Abstract
Exacerbations in chronic obstructive pulmonary disease (COPD), which tend to occur in clusters and increase with disease severity, come with high societal and economic burdens. Prevention and delay of recurrent exacerbations is an unmet and significant therapeutic need for patients with COPD. GALATHEA (NCT02138916) and TERRANOVA (NCT02155660) were trials assessing efficacy of benralizumab in patients with frequent COPD exacerbations despite treatment. Although these studies found that benralizumab given as an add-on treatment did not significantly reduce annual rates of COPD exacerbations after 56 weeks of treatment, in the following exploratory post hoc analysis of the GALATHEA and TERRANOVA trials we identified a potential responder population in which treatment with benralizumab prevents recurrent COPD exacerbations during 30- and 90-day periods following an initial exacerbation, a vulnerable period for an exacerbation to occur. This responder population was characterized by high blood eosinophil counts and frequent previous exacerbations despite optimized triple therapy. These results highlight the importance of targeted therapies for high-risk populations and merit further research into the benefits of biologic therapies for COPD exacerbations.
Introduction
Preventing and delaying recurrent exacerbations is an unmet need and crucial therapeutic goal in chronic obstructive pulmonary disease (COPD). Studies have shown that COPD exacerbations often occur in clusters,Citation1–3 with a US study showing that approximately 20% of patients hospitalized for acute COPD exacerbations are readmitted to hospital within 30 days,Citation4 and a European study showing that over a third of patients are readmitted within 90 days.Citation5 Benralizumab is a humanized afucosylated (IgG1) anti-interleukin-5 (IL-5) receptor α monoclonal antibody that has been shown to rapidly deplete eosinophils in blood, airway, and sputum by inducing apoptosis following recruitment of natural killer cells.Citation6–8
GALATHEA (NCT02138916) and TERRANOVA (NCT02155660) were 2 randomized, double-blind, placebo-controlled Phase 3 trials assessing the efficacy of benralizumab in patients experiencing frequent COPD exacerbations despite treatment.Citation9 These trials showed that benralizumab added to inhaled maintenance treatments did not significantly reduce the annual COPD exacerbation rate after 56 weeks.Citation9 However, a post hoc analysis identified a potential responder subgroup with distinct characteristics, including a history of ≥3 COPD exacerbations in the previous year despite receiving triple therapy (inhaled corticosteroid, a long-acting ß2-agonist, and a long-acting muscarinic antagonist) and a baseline blood eosinophil (bEOS) count ≥300 cells/μL.Citation10
Methods
In this exploratory post hoc analysis of the GALATHEA and TERRANOVA trials, we examined the effect of benralizumab 100 mg versus placebo on recurrent COPD exacerbations in the potential responder population (≥3 COPD exacerbations in the previous year while receiving triple therapy plus bEOS ≥ 300 cells/μL) who had ≥1 exacerbation following randomization despite treatment. In this subpopulation, we evaluated recurrent COPD exacerbations within 30 and 90 days following an initial moderate or severe exacerbation (a vulnerable period for another exacerbation to occur). Moderate COPD exacerbations were defined as increased respiratory symptoms leading to systemic corticosteroid and/or antibiotic use; severe exacerbations were defined as hospitalizations. Severe exacerbations resulting in death were excluded from these analyses. Statistical analyses were performed using a negative binomial model, including the following covariates: treatment, study (GALATHEA or TERRANOVA), geographic region, and the number of prior exacerbations.
Overall, 145 patients (benralizumab, n = 73; placebo, n = 72) were included in this post hoc analysis, representing approximately 6.5% of the relevant population from GALATHEA and TERRANOVA (benralizumab, 73/1114; placebo, 72/1118). Baseline demographics and characteristics of the subpopulation included in these analyses are shown in . The mean (SD) patient age was 65.3 (8.4) years, most patients were male (n = 92; 63.4%) and white (n = 119; 82.1%), and 30.3% were current smokers. The median bEOS count was 450.0 (range: 300–3860) cells/μL, and the mean (SD) pre-bronchodilator forced expiratory volume in 1 second (FEV1) was 37.4% (12.3) of predicted. Patient demographics and clinical characteristics were similar between groups, although there was a higher number of exacerbations in the previous year in the placebo group (4.3 [1.9]) compared to the benralizumab group (3.9 [1.3]). By design, the patients in this analysis had more exacerbations in the past 12 months (4.1 [1.7]) compared to the overall patient population in the GALATHEA and TERRANOVA trials.Citation9
Table 1 Baseline Patient Demographics and Characteristics
Results
Within 30 days of an initial exacerbation, 14 moderate or severe exacerbations occurred in the benralizumab group versus 62 in the placebo group. Benralizumab reduced the risk of recurrent moderate or severe COPD exacerbations within 30 days by 60% compared with placebo (rate ratio [RR], 0.40; 95% CI: 0.22–0.72; p=0.0022) ().
Figure 1 Risk of recurrent COPD exacerbations within 30a and 90b days.
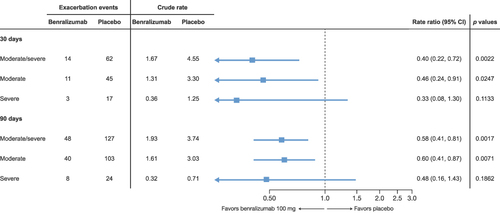
Within 90 days of an initial exacerbation, 48 moderate or severe exacerbations occurred in the benralizumab group versus 127 in the placebo group. Benralizumab reduced the risk for recurrent moderate or severe COPD exacerbations within 90 days by 42% compared to placebo (RR, 0.58; 95% CI: 0.41–0.81; p=0.0017) (). Additionally, fewer recurrent severe exacerbations occurred in the benralizumab group compared to placebo after 30 days (benralizumab = 3, placebo = 17; RR, 0.33; 95% CI: 0.08–1.30; p=0.1133) and 90 days (benralizumab = 8, placebo = 24; RR, 0.48; 95% CI: 0.16–1.43; p=0.1862), although the differences were not statistically significant.
Discussion
We acknowledge that a potential limitation of this post hoc analysis is the risk of a type I error. Yet, the effects of benralizumab on this patient population appear clinically significant and merit further investigation; indeed, the ongoing RESOLUTE (NCT04053634) phase 3 trial is designed to evaluate the efficacy of benralizumab specifically in this patient population. In addition, the ABRA trial (NCT04098718) will evaluate bEOS-guided benralizumab therapy in COPD patients with eosinophilic exacerbations in the acute setting. The results from these studies will examine the potential benefits of benralizumab therapy in COPD patients with an eosinophilic phenotype.
Conclusion
In conclusion, the results of this post hoc analysis support the hypothesis that benralizumab reduces the risk for recurrent COPD exacerbations within the vulnerable 30-to-90–day period in the previously identified responder population: those with high bEOS counts and frequent previous exacerbations despite optimized triple therapy. This is a promising finding, as recurrent COPD exacerbations carry serious risks for patients, including hospital readmittance 30 and 90 days following an event,Citation11,Citation12 treatment failure,Citation12 inpatient mortality of 10%, and up to 50% all-cause mortality 2 years after an event.Citation12 The results of this post hoc analysis highlight the importance of targeted interventions to reduce the risk for recurrent COPD exacerbations in high-risk patients, as well as the beneficial impact of biologic therapy on COPD exacerbations overall.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics Approval and Consent to Participate
Ethical review and approval was not required for this post hoc analysis of data from previously completed trials. As described in the previous primary publication of that study, independent ethics committees of the trial centers or central institutional review boards approved the trial protocols for the GALATHEA and TERRANOVA trials.Citation9 Both trials were conducted in accordance with the principles of the Declaration of Helsinki; all patients provided written informed consent.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
DS has received personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Synairgen, Teva, Theravance, and Verona. GJC has received grants from NIH-NHLBI, PA-DOH, GSK, Boehringer Ingelheim, Novartis, AstraZeneca, Respironics, MedImmune, Novartis, Pearl, PneumRx, Pulmonx, Broncus, Spiration, Olympus, Fisher-Paykel Healthcare, Chiesi, Gilead, Pfizer, Corvus, Lilly, Regeneron, Genentech, and Roche and is a consultant for Almirall, AstraZeneca, Nuvaira, GSK, CSA Medical, PneumRx, BTG, Mereo, Broncus, Pulmonx, and EOLO. AA has received grants and private fees from AstraZeneca, Chiesi, GlaxoSmithKline, and Menarini and is a member of the GOLD Science Committee and Board of Directors. MB has received fees from AstraZeneca, Boehringer Ingelheim, Chiesi, and GlaxoSmithKline, and grants from AstraZeneca, Roche, and other support from Asthma & Lung UK, Albus Health and ProAxsis to the institution, outside the submitted work. JS, GLS, YS, MJ, UJM, and IP are or were employees of AstraZeneca at the time these analyses were conducted and may own stock/stock options in AstraZeneca. IL was a contractor for AstraZeneca at the time these analyses were conducted and affiliated with Cytel Inc. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors thank Peter Barker, Ying Fan, and Yasa Reddy (AstraZeneca) for statistical discussions and programming support. Medical writing support was provided by Alexia Williams, PhD, and Dan Jackson, PhD, CMPP, of CiTRUS Health Group (United States), which was in accordance with Good Publication Practice (GPP 2022) guidelines and funded by AstraZeneca (Cambridge, UK).
Additional information
Funding
References
- Fidahussein SS, Croghan IT, Cha SS, Klocke DL. Posthospital follow-up visits and 30-day readmission rates in chronic obstructive pulmonary disease. Risk Manag Healthc Policy. 2014;28:105–112.
- Hurst JR, Donaldson GC, Quint JK, Goldring JJP, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(5):369–374. doi:10.1164/rccm.200807-1067OC
- Kong CW, Wilkinson TMA. Predicting and preventing hospital readmission for exacerbations of COPD. ERJ Open Res. 2020;6(2):00325–02019. doi:10.1183/23120541.00325-2019
- Shah T, Churpek MM, Perraillon MC, Konetzka RT. Understanding why patients with COPD get readmitted. Chest. 2015;147(5):1219–1226. doi:10.1378/chest.14-2181
- Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD Audit. Eur Respir J. 2016;47(1):113–121. doi:10.1183/13993003.01391-2014
- Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125(6):1237–1244.e2. doi:10.1016/j.jaci.2010.04.005
- Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–1096.e5. doi:10.1016/j.jaci.2013.05.020
- Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–1353.e2. doi:10.1016/j.jaci.2010.04.004
- Criner GJ, Celli BR, Brightling CE, et al. GALATHEA Study Investigators; TERRANOVA Study Investigators. Benralizumab for the prevention of COPD exacerbations. N Engl J Med. 2019;381(11):1023–1034. doi:10.1056/NEJMoa1905248
- Criner GJ, Celli BR, Singh D, et al. Predicting response to benralizumab in chronic obstructive pulmonary disease: analyses of GALATHEA and TERRANOVA studies. Lancet Respir Med. 2020;8(2):158–170. doi:10.1016/S2213-2600(19)30338-8
- Alqahtani JS, Aldabayan YS, Aldhahir AM, Al Rajeh AM, Mandal S, Hurst JR. Predictors of 30- and 90-day COPD exacerbation readmission: a prospective cohort study. Int J Chron Obstruct Pulmon Dis. 2021;16:2769–2781. doi:10.2147/COPD.S328030
- Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022;400(10356):921–972. doi:10.1016/S0140-6736(22)01273-9
