRWHensonPMDouglasISBurying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung diseaseChest200612961673168216778289
- RyterSWChoiAMAutophagy in the lungProc Am Thorac Soc201071132120160144
- PisetskyDSGauleyJUllalAJHMGB1 and microparticles as mediators of the immune response to cell deathAntioxid Redox Signal20111582209221921194388
- TheryCOstrowskiMSeguraEMembrane vesicles as conveyors of immune responsesNat Rev Immunol20099858159319498381
- GyorgyBSzaboTGPasztoiMMembrane vesicles, current state-of-the-art: emerging role of extracellular vesiclesCell Mol Life Sci201168162667268821560073
- CocucciERacchettiGMeldolesiJShedding microvesicles: artefacts no moreTrends Cell Biol2009192435119144520
- NomuraSOzakiYIkedaYFunction and role of microparticles in various clinical settingsThromb Res2008123182318667228
- MartinezMCTual-ChalotSLeonettiDAndriantsitohainaRMicroparticles: targets and tools in cardiovascular diseaseTrends Pharmacol Sci2011321165966521794929
- RautouPEVionACAmabileNMicroparticles, vascular function, and atherothrombosisCirc Res2011109559360621852557
- BoilardENigrovicPALarabeeKPlatelets amplify inflammation in arthritis via collagen-dependent microparticle productionScience2010327596558058320110505
- RubinOCanelliniGDelobelJLionNTissotJDRed blood cell microparticles: clinical relevanceTransfus Med Hemother201239534234723801926
- SmalleyDMShemanNENelsonKTheodorescuDIsolation and identification of potential urinary microparticle biomarkers of bladder cancerJ Proteome Res2008752088209618373357
- ValentiRHuberVIeroMFilipazziPParmianiGRivoltiniLTumor-released microvesicles as vehicles of immunosuppressionCancer Res20076772912291517409393
- MostefaiHAMezianiFMastronardiMLCirculating microparticles from patients with septic shock exert protective role in vascular functionAm J Respir Crit Care Med2008178111148115518723433
- BoulangerCMAmabileNTedguiACirculating microparticles: a potential prognostic marker for atherosclerotic vascular diseaseHypertension200648218018616801490
- SidhuSSMengistabATTauscherANLaVailJBasbaumCThe microvesicle as a vehicle for EMMPRIN in tumor-stromal interactionsOncogene200423495696314749763
- MacKenzieAWilsonHLKiss-TothEDowerSKNorthRASurprenantARapid secretion of interleukin-1beta by microvesicle sheddingImmunity200115582583511728343
- WilsonHLFrancisSEDowerSKCrossmanDCSecretion of intracellular IL-1 receptor antagonist (type 1) is dependent on P2X7 receptor activationJ Immunol200417321202120815240711
- GasserOHessCMiotSDeonCSanchezJCSchifferliJACharacterisation and properties of ectosomes released by human polymorphonuclear neutrophilsExp Cell Res2003285224325712706119
- BaroniMPizziraniCPinottiMStimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticlesFASEB J20072181926193317314141
- PisetskyDSUllalAJGauleyJNingTCMicroparticles as mediators and biomarkers of rheumatic diseaseRheumatology (Oxford)201251101737174622403183
- MartinezMCTesseAZobairiFAndriantsitohainaRShed membrane microparticles from circulating and vascular cells in regulating vascular functionAm J Physiol Heart Circ Physiol20052883H1004H100915706036
- LeyKLaudannaCCybulskyMINoursharghSGetting to the site of inflammation: the leukocyte adhesion cascade updatedNat Rev Immunol20077967868917717539
- LovrenFVermaSEvolving role of microparticles in the pathophysiology of endothelial dysfunctionClin Chem20135981166117423529703
- RyterSWChenZHKimHPChoiAMAutophagy in chronic obstructive pulmonary disease: homeostatic or pathogenic mechanism?Autophagy20095223523719066468
- TakahashiTKobayashiSFujinoNDifferences in the released endothelial microparticle subtypes between human pulmonary microvascular endothelial cells and aortic endothelial cells in vitroExp Lung Res2013394–515516123550836
- KosmiderBMessierEMChuHWMasonRJHuman alveolar epithelial cell injury induced by cigarette smokePLoS One2011612e2605922163265
- BernhardDPfisterGHuckCWDisruption of vascular endothelial homeostasis by tobacco smoke: impact on atherosclerosisFASEB J200317152302230414525940
- LiMYuDWilliamsKJLiuMLTobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophagesArterioscler Thromb Vasc Biol20103091818182420558816
- LiCJLiuYChenYYuDWilliamsKJLiuMLNovel proteolytic microvesicles released from human macrophages after exposure to tobacco smokeAm J Pathol201318251552156223499464
- HeissCAmabileNLeeACBrief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide productionJ Am Coll Cardiol200851181760177118452782
- GordonCGudiKKrauseACirculating endothelial microparticles as a measure of early lung destruction in cigarette smokersAm J Respir Crit Care Med2011184222423221471087
- SundarIKYaoHRahmanIOxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseasesAntioxid Redox Signal201318151956197122978694
- TuderRMPetracheIPathogenesis of chronic obstructive pulmonary diseaseJ Clin Invest201212282749275522850885
- HelalODefoortCRobertSIncreased levels of microparticles originating from endothelial cells, platelets and erythrocytes in subjects with metabolic syndrome: relationship with oxidative stressNutr Metab Cardiovasc Dis201121966567120399083
- SethiSMaloneyJGroveLWronaCBerensonCSAirway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2006173999199816474030
- SeemungalTSykesAICEAD ContributorsRecent advances in exacerbations of COPDThorax2008631085085218820113
- MayneEFunderburgNTSiegSFIncreased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expressionJ Acquir Immune Defic Syndr201259434034622156911
- KornekMLynchMMehtaSHCirculating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitisGastroenterology2012143244845822537612
- GeSHertelBEmdenSHMicroparticle generation and leucocyte death in Shiga toxin-mediated HUSNephrol Dial Transplant20122772768277522234918
- NieuwlandRBerckmansRJMcGregorSCellular origin and procoagulant properties of microparticles in meningococcal sepsisBlood200095393093510648405
- DiamantMTushuizenMESturkANieuwlandRCellular microparticles: new players in the field of vascular disease?Eur J Clin Invest200434639240115200490
- FreyssinetJMCellular microparticles: what are they bad or good for?J Thromb Haemost2003171655166212871302
- SinghNVan CraeyveldETjwaMCirculating apoptotic endothelial cells and apoptotic endothelial microparticles independently predict the presence of cardiac allograft vasculopathyJ Am Coll Cardiol201260432433122813611
- JungCSorenssonPSalehNArhedenHRydenLPernowJCirculating endothelial and platelet derived microparticles reflect the size of myocardium at risk in patients with ST-elevation myocardial infarctionAtherosclerosis2012221122623122245039
- NozakiTSugiyamaSKogaHSignificance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart diseaseJ Am Coll Cardiol200954760160819660689
- ErdbrueggerUGrossheimMHertelBDiagnostic role of endothelial microparticles in vasculitisRheumatology (Oxford)200847121820182518927191
- DuvalAHelleyDCapronLEndothelial dysfunction in systemic lupus patients with low disease activity: evaluation by quantification and characterization of circulating endothelial microparticles, role of anti-endothelial cell antibodiesRheumatology (Oxford)20104961049105520211868
- JimenezJJJyWMauroLMHorstmanLLSoderlandCAhnYSEndothelial microparticles released in thrombotic thrombocytopenic purpura express von Willebrand factor and markers of endothelial activationBr J Haematol2003123589690214632781
- CombesVSimonACGrauGEIn vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulantJ Clin Invest199910419310210393703
- AmabileNGuerinAPLeroyerACirculating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failureJ Am Soc Nephrol200516113381338816192427
- FaureVDouLSabatierFElevation of circulating endothelial microparticles in patients with chronic renal failureJ Thromb Haemost20064356657316405517
- AmabileNGuerinAPTedguiABoulangerCMLondonGMPredictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: a pilot studyNephrol Dial Transplant20122751873188022036944
- PirroMSchillaciGPaltricciaRIncreased ratio of CD31+/CD42− microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemiaArterioscler Thromb Vasc Biol200626112530253516946129
- MooreXLMichellDLeeSIncreased carotid intima–media thickness and reduced distensibility in human class III obesity: independent and differential influences of adiposity and blood pressure on the vasculaturePLoS One201381e5397223342053
- StepanianABourguignatLHennouSMicroparticle increase in severe obesity: Not related to metabolic syndrome and unchanged after massive weight lossObesity2013212236224323512861
- VincentPAXiaoKBuckleyKMKowalczykAPVE-cadherin: adhesion at arm’s lengthAm J Physiol Cell Physiol20042865C987C99715075197
- BardinNAnfossoFMasseJMIdentification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesionBlood200198133677368411739172
- ShawSKBambaPSPerkinsBNLuscinskasFWReal-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endotheliumJ Immunol200116742323233011490021
- ConwayDSchwartzMALessons from the endothelial junctional mechanosensory complexF1000 Biol Rep20124122238515
- NewmanPJBerndtMCGorskiJPECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamilyScience19902474947121912221690453
- Angelillo-ScherrerALeukocyte-derived microparticles in vascular homeostasisCirc Res2012110235636922267840
- PatilSNewmanDKNewmanPJPlatelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagenBlood20019761727173211238114
- CicmilMThomasJMLeducMBonCGibbinsJMPlatelet endothelial cell adhesion molecule-1 signaling inhibits the activation of human plateletsBlood200299113714411756163
- FalatiSPatilSGrossPLPlatelet PECAM-1 inhibits thrombus formation in vivoBlood2006107253554116166583
- MullerWALeukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory responseTrends Immunol200324632733412810109
- WoodfinAVoisinMBNoursharghSPECAM-1: a multi-functional molecule in inflammation and vascular biologyArterioscler Thromb Vasc Biol200727122514252317872453
- TakahashiTKobayashiSFujinoNIncreased circulating endothelial microparticles in COPD patients: a potential biomarker for COPD exacerbation susceptibilityThorax201267121067107422843558
- WangZYanXCD146, a multi-functional molecule beyond adhesionCancer Lett2013330215016223266426
- LehmannJMRiethmullerGJohnsonJPMUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamilyProc Natl Acad Sci U S A19898624989198952602381
- ElshalMFKhanSSTakahashiYSolomonMAMcCoyJPJrCD146 (Mel-CAM), an adhesion marker of endothelial cells, is a novel marker of lymphocyte subset activation in normal peripheral bloodBlood200510682923292416204154
- MaierCLShepherdBRYiTPoberJSExplant outgrowth, propagation and characterization of human pericytesMicrocirculation201017536738020618694
- BlannADWoywodtABertoliniFCirculating endothelial cells. Biomarker of vascular diseaseThromb Haemost200593222823515711737
- BertoliniFShakedYMancusoPKerbelRSThe multifaceted circulating endothelial cell in cancer: towards marker and target identificationNat Rev Cancer200661183584517036040
- BevilacquaMButcherEFurieBSelectins: a family of adhesion receptorsCell19916722331717161
- JimenezJJJyWMauroLMSoderlandCHorstmanLLAhnYSEndothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosisThromb Res2003109417518012757771
- SosnoskiDMEmanuelBSHawkinsALChromosomal localization of the genes for the vitronectin and fibronectin receptors alpha subunits and for platelet glycoproteins IIb and IIIaJ Clin Invest1988816199319982454952
- KokuboTUchidaHChoiETIntegrin alpha(v)beta(3) as a target in the prevention of neointimal hyperplasiaJ Vasc Surg200745Suppl AA33A3817544022
- SheppardDRoles of alphav integrins in vascular biology and pulmonary pathologyCurr Opin Cell Biol200416555255715363806
- MinagarAJyWJimenezJJElevated plasma endothelial microparticles in multiple sclerosisNeurology200156101319132411376181
- Bernal-MizrachiLJyWJimenezJJHigh levels of circulating endothelial microparticles in patients with acute coronary syndromesAm Heart J2003145696297012796750
- ThomashowMAShimboDParikhMAEndothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The Multi-Ethnic Study of Atherosclerosis Chronic Obstructive Pulmonary Disease studyAm J Respir Crit Care Med20131881606823600492
- TakahashiTSuzukiSKuboHYamayaMKurosawaSKatoMImpaired endothelial progenitor cell mobilization and colony-forming capacity in chronic obstructive pulmonary diseaseRespirology201116468068721355963
- YoungRPHopkinsREatonTEForced expiratory volume in one second: not just a lung function test but a marker of premature death from all causesEur Respir J200730461662217906084
- PeinadoVIPizarroSBarberaJAPulmonary vascular involvement in COPDChest2008134480881418842913
- HunninghakeDBCardiovascular disease in chronic obstructive pulmonary diseaseProc Am Thorac Soc200521444916113468
- BarrRGMesia-VelaSAustinJHImpaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) StudyAm J Respir Crit Care Med2007176121200120717761614
- IwamotoHYokoyamaAKitaharaYAirflow limitation in smokers is associated with subclinical atherosclerosisAm J Respir Crit Care Med20091791354018931335
- RyuCWJahngGHChoiCWMicrostructural change of the brain in chronic obstructive pulmonary disease: a voxel-based investigation by MRICOPD201310335736623713596
- DoddJWChungAWvan den BroekMDBarrickTRCharltonRAJonesPWBrain structure and function in chronic obstructive pulmonary disease: a multimodal cranial magnetic resonance imaging studyAm J Respir Crit Care Med2012186324024522652026
- PalangePTestaUHuertasACirculating haemopoietic and endothelial progenitor cells are decreased in COPDEur Respir J200627352954116507853
- MullerAMHermannsMISkrzynskiCNesslingerMMullerKMKirkpatrickCJExpression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitroExp Mol Pathol200272322122912009786
- KawanamiOJinEGhazizadehMMosaic-like distribution of endothelial cell antigens in capillaries and juxta-alveolar microvessels in the normal human lungPathol Int200050213614110792772
- DonaldsonGCHurstJRSmithCJHubbardRBWedzichaJAIncreased risk of myocardial infarction and stroke following exacerbation of COPDChest201013751091109720022970
- ManninoDMThornDSwensenAHolguinFPrevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPDEur Respir J200832496296918579551
- Soler-CatalunaJJMartinez-GarciaMARoman SanchezPSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax2005601192593116055622
- SeemungalTADonaldsonGCPaulEABestallJCJeffriesDJWedzichaJAEffect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981575 Pt 1141814229603117
- PatelARKowlessarBSDonaldsonGCCardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201318891091109924033321
- TanabeNMuroSHiraiTImpact of exacerbations on emphysema progression in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2011183121653165921471102
- MarchettiNCiccolellaDEJacobsMRHospitalized acute exacerbation of COPD impairs flow and nitroglycerin-mediated peripheral vascular dilationCOPD201182606521495833
- MaclayJDMcAllisterDAJohnstonSIncreased platelet activation in patients with stable and acute exacerbation of COPDThorax201166976977421507906
- WangRTLiJYCaoZGLiYMean platelet volume is decreased during an acute exacerbation of chronic obstructive pulmonary diseaseRespirology20131881244124823786593
- PolatliMCakirACildagOBolamanAZYeniseyCYeniceriogluYMicroalbuminuria, von Willebrand factor and fibrinogen levels as markers of the severity in COPD exacerbationJ Thromb Thrombolysis20082629710217622488
- WedzichaJASeemungalTAMacCallumPKAcute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levelsThromb Haemost200084221021510959691
- KoutsokeraAKiropoulosTSNikoulisDJClinical, functional and biochemical changes during recovery from COPD exacerbationsRespir Med2009103691992619121927
- ValipourASchrederMWolztMCirculating vascular endothelial growth factor and systemic inflammatory markers in patients with stable and exacerbated chronic obstructive pulmonary diseaseClin Sci (Lond)2008115722523218307413
- OguraHTanakaHKohTEnhanced production of endothelial microparticles with increased binding to leukocytes in patients with severe systemic inflammatory response syndromeJ Trauma2004564823830 discussion 830–82115187749
- RautouPELeroyerASRamkhelawonBMicroparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migrationCirc Res2011108333534321164106
- BoulangerCMScoazecAEbrahimianTCirculating microparticles from patients with myocardial infarction cause endothelial dysfunctionCirculation2001104222649265211723013
- WitwerKWBuzasEIBemisLTStandardization of sample collection, isolation and analysis methods in extracellular vesicle researchJ Extracell Vesicles20132
- MorelOTotiFMorelNFreyssinetJMMicroparticles in endothelial cell and vascular homeostasis: are they really noxious?Haematologica200994331331719252173
- van der ZeePMBiroEKoYP-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarctionClin Chem200652465766416439610
- BastaracheJAFremontRDKropskiJABossertFRWareLBProcoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndromeAm J Physiol Lung Cell Mol Physiol20092976L1035L104119700643
- AmabileNHeissCRealWMCirculating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertensionAm J Respir Crit Care Med2008177111268127518310479
- AmabileNHeissCChangVIncreased CD62e(+) endothelial microparticle levels predict poor outcome in pulmonary hypertension patientsJ Heart Lung Transplant200928101081108619782291
- AyersLFerryBCraigSNicollDStradlingJRKohlerMCirculating cell-derived microparticles in patients with minimally symptomatic obstructive sleep apnoeaEur Respir J200933357458019047314
- SimakJGeldermanMPYuHWrightVBairdAECirculating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcomeJ Thromb Haemost2006461296130216706974