?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aerosols delivered by Respimat® Soft Mist™ Inhaler (SMI) are slower-moving and longer-lasting than those from pressurized metered-dose inhalers (pMDIs), improving the efficiency of pulmonary drug delivery to patients. In this four-way cross-over study, adults with chronic obstructive pulmonary disease (COPD) and with poor pMDI technique received radiolabelled Berodual® (fenoterol hydrobromide 50 μg/ipratropium bromide 20 μg) via Respimat® SMI or hydrofluoroalkane (HFA)-MDI (randomized order) on test days 1 and 2, with no inhaler technique training. The procedure was repeated on test days 3 and 4 after training. Deposition was measured by gamma scintigraphy. All 13 patients entered (9 males, mean age 62 years; FEV1 46% of predicted) inhaled too fast at screening (peak inspiratory flow rate [IF]: 69–161 L/min). Whole lung deposition was higher with Respimat® SMI than with pMDI for untrained (37% of delivered dose vs 21% of metered dose) and trained patients (53% of delivered vs 21% of metered dose) (pSign-Test].15; pANOVA < = 0.05). Training also improved inhalation profiles (slower average and peak IF as well as longer breath-hold time). Drug delivery to the lungs with Respimat® SMI is more efficient than with pMDI, even with poor inhaler technique. Teaching patients to hold their breath as well as to inhale slowly and deeply increased further lung deposition using Respimat® SMI.
Introduction
Ever since pressurized metered-dose inhalers (pMDIs) were introduced in 1956, patients have had difficulty in using them correctly (CitationEpstein et al 1979; CitationCrompton 1982; CitationMolimard et al 2003). Mistakes are made in preparation, such as failing to remove the dust cap or, in the case of chlorofluorocarbon (CFC)-propelled MDIs, not shaking the inhaler. A common error is that the inhaler is fired before inhalation begins or after it ends (CitationMcFadden 1995; CitationNewman 2005). In asthma patients, incorrect use of pMDIs has been shown to reduce the efficacy of an inhaled bronchodilator (CitationLindgren et al 1987) and was associated with poorer disease control, particularly in patients who did not correctly co-ordinate inhaler firing and inhalation (CitationGiraud and Roche 2002).
A feature of pMDIs that makes co-ordination difficult is the rapid speed at which they deliver the aerosol cloud, which may only last for 0.15 sec for CFC-MDIs, although aerosol clouds from the newer hydrofluoroalkane (HFA)-propelled MDIs last for up to 0.36 sec (CitationHochrainer et al 2005). The Respimat® Soft Mist™ Inhaler (SMI), a propellant-free metered-dose inhaler, delivers aerosols that are slower-moving and last 4–10 times longer than aerosols from pMDIs (CitationHochrainer et al 2005). This is an innovation that has been shown to deliver a higher proportion of the emitted dose to the lungs than with CFC-MDIs with and without spacer in healthy subjects (CitationNewman et al 1996, Citation1998). This enables the nominal dose of bronchodilator to be reduced at least 2-fold while maintaining efficacy and safety in patients with chronic obstructive pulmonary disease (COPD) and asthma (CitationKilfeather et al 2004; Citationvon Berg et al 2004).
Teaching the correct inhaler technique was shown to improve drug delivery and bronchodilator response in asthma patients using a CFC-MDI (CitationNewman et al 1991). The purpose of our study was to compare the efficiency of lung deposition in patients with COPD using Respimat® SMI and a pMDI. By selecting patients with a poor inhaler technique and including a training step, the influence of inhaler technique on lung deposition was assessed.
Methods
Study design
This lung deposition study employed a four-way cross-over design, and was randomized with regard to drug dosing sequence. It was done at a single investigative centre in Germany (Inamed Research, Gauting, Germany), where patients with doctor-diagnosed COPD were recruited for screening visit followed by four test days at least two days (but no more than 14 days) apart.
The study protocol complied with German federal drug laws, met radiation protection requirements and was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice. Local and federal ethics approval was obtained and all patients gave written informed consent for participation.
Patients
To be eligible for entry to the study, adults aged at least 40 years with doctor-diagnosed COPD, a forced expiratory volume in 1 second (FEV1) of no more than 65% of predicted, a ratio of FEV1 to forced vital capacity (FVC) of no more than 0.70 and who were current or former smokers (at least 10 pack years) had to show a poor pMDI technique. This was defined as at least one of the following inhalation errors, based on descriptions of the ideal technique in the published literature (CitationNewman et al 1980, Citation1981; CitationDolovich et al 1981): failure to co-ordinate firing of a pMDI with inhalation (those who pressed the button less than 0.2 sec before inhalation but did inhale more than 1 L in volume actuation); an average inspiratory flow (IF) of at least 40 L/min and a peak IF of at least 60 L/min; and reporting by the patient of a “cold freon” effect, where the patient abruptly stops inhaling because of the uncomfortable feeling of the aerosol spray hitting the back of the throat.
Interventions
Within 21 days of the screening visit, eligible patients attended the study centre for the first of four test days. On each test day, patients received a single dose of 99mTechnetium (Tc)-radiolabeled Berodual® (fenoterol hydrobromide 50 μg/ipratropium bromide 20 μg per actuation) via either Respimat® SMI (1 actuation of 50/20 μg) or HFA-MDI (2 actuations of 50/20 μg each). All test doses were open-label; no blinding procedure was used. For both inhalers, the dose of radiolabel in one actuation was calculated such that the total amount of radioactivity administered on each test day would not exceed 4 MBq (although this limit was exceeded for 3 patients; see below). For both test inhalers, the radiolabeled formulations were tested in vitro to ensure that the particle size distribution was equivalent to that of an unlabelled product.
On each test day, the fine particle dose of radiolabelled product was 14%–32% of the mean radioactive output from HFA-MDI and 50%–80% from Respimat® SMI, as measured by gamma counts on the appropriate stages of an Andersen cascade impactor (Shibata Scientific Technology Ltd., Japan), as well as by chemical assay.
On test days 1 and 2, patients inhaled with Respimat® SMI or HFA-MDI using their usual technique, ie, no inhaler training was given. On test days 3 and 4, patients received instruction by a technician, physician or study nurse on the correct use of the test inhaler according to the instructions of the manufacturers, and were given time to practice with a placebo formulation before they inhaled any study drug. They were taught to inhale slowly and deeply from residual volume and to hold their breath for at least 10 sec. When patients could perform the inhalation technique correctly at a target rate of 30 L/min, the placebo was replaced with the radiolabelled formulation. The sequence in which a patient received Respimat® SMI or HFA-MDI on the “untrained” and “trained” test days was randomly allocated at the start of test day 1. The four possible sequences are shown in .
Table 1 Allocation of test inhaler on each of the four test days
Assessments
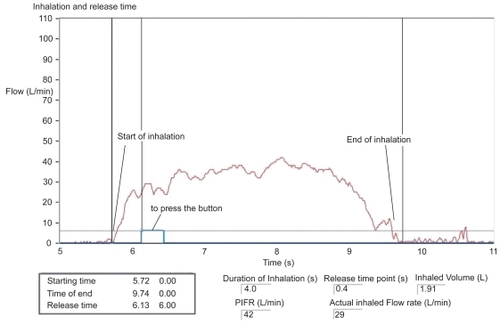
Before each patient used an inhaler on any of the test days, 3 actuations (puffs) were fired onto 3 separate inhalation filters, to ensure that the output of the inhaler was constant. To minimize contamination of the test environment, the patients used a nose clip for all test inhalations and all post-dose exhalations were made through a filter. A flow meter was used to record the inhalation profile over time (inhalation duration and volume, average and peak inhaled flow rate, and duration of breath hold). Calibration curves were defined using tube fittings which were adapted for each investigated device. The flow profiles were derived from pressure values of each test trial considering these device-depending calibration curves. These profiles were recorded on an inhalation trace (example shown in ). This showed the time points when inhalation started and when the inhaler was fired, and allowed lag time between these two events to be recorded. The breath-hold duration was also measured by an observer with a stopwatch.
Figure 1 Inspiratory flow profile during inhalation from Respimat® Soft Mist™ Inhaler (using trained technique). Courtesy Inamed Research.
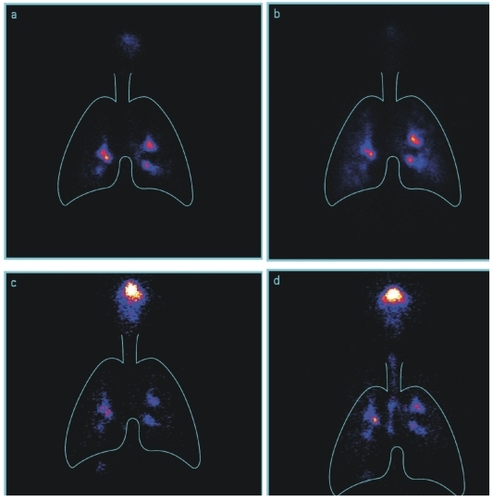
Immediately after dosing, lung radiograms were taken to measure deposition of radiolabel by gamma scintigraphy using a Siemens Diacam gamma camera. For imaging, patients sat upright with their backs to the camera (posterior view), and a sequence of images was collected over a 30-second period. Radioactivity on the inhalation and exhalation filters and mouthpiece was measured with a scintillation counter. The mean of the radioactivity on the three filters was taken as the total emitted activity from each inhaler; deposited activity was derived by subtracting the activity of exhalation filters from the emitted activity (as mean of the results of the three filters) (CitationPhipps et al 1989; CitationSnell and Ganderton 1999).
To determine the regional distribution of radioactivity, all regions of interest were delineated on each radiogram using lung outlines from a posterior krypton (81mKr) ventilation scan performed on each patient on one of the four test days. This scan was used to define the edges of the lung fields and further subdivision into central, intermediate and peripheral regions. The remaining regions defined the oropharynx, esophagus, and stomach. Counts in all regions were corrected for background radiation count, radioactive decay, and for the effect of attenuation of gamma rays by overlying tissue (CitationPitcairn and Newman 1997).
Endpoints and statistical analysis
The primary endpoint was whole lung deposition, ie, the proportion of the dose deposited in whole lung expressed as a fraction (%) of the delivered dose (ex-mouthpiece) for Respimat® SMI and of the metered dose for HFA-MDI. Secondary endpoints were the deposition of radiolabel in 3 lung regions (central, intermediate, and peripheral) and in the oropharynx. The ratio of peripheral deposition to central deposition (penetration index [PI]) was calculated and normalized to the ratio calculated from the krypton scans as a measure of the topographical distribution of the deposited aerosol in the lungs.
Deposition data were analyzed for all patients who completed all four test days (the full analysis set). Statistical analysis of the primary endpoint was done using the Sign test, a non-parametric test. The null hypothesis tested was that there is no difference between Respimat® SMI and HFA-MDI with respect to the improvement in whole lung deposition achieved after training compared with that achieved with the patient’s untrained technique. For each patient, the following sum was calculated and its sign (negative or positive) noted:
If this sum (median value for all patients) was positive, it would indicate that training was more effective for HFA-MDI, and if negative, it would indicate that training was more effective for Respimat® SMI. To assess the statistical significance of the difference between inhalers, the number of positive and negative differences between them were compared with those of a binomial distribution (in which the probabilities of a positive and a negative difference are the same, ie, 50% each). Statistical significance was assessed at the two-sided 5% level.
An additional supportive analysis was done using analysis of variance (ANOVA), for which whole lung deposition data were transformed logarithmically before use. The same mathematical construct was used as for the Sign test except that for each inhaler, the trained value was subtracted from the untrained value. Statistical analysis of the results was done using the SAS software (version 9.1, SAS Institute Inc, Cary, North Carolina, USA).
Results
The characteristics at screening of the 13 patients who entered the study are shown in . Inhaler technique assessment at the screening visit showed that all 13 patients inhaled too fast (inspiratory flow of 26–105 L/min [average] and 69–161 L/min [peak]). None showed poor co-ordination of inhaler firing, the lag time between inhalation start and inhaler firing ranging from −0.1 to +0.4 sec. None reported “cold freon” effect.
Table 2 Characteristics of 13 COPD patients at screening
One patient did not complete the study because of an acute COPD exacerbation that was judged not to be related to study medication. Therefore, the full analysis set consisted of 12 patients.
In 3 patients, the radioactivity inhaled in 1 actuation was more than the intended maximum of 4 MBq (4.1, 4.4, and 4.5 MBq). The Radiological Protection Board was notified of this.
Inhalation technique
The instruction in correct inhaler technique for the two inhalers on test days 3 and 4 produced appropriate improvements in key attributes of technique for both test inhalers. The mean average and peak IF rates were numerically slower, the mean duration of the inspiratory breath was numerically longer, and the mean breath-hold increased to more than 10 sec ().
Table 3 Characteristics of inhalation technique before and after training with both inhalers studied. Values are mean (standard deviation in parentheses) for full analysis set (n = 12)
Deposition profiles
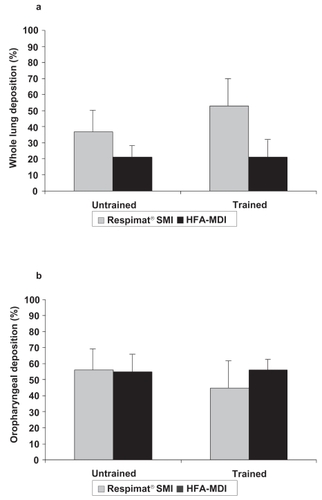
When patients used their own (poor) technique, there was trend towards higher values of mean whole lung deposition with Respimat® SMI (37% of delivered dose, standard deviation [SD] 14%) than with HFA-MDI (21% of metered dose, SD 7%). After training, the mean value for Respimat® SMI increased further to 53% of delivered dose (SD 17%), but the value for HFA-MDI was virtually unchanged (21% of metered dose, SD 10%) (). Analysis of the difference between inhalers by Sign test showed that more of the differences were negative than positive, suggesting that training had been more effective for Respimat® SMI. However, the difference was not significant (median value –0.38; pSign test = 0.15). An ANOVA verified same direction of difference, but resulted in a statistically significant difference (pANOVA < 0.05). In a similar pattern to the whole deposition results, mean oropharyngeal deposition from Respimat® SMI was reduced by training (56% of delivered dose before to 45% of delivered dose after it), but the mean value for HFA-MDI after training (56% of metered dose) was very similar to that before training (55% of metered dose; ).
Figure 2 Mean deposition values (as % of delivered dose for Respimat® Soft Mist™ Inhaler and % of metered dose for pMDI) from the two inhalers before and after training (standard deviation shown as error bar): a) whole lung deposition, b) oropharyngeal deposition.
For both inhalers, the distribution of deposited aerosol in different lung regions after training showed few changes (). With Respimat® SMI, the mean deposition values in all three lung regions were numerically higher after training than before, but distributed in a very similar proportion to the pre-training results. With HFA-MDI, the deposition values were unchanged after training. Consequently, the changes in PI for both inhalers were small and of no clinical relevance.
Table 4 Mean deposition (standard deviation in parentheses) [% of delivered dose for Respimat® SMI and % of metered dose for pMDI] of radiolabel in the three lung regions in full analysis set (n = 12)
Samples of scintigraphy images after inhalation with Respimat® SMI and HFA-MDI before and after technique training are shown in .
Discussion
Mean deposition of a combination bronchodilator in whole lung from Respimat® SMI was about twice that from HFA-MDI after patients were trained in the optimal use of both inhalers. The deposition for Respimat® SMI was measured as percent of delivered dose contrary to the deposition of HFA-MDI measured as percent of metered dose; these are the declared doses for the two marketed products.
The improvement in deposition after training in this study was not statistically significant according to the prospectively defined analysis (Sign test). The direction of difference, however, indicated a greater effect of training with Respimat® SMI (3 patients had a greater training effect with HFA-MDI and 9 with Respimat), and analysis of the same data using an alternative prospectively defined test (ANOVA) did show a significantly greater effect of training with Respimat® SMI.
These findings suggest that training patients in correct inhaler technique is beneficial for Respimat® SMI, notwithstanding the good deposition results achieved before training. The lack of improvement after training with pMDI, however, contrasts with the findings of other studies (CitationKemp and Meltzer 1990; CitationNewman et al 1991; CitationMinai et al 2004). The reason for this may be that although all of the patients in our study were judged as having a poor technique, the error they committed was to inhale too quickly – none showed poor co-ordination of inhaler firing with inhalation. In asthma patients using CFC-MDIs, delivery was markedly improved after training in poor co-ordinators but not in those who were judged as good coordinators (CitationNewman et al 1991). Poor co-ordination might therefore be a more crucial error of technique with pMDIs than inhaling too fast – asthma control was found to be worse in asthma patients who misused pMDIs than in those with good technique, and the worst level of control was in poor coordinators (CitationGiraud and Roche 2002).
The mean lung deposition values recorded with both inhalers in our study are higher than those of two studies in healthy non-smoking volunteers who were trained in optimal inhalation technique for the final prototype of Respimat® SMI and a CFC-MDI. Mean whole lung deposition from Respimat® SMI was 39% for an aqueous solution of fenoterol, 11% from CFC-MDI used without a spacer and 10% from CFC-MDI used with an AeroChamber spacer (CitationNewman et al 1998). When comparing these results, however, account must be taken of the different methods for expressing the delivered dose from Respimat® SMI. In our study, this was expressed as the dose that leaves the mouthpiece (which is the declared dose for the product), whereas in the earlier study, it was expressed as the dose leaving the nozzle, ie, including an amount deposited on the mouthpiece; restating the results of the earlier study using ex-mouthpiece doses would produce slightly higher values, reducing the discrepancy between the two studies. Observation of Newman et al with CFC-MDIs resulted in similar distribution as we have shown in this investigation (mean whole lung deposition in healthy subjects of 26% of metered dose (SD 6%) versus mean whole lung deposition in patients with COPD before training of 21% of metered dose (7%) and after training of 21% of metered dose (10%) (CitationNewman et al 1998). These findings are consistent with findings of other studies in volunteers and in patients with asthma or COPD (CitationLeach et al 2002; CitationHäussermann et al 2007) probably because HFA-MDIs have a higher fraction of fine particles that are more easily respirable, and because these are emitted at a lower velocity.
The measurements of inspiratory performance in our study gave immediate feedback on the degree of success of the inhaler technique training, and provided more context for the observed deposition performance of the two inhalers. The mean breath-hold duration increased from 9 to over 10 seconds after training. Average and peak IF were similar for both inhalers after training (33–35 L/min), and much slower than before training, but although these slower flows seemed to improve mean deposition from Respimat® SMI, they had no effect on deposition from pMDI. This suggests that training patients to inhale more slowly and deeply may make little difference to lung delivery in patients using pMDI, but will have greater benefits for those using products delivered via Respimat® SMI.
Patients who use dry powder inhalers (DPIs) do not have the challenge of having to co-ordinate inhalation with inhaler firing, and are trained to inhale with a higher peak IF, to ensure full de-agglomeration of the powder. A comparison in asthma patients showed that deposition of budesonide from the Turbuhaler® at a fast peak IF (29%) was higher than at low peak IF (18%), but optimal inhalation from Respimat® SMI (slow average IF) produced significantly higher deposition (52%) (CitationPitcairn et al 2005).
A key limitation of our investigation, in common with other scintigraphy studies, was the small number of patients, which produced a large variability in both deposition results and inhalation profiles as shown by standard deviations around our mean estimates. Using larger patient numbers in trials involving exposure to ionizing radiation is very difficult to justify. This constraint was mitigated by using a four-way crossover design that allowed the widest possible comparative analysis from the small sample. Although possible period bias was controlled for by randomizing the treatment sequence, it was not possible to blind the inhalers to investigators or patients because of differences in their geometry, and this might have introduced a preference bias.
In summary, drug delivery to the lungs with Respimat® SMI was found to be more efficient than with HFA-MDI, even in patients who have a poor inhaler technique. Inhaler training further improved the deposition profile for Respimat® SMI, but made almost no difference to the profile for pMDI. This is in keeping with clinical trials seen in patients with asthma and COPD who were treated at home (without supervision) with Berodual® administered from either the Respimat® SMI or from a pMDI. These studies showed that reducing the dose in the Respimat® SMI to half, or even one quarter, of the nominal dose of the pMDI resulted in comparable efficacy and safety (CitationKassner et al 2004; CitationVincken 2008). Teaching patients to inhale slowly and deeply and hold their breath for as long as they can increases further lung deposition from Respimat® SMI.
Disclosures
BH, GA, and HD are employees of Boehringer Ingelheim. TM is an employee of Inamed Research. PB has no conflicts to disclose.
References
- CromptonGK1982Problems patients have using pressurized aerosol inhalersEur J Respir DisSuppl 1191014
- DolovichMRuffinRE1981Optimal delivery of aerosols from metered dose inhalersChest806 Suppl91157307637
- EpsteinSWManningCP1979Survey of the clinical use of pressurized aerosol inhalersCan Med Assoc J1208136427689
- GiraudVRocheN2002Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stabilityEur Respir J192465111866004
- HäussermannSAcerbiD2007Lung deposition of formoterol HFA (Atimos/Forair) in healthy volunteers, asthmatic and COPD patientsJ Aerosol Med203314117894539
- HochrainerDHolzH2005Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalersJ Aerosol Med182738216181002
- KassnerFHodderR2004A review of ipratropium bromide/fenoterol hydrobromide (Berodual) delivered via Respimat Soft Mist Inhaler in patients with asthma and chronic obstructive pulmonary diseaseDrugs6416718215257628
- KempJPMeltzerEO1990Beta 2 adrenergic agonists – oral or aerosol for the treatment of asthma?J Asthma27149571973417
- KilfeatherSAPonitzHH2004Improved delivery of ipratropium bromide/fenoterol from Respimat Soft Mist Inhaler in patients with COPDRespir Med983879715139567
- LeachCLDavidsonPJ2002Lung deposition of hydrofluoroalkane-134a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chlorofluorocarbon beclomethasone: a cross-over study in healthy volunteersChest122510612171824
- LindgrenSBakeB1987Clinical consequences of inadequate inhalation technique in asthma therapyEur J Respir Dis709383817076
- McFaddenERJr1995Improper patient techniques with metered dose inhalers: clinical consequences and solutions to misuseJ Allergy Clin Immunol96278837636071
- MinaiBAMartinJE2004Results of a physician and respiratory therapist collaborative effort to improve long-term metered-dose inhaler technique in a pediatric asthma clinicRespir Care49600515165293
- MolimardMRaherisonC2003Assessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary careJ Aerosol Med162495414572322
- NewmanSP2005Inhaler treatment options in COPDEur Respir Rev141028
- NewmanSPBrownJ1998Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devicesChest113957639554631
- NewmanSPPaviaD1980Simple instructions for using pressurized aerosol bronchodilatorsJ R Soc Med7377697241435
- NewmanSPPaviaD1981How should a pressurized beta-adrenergic bronchodilator be inhaled?Eur J Respir Dis623216112156
- NewmanSPSteedKP1996Efficient delivery to the lungs of flunisolide aerosol from a new portable handheld multidose nebuliserJ Pharm Sci8596048877887
- NewmanSPWeiszAW1991Improvement of drug delivery with a breath actuated pressurised aerosol for patients with poor inhaler techniqueThorax4671261750017
- PhippsPRGondarI1989Comparison of planar tomographic gamma scintigraphy to measure the penetration index of inhaled aerosolsAm Rev Respir Dis1391516232786364
- PitcairnGReaderS2005Deposition of corticosteroid aerosol in the human lung by Respimat Soft Mist inhaler compared to deposition by metered dose inhaler or by Turbuhaler dry powder inhalerJ Aerosol Med182647216181001
- PitcairnGRNewmanSP1997Tissue attenuation corrections in gamma scintigraphyJ Aerosol Med1018798
- SnellNJCGandertonD1999Assessing lung deposition of inhaled medicationsRespir Med931233310464864
- VinckenW2008Clinical efficacy and safety of the combination of ipratropium bromide and fenoterol inhaled via Respimat Soft Mist inhaler for relief of airflow obstructionExpert Rev Resp Med21226
- von BergAJeenaPM2004Efficacy and safety of ipratropium bromide plus fenoterol inhaled via Respimat Soft Mist Inhaler vs a conventional metered dose inhaler plus spacer in children with asthmaPediatr Pulmonol372647214966821