Abstract
Chronic obstructive pulmonary disease (COPD) is one of the most common chronic illnesses in the world. The disease encompasses emphysema, chronic bronchitis, and small airway obstruction and can be caused by environmental exposures, primarily cigarette smoking. Since only a small subset of smokers develop COPD, it is believed that host factors interact with the environment to increase the propensity to develop disease. The major pathogenic factors causing disease include infection and inflammation, protease and antiprotease imbalance, and oxidative stress overwhelming antioxidant defenses. In this review, we will discuss the major environmental and host sources for oxidative stress; discuss how oxidative stress regulates chronic bronchitis; review the latest information on genetic predisposition to COPD, specifically focusing on oxidant/antioxidant imbalance; and review future antioxidant therapeutic options for COPD. The complexity of COPD will necessitate a multi-target therapeutic approach. It is likely that antioxidant supplementation and dietary antioxidants will have a place in these future combination therapies.
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease state characterized by airflow limitation that is not fully reversible;Citation1 it can include some combination of emphysema, chronic bronchitis (CB), and small airway obstruction.Citation2,Citation3 The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lungs. The mechanistic basis underlying COPD is complex and can involve recurrent inflammation, oxidative stress (ie, oxidant/antioxidant imbalance), protease/antiprotease imbalance, environmental insult, and host genetics.Citation4 Cigarette smoking serves as the major risk factor for developing COPD and is also the major source of oxidants/reactive oxygen species (ROS) to the lungs and the body in exposed individuals. It is this recurrent oxidative stress from environmental sources (eg, cigarette smoking) and persistent inflammation that can lead to extensive tissue damage and disease exacerbation susceptibility. This review focuses first on the relationship of cigarette smoking to COPD-associated oxidative stress. The second part addresses oxidant regulation of mucin gene expression and also genetic and epigenetic factors important for COPD-associated oxidative stress. Finally, we present an update about the current antioxidant nutritional and dietary supplements that have been evaluated for COPD therapy, along with alternative therapies such as acupuncture that have also been evaluated as potential COPD therapeutics.
Cigarette smoking and oxidative stress
Temperatures in the combustion zone of a burning cigarette (800°C–950°C) result in a complete pyrolysis of tobacco. However, immediately downstream, a rapid drop in temperature (200°C–600°C) and a lack of oxygen allow for an incomplete combustion. Subsequently, a complex aerosol is generated during smoking, which includes condensed liquid droplets (the particulate fraction or tar) suspended in a mixture of volatile/semivolatile compounds and combustion gases (the gas fraction). A single puff of such cigarette smoke was quantified to contain 1017 free radicals in the tar phase and 1015 in the gas phase.Citation5,Citation6 Numerous oxidant compounds have been identified among the 4,000 to 7,000 constituents in cigarette smoke.Citation7,Citation8 In the particulate fraction, phenols and semiquinones are recognized among these compounds, while superoxide (O2−), epoxides, peroxides, nitric oxide (NO; 500 to 1,000 ppm), nitrogen dioxide, peroxynitrite (ONOO−), and peroxynitrates are included in the gas phase.
Smoking produces a shift in the normal balance between oxidants and antioxidants to impact an oxidative stress both in the lungs and systemically. Oxidants included in cigarette smoke can directly injure cells and tissues, inactivate defense mechanisms, and initiate inflammation, which further elevates oxidative stress. It is difficult, if not impossible, to determine if the oxidants responsible for the stress are those originally included in cigarette smoke or those which result from the associated inflammatory response.
Specific endpoints of oxidative stress which have been quantified with smoking are numerous and diverse and have included: 1) generation of O2−, hydrogen peroxide (H2O2), and nitrite/nitrate; 2) levels of superoxide dismutase, catalase, myeloperoxidase, and cytochrome P450 (and their activities); 3) exhaled breath ethane/alkanes, thiobarbituric acid-reactive substances, and other indices of lipid peroxidation; 4) concentrations of oxidized proteins; 5) total, reduced, and oxidized glutathione; 6) activity of glutathione peroxidase, glutathione transferase, and glutathione reductase; 7) concentrations of prostanoids (F2-isoprostanes and prostaglandin F2-alpha), hydroxyeicosatetraenoic acid products, F(4)- neuroprostanes, 7-ketocholesterol, 24- and 27-hydroxycholesterol, low density lipoproteins, and other cholesterol oxidation products; 8) concentrations of uric acid and allantoin; 9) evidence of DNA damage (8-hydroxy-2′-deoxyguanosine and 8-oxo-2′- deoxyguanosine); 10) histopathology (eg, 4-hydroxy-2-nonenal and 8-hydroxydeoxyguanosine); 11) immunohistochemistry for specific proteins such as nitrotyrosine; 12) gene expression microarray analysis; 13) Trolox equivalent antioxidant capacity; 14) antioxidant reducing capacity; and 15) total radical trapping parameters. These measurements have been obtained in numerous different samples, including cells and their fractions, whole blood, serum, plasma, cord blood, sputum, exhaled breath, breath condensate, lavage fluid, lavage cells, urine, and tissues (eg, lung, vasculature, brain, muscle, testicles, and pancreas).
Oxidant generation with cigarette smoke exposure
Oxidants, including H2O2, can be directly measured in the particulate fraction of cigarette smoke.Citation9 Metals are also included in the particulate fraction of cigarette smoke, but concentrations appear to be low.Citation10 There is little evidence to support the assertion that iron and copper, introduced into the body by smoking, catalyze Fenton-type reactions in either the lung or any tissue.
Phagocytes (eg, macrophages and neutrophils) in smokers are elevated in number, both in the lung and systemically, and generate ROS at increased rates.Citation11 The major cell sources of O2− are likely the nicotine adenine disphosphonucleotide (NADPH) oxidoreductases, but others may also be increased (eg, xanthine oxidase).Citation12 Catalase, the enzyme responsible for the breakdown of H2O2, is downregulated in ex-smokers.Citation13 In vitro studies confirm that phagocytes collected from cigarette smokers spontaneously release increased amounts of oxidants such as O2− and H2O2 compared to those from nonsmokers.Citation14 H2O2 is elevated in bronchoalveolar lavage fluid and in exhaled breath condensate collected from cigarette smokers,Citation15 and some portion of this was demonstrated to be attributable to the elevated number of macrophages in the lower respiratory tract of smokers and their increased release of O2−.Citation16
Cigarette smoking is associated with lipid peroxidation and conversion of polyunsaturated fatty acids to hydroperoxides, endoperoxides, aldehydes (eg, malondialdehyde), and alkanes (eg, ethane and pentane). Levels of these end products are increased in smokers, including thiobarbituric acid-reactive products (in sputum, blood, and lung components), isoprostanes (in blood, urine, and breath condensate), 4-hydroxy-2-nonenal adducts, and breath alkanes.Citation17,Citation18
Cigarette smoking depletes antioxidants.Citation19 Concentrations of ascorbate and vitamin E are decreased among smokers.Citation20 Smokers have 15%–20% lower serum concentrations of ascorbate than do nonsmokers. These specific changes in ascorbate and vitamin E correspond to an elevated oxidative stress rather than a dietary intake. Values normalize after smoking cessation.Citation21 Glutathione metabolism appears to be particularly provoked by smoking. Despite glutathione being acutely depleted in cells and in smokers,Citation22,Citation23 levels of reduced glutathione are elevated in bronchoalveolar lavage fluid of chronic smokers.Citation24 It has been proposed that such an increase of lung glutathione in smokers may be an attempt, albeit insufficient, to counter excess oxidant production after cigarette smoke exposure.Citation25 Exogenous antioxidants appear to have a capacity to prevent some portion of the biological effect and injury following smoking.Citation26 Pretreatment with antioxidants can decrease lipid peroxidation following exposure to cigarette smoke.Citation27 There are studies which suggest that vitamins C and E diminish production of oxidants by inflammatory cells and improve pulmonary function in smokers.Citation14,Citation28 Supplementation with N-acetylcysteine (NAC) also diminishes in vitro cytotoxicity after cigarette smoke exposure.Citation29
Cigarette smoke is also a source of reactive nitrogen species and causes nitrosative stress. Nitric oxide, abundant in cigarette smoke and generated by inflammatory cells, has potent antioxidant and anti-inflammatory actions, but also contributes to oxidative reactions.Citation30 NO reacts with thiols to produce nitrosothiols associated with biological effects.Citation31 Nitrosothiol levels have been shown to be higher in breath condensate collected from smokers compared with subjects who do not smoke.Citation32 NO in cigarette smoke can react with O2− to form peroxynitrite,Citation33 which decreases antioxidant capacity and augments oxidative stress.Citation34 NO and peroxynitrite can cause the nitration of tyrosine to form nitrotyrosine products of proteins measurable in body fluids and tissues.Citation35 However, it must be pointed out that NO levels in smokers were also reported to be normal or even lower relative to nonsmokers.Citation36 Fractional exhaled nitric oxide was reported to be decreased in smokers.Citation37 Such reduced NO production was postulated to possibly elevate oxidative stress since this molecule can function as an antioxidant as well as being a prooxidant.Citation38
In addition to an elevated oxidant burden in the lung, there is increased systemic oxidative stress in smokers.Citation39 Plasma Trolox equivalent antioxidant capacity and total glutathione are decreased in cigarette smokers.Citation40 Peripheral blood neutrophils from smokers release more oxidants than those isolated from normal subjects.Citation41 The proposal that oxidants in cigarette smoke, whether in the particulate or gas phase, pass through the pulmonary alveolar wall into the blood to induce a disseminated systemic oxidative stress is improbable, as such radicals would rapidly react with molecules in the lung.Citation42
Oxidative stress and disease after cigarette smoke exposure
Oxidative stress resulting from an imbalance between oxidants and antioxidants is proposed as the basis for COPD following exposure to cigarette smoking. Cigarette smoke increases the burden of oxidants in the respiratory tract, either directly included in the cigarette smoke or generated by inflammatory cells, depleting antioxidant defenses and injuring lung cells.Citation43 Evidence of a relationship between oxidative stress and injury exists. Cell lysis and epithelial permeability are increased after exposure to cigarette smoke, and these effects can be inhibited by antioxidants (ie, glutathione).Citation44 In addition, there are increased numbers of activated inflammatory cells in the lungs of patients with COPD relative to healthy subjects, and these cells release greater quantities of O2− and H2O2.Citation45,Citation46 Importantly, a correlation has been demonstrated between O2− release by peripheral white blood cells and bronchial hyperreactivity in patients with COPD, supporting a role for such oxidants in lung disease after smoking.Citation45 As discussed later in this article, oxidants have been demonstrated to mediate mucous hypersecretion and impaired mucociliary clearance, which can contribute to injury in COPD.Citation47 Furthermore, nitrogen-based free radicals also participate in COPD,Citation48 and 3-nitrotyrosine, formed by nitration of tyrosine, is observed in greater concentrations in the lungs of those with COPD.Citation49
There is systemic disease after exposure to cigarette smoke.Citation50–Citation52 Regarding vascular disease, cigarette smoking is associated with an oxidation of low-density lipoproteins and their deposition in the vasculature with its resultant dysfunction.Citation53 A depletion of antioxidants has also been documented in atherosclerosis.Citation54
Cigarette smoke-induced oxidative stress is postulated to initiate a series of cellular and molecular reactions, which include activation of kinase cascades and transcription factors, release of inflammatory mediators, initiation of inflammation, and cell injury and apoptosis ().Citation55 Consequently, oxidative stress is the initiating factor in the pathway by which cigarette smoke exposure leads to disease. Key among the redox-sensitive transcription factors coordinating the inflammatory response to cigarette smoke are nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (pro-oxidative) and nuclear factor erythroid 2-related factor 2 (Nrf2; antioxidative). The activation of these transcription factors is observed in both the lung and extrapulmonary tissues.Citation56 An increased expression of proinflammatory mediators, including cytokines and peroxidation products of arachidonic acid (leukotrienes, prostanoids, and isoprostanes), occurs in smokers. The final product of this cascade of reactions is inflammation (pulmonary and systemic) and apoptosis; if the response is prolonged, fibrotic and neoplastic injuries can result.
Figure 1 Schematic depicting the cascade of events underlying the biological effects of cigarette smoking.
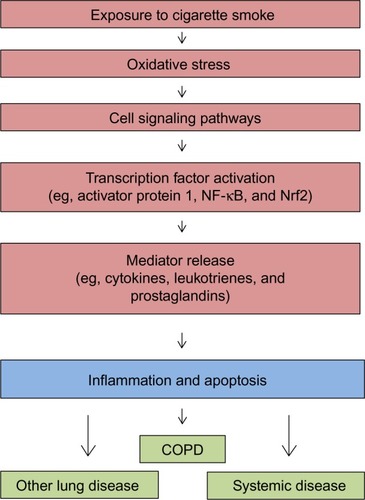
COPD due to smoking frequently does not reverse or improve after smoking cessation, but rather can progress in ex-smokers.Citation57 The reasons for persistence and progression of COPD despite cessation of the exposure are not fully appreciated. However, smoking cessation also does not eliminate the increased oxidative stress in the respiratory tract, suggesting that retained particles may continue to participate in oxidant generation.Citation58 To explain this incongruity, as well as the observation that COPD in smokers is particle related, it has been proposed that the particulate fraction of cigarette smoke (ie, tar) disrupts iron homeostasis to participate in oxidative stress. Retained particles in the lung effectively complex host iron to generate free radicals. In support of a role for disrupted iron homeostasis in disease after exposure to cigarette smoke, there is increased lavage iron concentrations in smokers.Citation59 Accumulation of iron in macrophages, proportional to the frequency and duration of cigarette smoking, has also been described among smokers.Citation60
Oxidative stress signaling pathways culminate in COPD as well as other lung and systemic disease. Oxidants influence many of these changes by modifying the expression of specific genes (eg, MUC5AC) and epigenetic factors pivotal to the development of disease.
Investigation has failed to delineate between the oxidative stress following cigarette smoking and that associated with COPD. Indices of oxidant generation can correspond to smoking alone (with no further change observed among COPD patients) and to COPD (with further change observed among COPD patients); results appear to vary with the specific endpoint, the methodology employed, and the study population.Citation61 Further development of biomarkers of oxidant burden may be required prior to delineating whether there are any true disparities in oxidative stress between smoking and COPD.Citation62
Exposures other than cigarette smoking and oxidative stress
Exposures other than cigarette smoking can impact the risk for COPD. Almost all of these exposures are particle related.Citation63,Citation64 Such exposures can include environmental tobacco smoke, burning of biomass, and air pollution particles. All these particle exposures, environmental and occupational, can result in oxidative stress. Production of ROS results either directly from the particle supporting an inappropriate electron transfer or from an interaction of the particle with cell proteins (eg, electron transport complexes in the mitochondria). Oxidative stress is accepted as the initial step in the biological effect after particle exposures.Citation65 In vitro exposures of cells to particles results in oxidant generation in a wide range of cell types, including phagocytic, epithelial, and endothelial cells. In vivo oxidant production following exposure to different particles has also been confirmed.Citation66 Oxidant production by these particles initiates a pathway of cell signaling, activation of transcription factors, and release of mediators, and culminates in an inflammatory lung injury.
Oxidative stress and mucin gene expression in COPD and CB
COPD is one of the most common respiratory diseases worldwide. This disease is defined by the presence of an irreversible airflow obstruction and is characterized by the presence of both oxidative stress and airway inflammation.Citation1 The disease encompasses several phenotypes: emphysema, small airway obstruction and obliteration,Citation2,Citation3 and CB. A majority of patients with COPD have CB, and this diagnosis is associated with a higher risk for respiratory-related death. CB is diagnosed in patients with a productive chronic cough for at least 3 months per year for 2 consecutive years.Citation1 Patients with CB have a greater risk of COPD exacerbations and more respiratory symptoms due to mucus obstruction of airways and secondary bacterial and viral bronchitis. Airway mucus obstruction in CB is due to several potential factors: 1) ciliated cells are injured by proteases and by oxidative injury, resulting in failure of the mucociliary escalator;Citation67 2) there is increased production and secretion of the major macromolecular constituent of mucus, namely, mucin glycoproteins; specifically, two respiratory tract gel-forming mucins, MUC5AC and MUC5B, are increased in the airway mucus and associated with hypertrophy and hyperplasia of mucous cells in the submucosal glands and superficial airway epithelium;Citation68–Citation70 3) the airway mucus is dehydrated due to tobacco smoke-triggered downregulation of the cell surface expression of the cystic fibrosis transmembrane conductance regulator;Citation71–Citation73 and 4) the presence of plasma proteins, glycosaminoglycans and proteoglycans,Citation74 DNA, lipids, and inflammatory cells and pathogens in CB airway mucus that change the normal biophysical properties of mucus (reviewed in Fahy and DickeyCitation75).
Oxidative stress, generated by tobacco smoke and augmented by ROS generated from both inflammatory cells and mitochondrial activity by cells resident in the respiratory tract, regulate mucin gene expression and mucous cell metaplasia. Tobacco smoke upregulates MUC5AC expression via epidermal growth factor receptor (EGFR)-dependent and -independent activation of activator protein 1 (AP-1),Citation76 or via specificity protein 1 (Sp1) activation.Citation77 Tobacco smoke also upregulates IL-8,Citation78 which may function as an autocrine/paracrine regulator of MUC5AC.Citation79 Tobacco smoke constituents, including aldehydes (eg, acrolein) and H2O2, also upregulate mucin gene expression. Acrolein-induced mucin production is mediated by matrix metalloproteinase (MMP)9Citation80 and by MMP14,Citation81 and monocyte-dominant inflammation augments acrolein-induced upregulation of MUC5AC.Citation82 Similarly, H2O2 exposure upregulates MUC5AC mRNA expression by an NADPH oxidoreductase 4 (NOX4)-dependent mechanism.Citation83 Smoking will trigger the influx of neutrophils into the airways and release of neutrophil elastase (NE) into the airways. We have reported that NE regulates MUC5AC gene expression via a mechanism dependent on ROSCitation84 and catalyzed by NADPH quinone oxidoreductase 1 (NQO1) activity.Citation85 In addition, oxidant-generated hyaluronan fragments upregulate both MUC5AC and MUC5B in airway epithelial cells.Citation86,Citation87 Importantly, tobacco smoke also sustains goblet cell metaplasia by suppressing Bik, a proapoptotic molecule, therefore preventing apoptosis of mucous cells.Citation88 Developmental determinants of goblet cell differentiation, SAM pointed domain ETS factor (Spdef) and FOXA3, are present in COPD airway epithelial cells,Citation89 but the regulatory mechanisms that govern their expression are not known (reviewed in Whitsett et alCitation90). Interestingly, several components of the Notch signaling pathway, the receptor Notch 3 and the effectors HES5 and HEY2, are decreased in healthy smokers compared to healthy nonsmokers,Citation91 suggesting that Notch suppression sustains airway mucous cell hyperplasia/hypertrophy.
Genetic and epigenetic regulation of oxidative stress in COPD
Tobacco smoke activates lipid peroxidation and carbonyl stress (reviewed in Kirkham and BarnesCitation92), resulting in DNA damageCitation93 and airway epithelial cell senescence and apoptosis (reviewed in Nyunoya et alCitation94); these are important pathways that contribute to both emphysema and airway remodeling. However, only a small portion of smokers develop COPD, emphasizing the importance of host factors in regulating tobacco smoke-induced oxidative stress and COPD. To determine the genetic factors predisposing to COPD, several approaches have been applied. Some investigators have focused on the transcriptional and epigenetic regulation of specific molecular and cellular pathways relevant to emphysema and CB phenotypes. In contrast, other investigators have leveraged consortia of large patient cohorts to interrogate unbiased genome-wide associations and discover single nucleotide polymorphisms (SNPs) that correlate with disease.
Using airway epithelial cells obtained by bronchoscopies from a large cohort of patients with and without COPD and segregated for pulmonary function test severity, signatures of gene expression have been identified by microarray in airway epithelia that are consistent with the gene expression data from lung tissues in patients with emphysema.Citation95 The expression of at least two oxidant-regulated genes are increased in COPD and include CYP2C18, a P450 enzyme, and aryl hydrocarbon receptor nuclear translocator-like 2 (ARNTL2), a basic helix-loop-helix transcription factor that is active under conditions of low oxygen tension.Citation95
Using a candidate gene approach, several genetic mutations in antioxidant genes are linked to COPD severity (reviewed inCitation96). Polymorphisms in glutathione S-transferase (GST) M1, GSTP1, superoxide dismutase 3 (SOD3), and microsomal epoxide hydrolase (EPHX1) are associated with more rapid decline in lung function in COPD.
Epigenetic regulatory pathways are also activated by oxidative stress. The effect of miRNAs on signaling pathways in COPD varies with biospecimen source and the mechanism of miRNA regulation. A good example of this complexity is the biology of miR-199a-5p in COPD. One report demonstrates that increased expression of miR-199a-5p in the lung tissue of COPD patients correlates with downregulation of hypoxia inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF); this function of miR-199a-p5 is demonstrated in human endothelial cells in vitro.Citation97 Another recent report demonstrates that promoter methylation of miR-199a-5p in monocytes occurs in α-1-antitrypsin (A1AT) ZZ symptomatic patients, resulting in suppression of miR-199a-p5 expression and subsequent upregulation of the unfolded protein response.Citation98 Both pathways engaging miR-199a-p5 result in emphysema, but by very different mechanisms.
Methylation of DNA CpG islands is broadly increased both in blood leukocytesCitation99 and in small airway epithelia of COPD patients. The genes targeted by differential methylation in the small airways downregulate Nrf2 expression, resulting in loss of phase II metabolizing enzymes that provide antioxidant protection.Citation100
Tobacco smoke downregulates histone deacetylases (HDACs), resulting in increased oxidation and protease activity. HDAC2 is decreased in COPD macrophages and lung tissues, and this is associated with increased H4 acetylation localized to the promoter of IL-8.Citation101 HDAC2 downregulation impairs Nrf2 activation in the lung by decreasing the half-life of Nrf2.Citation102 Sirtuin1 (SIRT1), a type III HDAC, is redox sensitive and can be post-translationally modified, leading to proteosomal degradation. Loss of SIRT1 correlates with tissue inhibitor of metalloproteinase (TIMP)-1 lysine acetylation and subsequent degradation of TIMP-1, the major anti-MMP protease, resulting in increased MMP-9 in human COPD lung tissue.Citation103
Genome-wide association studies (GWAS) have identified a few SNPs associated with COPD, but the frequency of these polymorphisms in the population do not explain the much larger frequency of disease occurrence, suggesting that environmental exposures and/or epigenetic regulation have a significantly greater impact on phenotype. Other challenges for GWAS analysis include potentially missing modifying genes that interact with GWAS-identified genes, missing genes specific to ethnic backgrounds, using too broad a phenotype to test associations, and not considering the impact of expression levels of identified genes in tissues (eQTL) which would strengthen the association of genetic polymorphisms to phenotype.Citation104
Two studies evaluated thousands of subjects phenotyped by the Global initiative for chronic Obstructive Lung Disease (GOLD) criteria for severity of COPD. The studies compared smokers with airflow obstruction to smokers with no airflow obstruction. Both studies detected significant levels of expression of SNPs on chromosome (chr) 15 spanning several genes including nicotinic acetylcholine receptors 3–5 (CHRNA3–5), and iron response element binding protein 2.Citation105,Citation106 One study identified family with sequence similarity 13 member A (FAM13A) on chromosome (chr) 4, a Rho A inhibitor, important for regulating oxidative stress and apoptosis in COPD lung cells. The following chromosomal localizations and gene candidates were determined to be related to forced expiratory volume in 1 second (FEV1): chr 4q24, glutathione S-transferase, C-terminal containing domain (GSTCD); chr 2q35, tensin 1 (TNS1); and chr 5q33, 5-hydroxytryptamine (serotonin) receptor 4 (HTR4). Similarly, for a decreased FEV1/forced vital capacity ratio, the following chromosomes and associated gene candidates were identified: chr 6p21, advanced glycosylation end product-specific receptor (AGER); and chr 15q23, thrombospondin, type I, domain containing 4 (THSD4).Citation107 Finally, hedgehog interacting protein (HHIP), a developmental transcription factor, was identified as a COPD susceptibility gene at the level of significance in these studies.
Using a consortia of large COPD cohorts that included the COPDGene study (non-Hispanic white and African-American ethnic origin) and the ECLIPSE, NETT/NAS, and Norway GenKOLS studies, 6,633 individuals with moderate-to-severe COPD and 5,704 control individuals were evaluated, and three known COPD GWAS gene candidates were identified: CHRNA3, FAM13A, and HHIP. In addition, three new associations were identified: Ras interacting/interference 3 (RIN3), MMP12, and TGFB2.Citation108
As phenotypes were characterized in detail and compared, new genome-wide associations for COPD became apparent. In one study, GWAS was conducted for a phenotype of emphysema defined by chest computed tomography scans in over 2,000 individuals with COPD. This analysis detected a near-significant association between the genomic region encompassing bicaudal D homolog 1 (BICD1) and emphysema severity.Citation109 BICD1 is associated with dynein function and telomere shortening. Another study evaluated computed tomography scan severity of emphysema across ethnic groups and determined the following GWAS related to ethnic background: small nuclear ribonucleoprotein polypeptide F (SNRPF) and palmitoyl protein thioesterase 2 (PPT2) in non-Hispanic whites; for Hispanic subjects, α-mannosidase-related gene (MAN2B1); for Chinese subjects, DEAH box helicase 15 (DHX15) and mannosyl–glucosaminyl transferase 5B (MGAT5B), which acts on α-linked mannose; and, for African Americans, a third α-mannosidase-related gene, MAN1C1.Citation110 The relation of GWAS to oxyhemoglobin saturations in two large cohorts, COPDGene and ECLIPSE, was tested. African Americans in COPDGene had SNP associations on chr 14 and 15. Candidate genes include forkhead box G1 (FOXG1) on chr 14, and, on chr 15, candidate genes include DNA topoisomerase-2-binding protein 1 (TOPBP1)-interacting checkpoint and replication regulator (TICRR), a regulator of cell cycle progression, and kinesin family member 7 (KIF7), a regulator of sonic hedgehog signaling. Chr 15 had no associations with COPD in non-Hispanic whites.Citation111 GWAS for chronic mucus hypersecretion was associated with special AT-rich sequence-binding protein 1 locus (SATB1) on chr 3. This gene regulates chromatin structure. Although the original correlation was less than usually accepted for statistical correlation, the GWAS SNP was independently verified in other large COPD GWAS cohorts, and there was eQTL evidence of increased expression of SATB1 mRNA in tissues of patients with chronic mucus hypersecretion.Citation112
Tremendous progress has been made in identifying environmental and inherited risk factors for COPD and CB. There are several target genes that increase susceptibility to COPD via oxidant-mediated pathways. Moreover, oxidant signaling pathways play a major role in airway remodeling relevant to CB. The next frontier to understanding how inheritance increases the risk of COPD disease susceptibility will be investigation of the early origins of COPD in childhood and infancy, including consideration of the role of inherited epigenetic factors. Factors that reduce ultimate lung growthCitation113,Citation114 or antioxidant capacity may be discovered, leading to new approaches to prevention or treatment of COPD.
Current and emerging antioxidants, diet, and alternative therapies
Studies have been undertaken to evaluate the clinical effectiveness of antioxidant therapy for COPD. Potential antioxidant therapies were reviewed in 2008,Citation115 and again in 2013,Citation116 with both reports suggesting that certain classes of antioxidants be evaluated for the clinical usefulness in COPD, including: thiol compounds (eg, NAC, carbocysteine), Nrf2 activators (eg, sulforaphane), antioxidant vitamins (eg, vitamin E and C), and food/diet-derived polyphenols (eg, curcumin and resveratrol) (see ). Some of these antioxidants have been evaluated in rodent and/or human studies. For example, in one study, carbocysteine, a thiol antioxidant, was evaluated for its impact on COPD exacerbation rate in a randomized, placebo-controlled trial.Citation117 In the Chinese COPD patients evaluated, the investigators reported that carbocysteine was well tolerated, and that it significantly decreased exacerbation rate over a 1-year period.Citation117 Similarly, in a more recent randomized, placebo-controlled study with stable COPD patients in Hong Kong, 1-year treatment with high-dose NAC therapy, another thiol antioxidant, also reduced exacerbation frequency.Citation118 This group also reported an improvement in the forced expiratory flow 25%–75% by 16 weeks of NAC therapy, which persisted for the 1-year treatment period, suggestive of decreased small airway obstruction.Citation118 However, other, earlier studiesCitation115,Citation116 did not demonstrate any impact of NAC therapy on lung function. The exact mechanism of action of NAC regulation of exacerbation frequency is not clear. Tse et al propose that it is the antioxidant and anti-inflammatory properties of NAC; however, one report suggests that it may also be that NAC helps restore the normal antiviral innate immune response that is suppressed by cigarette smoking.Citation119 Thiol antioxidants show promise as one component of the COPD therapeutic “toolbox”, with their ability to attenuate exacerbation frequency, which may potentially help slow disease progression.
Table 1 Clinical application of antioxidants summary table
Nrf2 plays an important role in protection against ROS, especially those triggered by smoking. Nrf2 is a transcription factor which can bind to the antioxidant response element of important stress response genes. It regulates many of the antioxidant and phase II cytoprotective genes. Nrf2 and its importance in COPD pathophysiology has been studied in rodent models and with cells from COPD patients. Sulforaphane, derived from broccoli sprouts, is an Nrf2 activator that is being considered for potential clinical trials in COPD. Sulforaphane has been evaluated in ex vivo/in vitro studies. In one study using alveolar macrophages from COPD patients, sulforaphane treatment helped restore glucocorticoid sensitivity, through a glutathione-dependent mechanism, to increase HDAC2 activity.Citation102 Food/plant-derived polyphenols, such as curcumin and resveratrol, have also been evaluated in a similar ex vivo/in vitro manner. Curcumin, the active constituent from turmeric, has anticancer and anti-inflammatory properties.Citation120,Citation121 Similar to sulforaphane, curcumin treatment of a monocytic cell line reversed cigarette smoke extract- or peroxide-induced steroid resistance by restoring HDAC2 activity and expression.Citation122 In a mouse model of cigarette smoke-induced emphysema, oral curcumin therapy significantly attenuated lung lavage neutrophils and macrophages as well as lung mean linear intercepts as a measure of emphysema.Citation123 Resveratrol, another polyphenolic compound that is derived from red wine, also has antioxidant and anti-inflammatory properties.Citation115,Citation124 Resveratrol treatment of alveolar macrophages from COPD patients significantly reduced bacterial endotoxin-induced cytokine production despite steroid insensitivity.Citation125
Dietary modification may be another avenue of promising intervention for COPD patients.Citation126 Two studies from the Netherlands suggest that diets rich in solid fruits may be beneficial in limiting COPD symptoms and/or mortality.Citation127,Citation128 The first study, by Tabak et al, focused on dietary intake of polyphenolic bioflavonoids and measured three subclasses: catechins, flavonols, and flavones. Total flavonoid intake was positively associated with FEV1 and inversely associated with cough and breathlessness.Citation127 The second study, by Walda et al, also included patients from European countries and evaluated the 20-year mortality rate in men. Increased dietary intake of fruit and vitamin E demonstrated an inverse trend in 20-year COPD mortality with a corresponding 24% lower COPD mortality risk.Citation128 In a follow-up to the Tabak et al study,Citation127 a 5-year follow-up was performed and a subset of these patients with valid initial study lung function measurements were included in a study to evaluate the relationship of dietary intake to FEV1 decline over time. McKeever et al reported that people eating a more traditional diet, with intake of meat and potatoes dominating, was associated with a lower FEV1 and an increased incidence of COPD.Citation129 These two studies focused on solid fruits, in particular apples and pears, but other fruits are rich in polyphenols and bioflavonoids. Guava is one fruit whose extract is very phenolic rich; it has been proposed as potential COPD therapy, but not yet evaluated in clinical trials.Citation130 Blueberries are another antioxidant bioflavonoid-rich food. In healthy men, blueberry intake has been shown to improve vascular function.Citation131 Two dietary-intervention randomized controlled trials reported differing results. One study, based in the United Kingdom, had patients increase fruit and vegetable intake to at least five servings per day, while the control group consumed two or less portions per day for 12 weeks.Citation132 Over this short period, there were no changes in lung function nor in markers of either oxidative stress or inflammation.Citation132 However, a study in Greece, in which the intervention group increased their fruit and vegetable consumption over a 3-year period, demonstrated a significant increase in FEV1 (% predicted) over time.Citation133 Furthermore, the intervention group also demonstrated a significant decrease in the mean annual exacerbation rate.Citation133 In addition, recent reports also highlight the importance of overall nutritional support, improved dietary quality, and increased dietary fiber for COPD management.Citation134–Citation136 Collectively, these studies suggest a diet rich in fruits providing polyphenolic bioflavonoids and other antioxidants (eg, proanthocyanidinsCitation137) along with dietary adviceCitation138 as important for the overall management of COPD.
Vitamins are generally obtained from our diets; however, is vitamin supplementation helpful for COPD management? Schols, in a recent translational paper,Citation126 felt that there is insufficient evidence to support taking high doses of single nutrient supplements to temper respiratory pathology; however, addressing vitamin deficiencies may be considered. It is important to note that caution must be used when using high-dose vitamin supplementation. There have been reports that, for some vitamins (eg, vitamin A), high-dose supplementation can increase the risk of lung cancer and mortality in susceptible individuals.Citation139–Citation141 In an ex vivo/in vitro analysis, compared to placebo-treated patients, white blood cells from stable COPD patients who had been supplemented with vitamin E or C for 12 weeks had increased resistance to peroxide-induced DNA breakages.Citation142 In a different controlled study, ascorbate intravenous infusion improved skeletal muscle fatigue resistance in COPD patients.Citation143 The combination treatment of vitamin C, zinc, selenium, and Echinacea purpurea, a herb that has been used for treating the common cold, helped shorten COPD exacerbation episodes.Citation144
Many people have studied the relationship of vitamin D with COPD management with varying results. A summary of the information from several reports, listed in chronological order, is shown in . Collectively, these studies suggest that COPD patients with serum 25-hydroxyvitamin D (25-OH vitamin D) below 20 ng/mL are deficient and may be at increased risk of exacerbations, worse lung function, and decline in lung function with time. This concept is supported in a review about vitamin insufficiencies and supplementation for COPD, in which Tsiligianni and van der Molen point out that the majority of COPD patients have vitamin D deficiency and that supplementation might perhaps be beneficial.Citation145 Lehouck et al suggest that it is generally only the COPD patients with severe deficiencies of serum 25-OH vitamin D <10 ng/mL who may benefit from vitamin D supplementation.Citation146 Vitamin D is important as an immune system regulatorCitation145 and, given that low serum 25-OH vitamin D levels have been associated with many chronic diseases (eg, autoimmune disease, cardiovascular disease, and infections),Citation146 vitamin D supplementation may be an important therapeutic strategy for COPD that is worthy of further investigation.
Table 2 COPD and vitamin D reports
Therapeutic strategies that can attenuate the inflammatory response can, in turn, attenuate oxidative stress. Alternative therapies such as herbal preparations, acupuncture, and tai chi or qigong may each help mitigate the overall inflammatory status of the COPD patient, either directly or indirectly. In the past few years, there have been multiple systematic reviews published relating to herbal therapies for COPD. One of these reports focused specifically on the combination of Tanreqing injection along with conventional therapy.Citation147 Tanreqing is a standardized herbal formula administered by intravenous drip, which has been shown to have some antibacterial and antiviral effects along with anti-inflammatory actions.Citation147 Tanreqing combined with conventional therapies demonstrated improvements in lung function and oxygenation, as well as decreased length of hospital stays, with no serious adverse events reported.Citation147 Ginseng is a single herbal product that has also been evaluated for use with COPD patients. It is known for supporting qi of the lung and spleen to improve the functions of these organs and has been used for respiratory diseases.Citation148 Studies were performed in a similar manner to that involving TanreqingCitation147 with ginseng given in combination with other therapies. As with Tanreqing, systematic review of the ginseng studies reported improvements in lung function and also quality of life (Qol) scores based on the St George’s Respiratory Questionnaire (SGRQ).Citation148 Other studies have also focused on herbal therapies for improvement of Qol for COPD patients. Based on systematic review of the literature, oral Chinese herbal medicines resulted in improvement in Qol scores when compared to no treatment or routine therapies.Citation148 A recent systematic review addressed the impact of Chinese herbal medicines on the body mass index, airflow obstruction, dyspnea, and exercise (BODE) index and the 6-minute walk test (6MWT).Citation149 The 6MWT provides the exercise capacity score for the BODE index, while the BODE index correlates well overall with COPD mortality. Results from the systematic review suggest a benefit for the BODE index and the 6MWT by the combination of Chinese herbal medicine with routine therapies, suggesting an overall clinical benefit, too.Citation149 The main herbs used for the studies were Astragalus membranaceus, Panax ginseng, and Cordyceps sinensis alone or, sometimes, in combination with other herbs.Citation149 Collectively, these systematic reviews suggest a benefit of Chinese medicine herbal preparations for treatment of specific aspects of the overall COPD disease complex and that further large-scale high quality studies are warranted.
Acupuncture is another Chinese modality that has been evaluated as adjunctive therapy for COPD patients. Acupuncture can be administered in different ways and may be different for each patient. To control for these types of variability, Suzuki et al, in their studies, standardized the acupuncture treatment protocol and used the same acupuncture points for all patients. In a small study, all patients received conservative treatment and one-half also received acupuncture for 10 weeks. The control group was matched to the treatment group but did not receive any sham treatment. The patients receiving acupuncture showed improvement in the modified Borg dyspnea scale and in oxygen saturation during the 6MWT.Citation150 The next study by the same group was also a small trial, which showed similar improvements after 10 weeks of acupuncture.Citation151 Suzuki et al then performed a slightly larger trial, now placebo-controlled, that extended the acupuncture to 12 weeks and included eleven different acupuncture points similar to those used in previous studies.Citation151,Citation152 Similar results were reported in this controlled trial, with improvement in the Borg scale score in the acupuncture-treated patients.Citation152 The reports from Suzuki et al were without electrical stimulation through the acupuncture needles. Studies were also performed using Acu-TENS, which is transcutaneous electrical nerve stimulation on acupuncture point sites. One study was a double-blinded, randomized controlled trial with Acu-TENS or placebo-TENS on a single acupuncture point site, once for 45 minutes. FEV1 improved in the Acu-TENS-treated patients immediately posttreatment.Citation153 There was also a corresponding increase in blood β-endorphin levels. In a subsequent study, which was also a randomized controlled trial, the patients received 4 weeks of Acu-TENS treatment.Citation154 This study had both placebo-TENS and sham-TENs control groups. Placebo is where the TENS stimulation electrodes are placed on the patient over the appropriate acupuncture point but without electrical stimulation applied. For the sham treatment group, electrical stimulation is applied to another part of the body that is a non-acupuncture point. Similarly to the previously mentioned study,Citation153 Ngai et al demonstrated improvement in FEV1. They also demonstrated improvements in oxygen saturation and distance for the 6MWT and improvement in the SGRQ Qol score, with corresponding increases in blood β-endorphin levels.Citation154 Collectively, these studies suggest that acupuncture with or without electrical stimulation promotes improved oxygenation in COPD patients.
Tai chi and qigong (TCQ) are traditional Chinese exercises with emphasis on specific focused movements, meditation, and breathing exercises. In a qigong randomized controlled study presented as a mind–body exercise intervention, the patients participating in the qigong exercises demonstrated significant improvement on the monitored functional task evaluation and the 6MWT by the 6-month follow-up.Citation155 In a separate randomized controlled trial, TCQ were compared to other breathing and self-paced walking exercises or normal routine (ie, no extra exercise), demonstrating, by the end of the 3-month treatment period, improved lung function and 6MWT and decreased exacerbation rate in the TCQ group compared to the two other groups.Citation156 In a systematic review of TCQ COPD trials, compared to no exercise, TCQ therapy promoted significant improvements in 6MWT, FEV1, and FEV1% predicted as well as SGRQ Qol score; however, compared to other exercise training, there was only improvement in the 6MWT.Citation157 Similar to acupuncture, TCQ is an alternative therapy that shows potential benefit for COPD patients and is worthy of further investigation with high-quality trials. It is important to note that the placebo effect does not improve physiologic measures, but is associated with significant improvement in well-being as measured by surveys.Citation158 Thus, the randomized, placebo-controlled trials show that complementary medicines do have a significant physiologic effect on pulmonary function outcomes in COPD.
COPD is a complex disease process and no single treatment can treat the whole disease. Clearly, from the different studies discussed, antioxidant supplementation, dietary antioxidants, Chinese herbal preparations, acupuncture, and TCQ may each have their place in COPD therapy, necessitating the use of combination and complementary therapies. One area that has not been addressed but is worth mentioning is the microbiome. The intestinal microbiome is critical to the regulation of our immune system, and alterations in the intestinal microbiome and dysbiosis may be a component of the pathogenesis of many diseases.Citation159 Furthermore, our diets can be a major influence on the intestinal microbial composition, potentially impacting our health.Citation160 One study in rats demonstrated that a diet rich in blueberries promoted gut health, evaluated by colonic metabolism measurements, and minimized invasive bacterial species.Citation161 Similarly, modification to a Paleolithic hunter-gatherer-type diet might be worth considering. This type of diet promotes fruit and vegetables and eliminates cereal grains, legumes, and dairy, and has been shown to promote metabolic and physiologic improvements in sedentary adults.Citation162 Serial analyses of gut and respiratory microbiomes in infants with cystic fibrosis have shown that nutritional factors and gut microbial colonization patterns are determinants of respiratory tract microbial development, suggesting that dietary alterations and probiotic strategies may provide early intervention opportunities.Citation163 Similar considerations are worth investigating as potential early intervention opportunities for control of COPD progression.Citation164
Disclosure
The authors report no conflicts of interest in this work.
References
- KimVCrinerGJChronic bronchitis and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187322823723204254
- HoggJCChuFUtokaparchSThe nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med2004350262645265315215480
- McDonoughJEYuanRSuzukiMSmall-airway obstruction and emphysema in chronic obstructive pulmonary diseaseN Engl J Med2011365171567157522029978
- FischerBMPavliskoEVoynowJAPathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammationInt J Chron Obstruct Pulmon Dis2011641342121857781
- PryorWACigarette smoke and the involvement of free radical reactions in chemical carcinogenesisBr J Cancer Suppl1987819233307870
- ChurchDFPryorWAFree-radical chemistry of cigarette smoke and its toxicological implicationsEnviron Health Perspect1985641111263007083
- RodgmanAPerfettiTAThe Chemical Components of Tobacco and Tobacco SmokeBoca Raton, FLCRC Press Inc2009
- PryorWACigarette smoke radicals and the role of free radicals in chemical carcinogenicityEnviron Health Perspect1997105Suppl 48758829255574
- ZangLYStoneKPryorWADetection of free radicals in aqueous extracts of cigarette tar by electron spin resonanceFree Radic Biol Med19951921611677649487
- GhioAJHilbornEDStonehuernerJGParticulate matter in cigarette smoke alters iron homeostasis to produce a biological effectAm J Respir Crit Care Med2008178111130113818723436
- HempelSLBuettnerGRO’MalleyYQWesselsDAFlahertyDMDihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123Free Radic Biol Med1999271–214615910443931
- HeunksLMViñaJvan HerwaardenCLFolgeringHTGimenoADekhuijzenPNXanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary diseaseAm J Physiol19992776 Pt 2R1697R170410600916
- HoSPChan-YeungMChowKKIpMSMakJCAntioxidant enzyme activities in healthy Chinese adults: influence of age, gender and smokingRespirology200510330530915955142
- van AntwerpenVLTheronAJRichardsGAVitamin E, pulmonary functions, and phagocyte-mediated oxidative stress in smokers and nonsmokersFree Radic Biol Med19951859359417797104
- DekhuijzenPNAbenKKDekkerIIncreased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19961543 Pt 18138168810624
- SchabergTHallerHRauMKaiserDFassbenderMLodeHSuperoxide anion release induced by platelet-activating factor is increased in human alveolar macrophages from smokersEur Respir J1992543873931314191
- MorrowJDFreiBLongmireAWIncrease in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damageN Engl J Med199533218119812037700313
- MontuschiPCollinsJVCiabattoniGExhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokersAm J Respir Crit Care Med20001623 Pt 11175117710988150
- HalliwellBAntioxidants in human health and diseaseAnnu Rev Nutr19961633508839918
- DuthieGGArthurJRJamesWPEffects of smoking and vitamin E on blood antioxidant statusAm J Clin Nutr199153Suppl 41061S1063S2012019
- LykkesfeldtJPrieméHLoftSPoulsenHEEffect of smoking cessation on plasma ascorbic acid concentrationBMJ19963137049918688760
- BurlakovaEBZhizhinaGPGurevichSMBiomarkers of oxidative stress and smoking in cancer patientsJ Cancer Res Ther201061475320479547
- WickendenJAClarkeMCRossiAGCigarette smoke prevents apoptosis through inhibition of caspase activation and induces necrosisAm J Respir Cell Mol Biol200329556257012748058
- CantinAMNorthSLHubbardRCCrystalRGNormal alveolar epithelial lining fluid contains high levels of glutathioneJ Appl Physiol (1985)19876311521573040659
- LiXYDonaldsonKRahmanIMacNeeWAn investigation of the role of glutathione in increased epithelial permeability induced by cigarette smoke in vivo and in vitroAm J Respir Crit Care Med19941496151815258004308
- CantinACrystalRGOxidants, antioxidants and the pathogenesis of emphysemaEur J Respir Dis Suppl19851397172995106
- ChurgACherukupalliKCigarette smoke causes rapid lipid peroxidation of rat tracheal epitheliumInt J Exp Pathol19937421271328499312
- MacNeeWWiggsBBelzbergASHoggJCThe effect of cigarette smoking on neutrophil kinetics in human lungsN Engl J Med1989321149249282779614
- SelbyCDrostEWraithPKMacNeeWIn vivo neutrophil sequestration within lungs of humans is determined by in vitro “filterability”J Appl Physiol (1985)1991715199620031761502
- RahmanIMacNeeWRole of oxidants/antioxidants in smoking-induced lung diseasesFree Radic Biol Med19962156696818891669
- GastonBReillyJDrazenJMEndogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airwaysProc Natl Acad Sci U S A1993902310957109618248198
- CorradiMMontuschiPDonnellyLEPesciAKharitonovSABarnesPJIncreased nitrosothiols in exhaled breath condensate in inflammatory airway diseasesAm J Respir Crit Care Med2001163485485811282756
- PadmajaSHuieREThe reaction of nitric oxide with organic peroxyl radicalsBiochem Biophys Res Commun199319525395448373394
- Van der VlietASmithDO’NeillCAInteractions of peroxynitrite with human plasma and its constituents: oxidative damage and antioxidant depletionBiochem J1994303Pt 12953017945255
- PetruzzelliSPuntoniRMimottiPPlasma 3-nitrotyrosine in cigarette smokersAm J Respir Crit Care Med19971566190219079412573
- CorradiMMajoriMCaccianiGCConsigliGFde’MunariEPesciAIncreased exhaled nitric oxide in patients with stable chronic obstructive pulmonary diseaseThorax199954757257510377199
- RytiläPRehnTIlumetsHIncreased oxidative stress in asymptomatic current chronic smokers and GOLD stage 0 COPDRespir Res200676916646959
- ZhangWZVenardosKChin-DustingJKayeDMAdverse effects of cigarette smoke on NO bioavailability: role of arginine metabolism and oxidative stressHypertension200648227828516801489
- LangenRCKornSHWoutersEFROS in the local and systemic pathogenesis of COPDFree Radic Biol Med200335322623512885585
- BloomerRJDecreased blood antioxidant capacity and increased lipid peroxidation in young cigarette smokers compared to nonsmokers: impact of dietary intakeNutr J200763917996062
- TalukderMAJohnsonWMVaradharajSChronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in miceAm J Physiol Heart Circ Physiol20113001H388H39621057039
- YamaguchiYNasuFHaradaAKunitomoMOxidants in the gas phase of cigarette smoke pass through the lung alveolar wall and raise systemic oxidative stressJ Pharmacol Sci2007103327528217332694
- CanoMThimmalappulaRFujiharaMCigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and age-related macular degenerationVision Res201050765266419703486
- LannanSDonaldsonKBrownDMacNeeWEffect of cigarette smoke and its condensates on alveolar epithelial cell injury in vitroAm J Physiol19942661 Pt 1L92L1008304473
- RahmanIMorrisonDDonaldsonKMacNeeWSystemic oxidative stress in asthma, COPD, and smokersAm J Respir Crit Care Med19961544 Pt 1105510608887607
- HoidalJRFoxRBLeMarbePAPerriRRepineJEAltered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokersAm Rev Respir Dis1981123185896257154
- RogersDFMucus hypersecretion in chronic obstructive pulmonary diseaseChadwickDGoodeJAChronic Obstructive Pulmonary Disease: Pathogenesis to TreatmentChichesterJohn Wiley & Sons20016583
- KharitonovSABarnesPJNitric oxide, nitrotyrosine, and nitric oxide modulators in asthma and chronic obstructive pulmonary diseaseCurr Allergy Asthma Rep20033212112912562551
- RicciardoloFLCaramoriGItoKNitrosative stress in the bronchial mucosa of severe chronic obstructive pulmonary diseaseJ Allergy Clin Immunol200511651028103516275371
- Varela-CarverAParkerHKleinertCRimoldiOAdverse effects of cigarette smoke and induction of oxidative stress in cardiomyocytes and vascular endotheliumCurr Pharm Des201016232551255820550505
- Müller-SchweinitzerEMüllerSEReinekeDCReactive oxygen species mediate functional differences in human radial and internal thoracic arteries from smokersJ Vasc Surg201051243844420036100
- BurkeAFitzgeraldGAOxidative stress and smoking-induced vascular injuryProg Cardiovasc Dis2003461799012920701
- HaratsDBen-NaimMDabachYHollanderGSteinOSteinYCigarette smoking renders LDL susceptible to peroxidative modification and enhanced metabolism by macrophagesAtherosclerosis1989792–32452522597232
- CherubiniAVignaGBZulianiGRuggieroCSeninUFellinRRole of antioxidants in atherosclerosis: epidemiological and clinical updateCurr Pharm Des200511162017203215974956
- RahmanIOxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanismsCell Biochem Biophys200543116718816043892
- KhannaAGuoMMehraMRoyalW3rdInflammation and oxidative stress induced by cigarette smoke in Lewis rat brainsJ Neuroimmunol20132541–2697523031832
- RutgersSRPostmaDSten HackenNHOngoing airway inflammation in patients with COPD who do not currently smokeChest20001175 Suppl 1262S10843943
- LouhelainenNRytilaPHaahtelaTKinnulaVLDjukanovićRPersistence of oxidant and protease burden in the airways after smoking cessationBMC Pulm Med200992519473482
- ThompsonABBohlingTHeiresALinderJRennardSILower respiratory tract iron burden is increased in association with cigarette smokingJ Lab Clin Med199111764934992045717
- WesseliusLJNelsonMESkikneBSIncreased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokersAm J Respir Crit Care Med199415036906958087339
- InonuHDorukSSahinSOxidative stress levels in exhaled breath condensate associated with COPD and smokingRespir Care201257341341921968597
- LouhelainenNMyllarniemiMRahmanIKinnulaVLAirway biomarkers of the oxidant burden in asthma and chronic obstructive pulmonary disease: current and future perspectivesInt J Chron Obstruct Pulmon Dis20083458560319281076
- BalmesJBecklakeMBlancPEnvironmental and Occupational Health Assembly, American Thoracic SocietyAmerican Thoracic Society Statement: occupational contribution to the burden of airway diseaseAm J Respir Crit Care Med2003167578779712598220
- SanganiRGGhioAJLung injury after cigarette smoking is particle relatedInt J Chron Obstruct Pulmon Dis2011619119821660296
- TaoFGonzalez-FlechaBKobzikLReactive oxygen species in pulmonary inflammation by ambient particulatesFree Radic Biol Med200335432734012899936
- KadiiskaMBMasonRPDreherKLCostaDLGhioAJIn vivo evidence of free radical formation in the rat lung after exposure to an emission source air pollution particleChem Res Toxicol19971010110411089348432
- AmitaniRWilsonRRutmanAEffects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epitheliumAm J Respir Cell Mol Biol1991426321898852
- CaramoriGDi GregorioCCarlstedtIMucin expression in peripheral airways of patients with chronic obstructive pulmonary diseaseHistopathology20044547748415500651
- CaramoriGCasolariPDi GregorioCMUC5AC expression is increased in bronchial submucosal glands of stable COPD patientsHistopathology200955332133119723147
- KirkhamSKolsumURousseauKSinghDVestboJThorntonDJMUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2008178101033103918776153
- CantinAMHanrahanJWBilodeauGCystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokersAm J Respir Crit Care Med2006173101139114416497995
- ClunesLADaviesCMCoakleyRDCigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydrationFASEB J201226253354521990373
- RabARoweSMRajuSVBebokZMatalonSCollawnJFCigarette smoke and CFTR: implications in the pathogenesis of COPDAm J Physiol Lung Cell Mol Physiol20133058L530L54123934925
- GirodSGalabertCLecuireAZahmJMPuchelleEPhospholipid composition and surface-active properties of tracheobronchial secretions from patients with cystic fibrosis and chronic obstructive pulmonary diseasesPediatr Pulmonol199213122271589308
- FahyJVDickeyBFAirway mucus function and dysfunctionN Engl J Med2010363232233224721121836
- GenschEGallupMSucherATobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNKJ Biol Chem2004279390853909315262961
- DiYPZhaoJHarperRCigarette smoke induces MUC5AC protein expression through the activation of Sp1J Biol Chem201228733279482795822700966
- MorettoNBertoliniSIadiciccoCCigarette smoke and its component acrolein augment IL-8/CXCL8 mRNA stability via p38 MAPK/MK2 signaling in human pulmonary cellsAm J Physiol Lung Cell Mol Physiol201230310L929L93822983351
- BautistaMVChenYIvanovaVSRahimiMKWatsonAMRoseMCIL-8 regulates mucin gene expression at the posttranscriptional level in lung epithelial cellsJ Immunol200918332159216619596978
- DeshmukhHSShaverCCaseLMAcrolein-activated matrix metalloproteinase 9 contributes to persistent mucin productionAm J Respir Cell Mol Biol200838444645418006877
- DeshmukhHSMcLachlanAAtkinsonJJMatrix metalloproteinase-14 mediates a phenotypic shift in the airways to increase mucin productionAm J Respir Crit Care Med2009180983484519661247
- BorchersMTWesselkamperSWertSEShapiroSDLeikaufGDMonocyte inflammation augments acrolein-induced Muc5ac expression in mouse lungAm J Physiol1999277L489L49710484456
- KimHJParkYDMoonUYThe role of Nox4 in oxidative stress-induced MUC5AC overexpression in human airway epithelial cellsAm J Respir Cell Mol Biol200839559860918539955
- FischerBMVoynowJANeutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen speciesAm J Respir Cell Mol Biol200226444745211919081
- ZhengSByrdASFischerBMGroverARGhioAJVoynowJARegulation of MUC5AC expression by NAD(P)H:quinone oxidoreductase 1Free Radic Biol Med20074291398140817395013
- Casalino-MatsudaSMMonzónMEFortezaRMEpidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epitheliumAm J Respir Cell Mol Biol200634558159116424381
- Casalino-MatsudaSMMonzonMEDayAJFortezaRMHyaluronan fragments/CD44 mediate oxidative stress-induced MUC5B up-regulation in airway epitheliumAm J Respir Cell Mol Biol200940327728518757307
- MebratuYASchwalmKSmithKRSchuylerMTesfaigziYCigarette smoke suppresses Bik to cause epithelial cell hyperplasia and mucous cell metaplasiaAm J Respir Crit Care Med2011183111531153821317312
- ChenGKorfhagenTRKarpCLFoxa3 induces goblet cell metaplasia and inhibits innate antiviral immunityAm J Respir Crit Care Med2014189330131324392884
- WhitsettJAHaitchiHMMaedaYIntersections between pulmonary development and diseaseAm J Respir Crit Care Med2011184440140621642246
- TilleyAEHarveyBGHeguyADown-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009179645746619106307
- KirkhamPABarnesPJOxidative stress in COPDChest2013144126627323880677
- AoshibaKZhouFTsujiTNagaiADNA damage as a molecular link in the pathogenesis of COPD in smokersEur Respir J20123961368137622267761
- NyunoyaTMebratuYContrerasADelgadoMChandHSTesfaigziYMolecular processes that drive cigarette smoke-induced epithelial cell fate of the lungAm J Respir Cell Mol Biol201450347148224111585
- SteilingKLenburgMESpiraAPersonalized management of chronic obstructive pulmonary disease via transcriptomic profiling of the airway and lungAnn Am Thorac Soc2013Suppl 10S190S19624313772
- PostmaDSKerkhofMBoezenHMKoppelmanGHAsthma and chronic obstructive pulmonary disease: common genes, common environments?Am J Respir Crit Care Med2011183121588159421297068
- MizunoSBogaardHJGomez-ArroyoJMicroRNA-199a-5p is associated with hypoxia-inducible factor-1α expression in lungs from patients with COPDChest2012142366367222383663
- HassanTCarrollTPBuckleyPGmiR-199a-5p silencing regulates the unfolded protein response in chronic obstructive pulmonary disease and α1-antitrypsin deficiencyAm J Respir Crit Care Med2014189326327324299514
- QiuWBaccarelliACareyVJVariable DNA methylation is associated with chronic obstructive pulmonary disease and lung functionAm J Respir Crit Care Med2012185437338122161163
- VucicEAChariRThuKLDNA methylation is globally disrupted and associated with expression changes in chronic obstructive pulmonary disease small airwaysAm J Respir Cell Mol Biol201450591292224298892
- ItoKItoMElliottWMDecreased histone deacetylase activity in chronic obstructive pulmonary diseaseN Engl J Med2005352191967197615888697
- MalhotraDThimmulappaRKMercadoNDenitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patientsJ Clin Invest2011121114289430222005302
- SundarIKYaoHRahmanIOxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseasesAntioxid Redox Signal201318151956197122978694
- WeissSTWhat genes tell us about the pathogenesis of asthma and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2010181111170117320133923
- PillaiSGGeDZhuGICGN InvestigatorsA genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility lociPLoS Genet200953e100042119300482
- ChoMHBoutaouiNKlandermanBJVariants in FAM13A are associated with chronic obstructive pulmonary diseaseNat Genet201042320020220173748
- RepapiESayersIWainLVGenome-wide association study identifies five loci associated with lung functionNat Genet2010421364420010834
- ChoMHMcDonaldMLZhou X, et al; NETT Genetics, ICGN, ECLIPSE and COPDGene Investigators. Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysisLancet Respir Med20142321422524621683
- KongXChoMHAndersonWECLIPSE Study NETT InvestigatorsGenome-wide association study identifies BICD1 as a susceptibility gene for emphysemaAm J Respir Crit Care Med20111831434920709820
- ManichaikulAHoffmanEASmolonskaJGenome-wide study of percent emphysema on computed tomography in the general population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource StudyAm J Respir Crit Care Med2014189440841824383474
- McDonaldMLChoMHSorheimICCommon genetic variants associated with resting oxygenation in chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol201451567868724825563
- DijkstraAESmolonskaJvan den BergeMLifeLines Cohort studySusceptibility to chronic mucus hypersecretion, a genome wide association studyPLoS One201494e9162124714607
- GillilandFDBerhaneKMcConnellRMaternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung functionThorax200055427127610722765
- SternDAMorganWJWrightALGuerraSMartinezFDPoor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort studyLancet2007370958975876417765525
- RahmanIAntioxidant therapeutic advances in COPDTher Adv Respir Dis20082635137419124382
- BiswasSHwangJWKirkhamPARahmanIPharmacological and dietary antioxidant therapies for chronic obstructive pulmonary diseaseCurr Med Chem201320121496153022963552
- ZhengJPKangJHuangSGEffect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled studyLancet200837196292013201818555912
- TseHNRaiteriLWongKYHigh-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE studyChest2013144110611823348146
- WuWPatelKBBoothJLZhangWMetcalfJPCigarette smoke extract suppresses the RIG-I-initiated innate immune response to influenza virus in the human lungAm J Physiol Lung Cell Mol Physiol20113006L821L83021335520
- AggarwalBBKumarABhartiACAnticancer potential of curcumin: preclinical and clinical studiesAnticancer Res2003231A36339812680238
- JagetiaGCAggarwalBB“Spicing up” of the immune system by curcuminJ Clin Immunol2007271193517211725
- MejaKKRajendrasozhanSAdenugaDCurcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2Am J Respir Cell Mol Biol200839331232318421014
- SuzukiMBetsuyakuTItoYCurcumin attenuates elastase- and cigarette smoke-induced pulmonary emphysema in miceAm J Physiol Lung Cell Mol Physiol20092964L614L62319168576
- WoodLGWarkPAGargMLAntioxidant and anti-inflammatory effects of resveratrol in airway diseaseAntioxid Redox Signal201013101535154820214495
- KnoblochJHagHJungckDUrbanKKochAResveratrol impairs the release of steroid-resistant cytokines from bacterial endotoxin-exposed alveolar macrophages in chronic obstructive pulmonary diseaseBasic Clin Pharmacol Toxicol2011109213814321447053
- ScholsAMNutrition as a metabolic modulator in COPDChest201314441340134524081345
- TabakCArtsICSmitHAHeederikDKromhoutDChronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: the MORGEN StudyAm J Respir Crit Care Med20011641616411435239
- WaldaICTabakCSmitHADiet and 20-year chronic obstructive pulmonary disease mortality in middle-aged men from three European countriesEur J Clin Nutr200256763864312080403
- McKeeverTMLewisSACassanoPAPatterns of dietary intake and relation to respiratory disease, forced expiratory volume in 1 s, and decline in 5-y forced expiratory volumeAm J Clin Nutr201092240841520554789
- FloresGDastmalchiKWuSBPhenolic-rich extract from the Costa Rican guava (Psidium friedrichsthalianum) pulp with antioxidant and anti-inflammatory activity. Potential for COPD therapyFood Chem2013141288989523790863
- Rodriguez-MateosARendeiroCBergillos-MecaTIntake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activityAm J Clin Nutr20139851179119124004888
- BaldrickFRElbornJSWoodsideJVEffect of fruit and vegetable intake on oxidative stress and inflammation in COPD: a randomised controlled trialEur Respir J20123961377138422088966
- KeranisEMakrisDRodopoulouPImpact of dietary shift to higher-antioxidant foods in COPD: a randomised trialEur Respir J201036477478020150206
- CollinsPFEliaMStrattonRJNutritional support and functional capacity in chronic obstructive pulmonary disease: a systematic review and meta-analysisRespirology201318461662923432923
- Fonseca WaldELvan den BorstBGoskerHRScholsAMDietary fibre and fatty acids in chronic obstructive pulmonary disease risk and progression: a systematic reviewRespirology201419217618424372903
- van de BoolCMattijssen-VerdonschotCvan MelickPPQuality of dietary intake in relation to body composition in patients with chronic obstructive pulmonary disease eligible for pulmonary rehabilitationEur J Clin Nutr201468215916524327123
- BagchiDSwaroopAPreussHGBagchiMFree radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: an overviewMutat Res Fundam Mol Mech Mutagen Epub4192014
- FarooqiNNordströmLLundgrenRSandströmTHåglinLChanges in body weight and physical performance after receiving dietary advice in patients with chronic obstructive pulmonary disease (COPD): 1-year follow-upArch Gerontol Geriatr2011531707520619471
- OmennGSGoodmanGEThornquistMDEffects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular diseaseN Engl J Med199633418115011558602180
- OmennGSGoodmanGEThornquistMDRisk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy TrialJ Natl Cancer Inst19968821155015598901853
- GoodmanGEThornquistMDBalmesJThe Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplementsJ Natl Cancer Inst200496231743175015572756
- WuTCHuangYCHsuSYWangYCYehSLVitamin E and vitamin C supplementation in patients with chronic obstructive pulmonary diseaseInt J Vitam Nutr Res200777427227918271282
- RossmanMJGartenRSGrootHJAscorbate infusion increases skeletal muscle fatigue resistance in patients with chronic obstructive pulmonary diseaseAm J Physiol Regul Integr Comp Physiol201330510R1163R117024068051
- IsbaniahFWiyonoWHYunusFSetiawatiATotzkeUVerbruggenMAEchinacea purpurea along with zinc, selenium and vitamin C to alleviate exacerbations of chronic obstructive pulmonary disease: results from a randomized controlled trialJ Clin Pharm Ther201136556857621062330
- TsiligianniIGvan der MolenTA systematic review of the role of vitamin insufficiencies and supplementation in COPDRespir Res20101117121134250
- LehouckAMathieuCCarremansCHigh doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trialAnn Intern Med2012156210511422250141
- ZhongYMaoBWangGTanreqing injection combined with conventional Western medicine for acute exacerbations of chronic obstructive pulmonary disease: a systematic reviewJ Altern Complement Med201016121309131921091297
- AnXZhangALYangAWOral ginseng formulae for stable chronic obstructive pulmonary disease: a systematic reviewRespir Med2011105216517621146973
- ChenXMayBDiYMOral Chinese herbal medicine combined with pharmacotherapy for stable COPD: a systematic review of effect on BODE index and six minute walk testPLoS One201493e9183024622390
- SuzukiMNamuraKOhnoYThe effect of acupuncture in the treatment of chronic obstructive pulmonary diseaseJ Altern Complement Med20081491097110519055335
- SuzukiMNamuraKOhnoYCombined standard medication and acupuncture for COPD: a case seriesAcupunct Med20123029610222516032
- SuzukiMMuroSAndoYA randomized, placebo-controlled trial of acupuncture in patients with chronic obstructive pulmonary disease (COPD): the COPD-acupuncture trial (CAT)Arch Intern Med20121721187888622905352
- JonesAYNgaiSPHui-ChanCWYuHPAcute effects of Acu-TENS on FEV1 and blood Β-endorphin level in chronic obstructive pulmonary diseaseAltern Ther Health Med201117581322314671
- NgaiSPJonesAYHui-ChanCWKoFWHuiDSEffect of 4 weeks of Acu-TENS on functional capacity and beta-endorphin level in subjects with chronic obstructive pulmonary disease: a randomized controlled trialRespir Physiol Neurobiol20101731293620601209
- NgBHTsangHWJonesAYSoCTMokTYFunctional and psychosocial effects of health qigong in patients with COPD: a randomized controlled trialJ Altern Complement Med201117324325121417809
- ChanAWLeeASuenLKTamWWTai chi Qigong improves lung functions and activity tolerance in COPD clients: a single blind, randomized controlled trialComplement Ther Med201119131121296261
- DingMZhangWLiKChenXEffectiveness of t’ai chi and qigong on chronic obstructive pulmonary disease: a systematic review and meta-analysisJ Altern Complement Med2014202798623961940
- WechslerMEKelleyJMBoydIOActive albuterol or placebo, sham acupuncture, or no intervention in asthmaN Engl J Med2011365211912621751905
- HillDAArtisDIntestinal bacteria and the regulation of immune cell homeostasisAnnu Rev Immunol20102862366720192812
- FlintHJScottKPLouisPDuncanSHThe role of the gut microbiota in nutrition and healthNat Rev Gastroenterol Hepatol201291057758922945443
- LacombeALiRWKlimis-ZacasDLowbush wild blueberries have the potential to modify gut microbiota and xenobiotic metabolism in the rat colonPLoS One201386e6749723840722
- FrassettoLASchloetterMMietus-SynderMMorrisRCJrSebastianAMetabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type dietEur J Clin Nutr200963894795519209185
- MadanJCKoestlerDCStantonBASerial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposuresMBio201234e002511222911969
- HanMKHuangYJLipumaJJSignificance of the microbiome in obstructive lung diseaseThorax201267545646322318161
- BlackPNScraggRRelationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination surveyChest200512863792379816354847
- GindeAAMansbachJMCamargoCAJrAssociation between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination SurveyArch Intern Med2009169438439019237723
- ShaheenSOJamesonKARobinsonSMRelationship of vitamin D status to adult lung function and COPDThorax201166869269821653927
- LangeNESparrowDVokonasPLitonjuaAAVitamin D deficiency, smoking, and lung function in the Normative Aging StudyAm J Respir Crit Care Med2012186761662122822023
- PuhanMASiebelingLFreiAZollerMBischoff-FerrariHTer RietGNo association of 25-hydroxyvitamin D with exacerbations in primary care patients with COPDChest20141451374324008868
