Abstract
Influencing the progression of COPD has long been an elusive goal of drug therapy. Directly or indirectly, this has again been investigated in two of the largest, long-term drug trials in COPD: Towards a Revolution in COPD Health (TORCH) and Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT®). Neither trial achieved statistical significance in their respective primary outcomes; however, both make considerable contributions to understanding of how the progression of COPD may be influenced. The objective of this article is to review the data from these different trials with a view to what can be learnt about the management of COPD. The long-term improvements in lung function, health-related quality of life, and possibly survival from the use of long-acting bronchodilators in these trials suggest an influence on progression of the disease. With the more optimistic view of benefits from drug treatment of COPD that these trials provide, a review of prescribing practices is warranted.
Burden of COPD
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality, and represents a substantial economic and social burden worldwide. Global prevalence of COPD in the general population has been reported to be between 7.5% and 10%,Citation1,Citation2 and is predominantly associated with smoking. In a meta-analysis of 67 population-based studies (representing >111,000 cases of COPD from 28 countries), prevalence of COPD was significantly higher among smokers (15.4%) and ex-smokers (10.7%) than people who had never smoked (4.3%).Citation2 Prevalence of COPD is therefore highest in countries where cigarette smoking is common.
Current prevalence data are likely to underestimate the true burden of COPD, since the disease is frequently misdiagnosed or not diagnosed until symptoms become clinically apparent at a more advanced stage. Of the people with COPD in Spain, for example, only an estimated 20% have their disease diagnosed.Citation3 In the US, it is believed that between 60% and 70% of patients with reduced forced expiratory volume in 1 second (FEV1) have never been diagnosed with COPD.Citation4
The burden of morbidity and mortality due to COPD is predicted to increase.Citation4 COPD is the fourth leading cause of mortality in the US and Europe,Citation5 and approximately 2.7 million deaths worldwide were attributable to COPD in 2000.Citation4 Age-adjusted mortality due to COPD doubled between 1970 and 2002 in the US,Citation6 and total deaths from COPD are projected to increase by more than 30% in the next 10 years,Citation7 with notable increases predicted in women.Citation4
It is unsurprising, therefore, that the economic costs attributed to COPD are substantial. For example, mean annual direct costs of COPD under usual clinical practice in Spain were calculated in a prospective study to be US$1876 per patient in 2003 (nearer US$3000 for severe COPD),Citation8 which is approximately twice the equivalent cost reported for asthma.Citation9 In the UK, direct costs were estimated to equate to approximately US$1900 per person per year in 1996, whilst in the US in the late 1990s, the annual cost of COPD was estimated to be US$23.9 billion, equating to approximately US$1500 per patient per year.Citation10
Evolution of the disease
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) and American Thoracic Society (ATS)/European Respiratory Society (ERS) consensus statements both define COPD as a progressive airflow limitation that is not fully reversible and recommend spirometry, particularly FEV1, as a means of diagnosing and staging COPD. Airflow limitation causes air trapping and hyperinflation as ventilation rate increases, for example during physical effort.Citation11 Hyperinflation causes or worsens breathlessness, as breathing becomes inefficient. Breathlessness encourages inactivity due to avoidance of exertion. Exercise capacity becomes reduced and deconditioning increases, which further worsens breathlessness on activity, and the cycle continues. Collectively, this contributes to reducing the patients’ health-related quality of life (HRQL). Exacerbations of COPD also contribute to worsen these disease outcomes. Currently, there is no universal definition of an exacerbation, though GOLD define an exacerbation as “an event in the natural course of the disease characterized by a change in the patient’s baseline dyspnea, cough and/or sputum that is beyond the normal day-to-day variations, is acute in onset, and my warrant a change in regular medication in a patient with underlying COPD.”Citation12
FEV1 decline as an outcome parameter
FEV1 decline has been considered a parameter of disease progression. FEV1 as a parameter of disease in individual patients has considerable advantages in that it is relatively simple to measure (with appropriate training) and is prognostic of mortality, both as a single measureCitation13–Citation15 and in terms of the rate of decline.Citation16
An increased rate of decline in FEV1 in susceptible smokers has been known for over 30 years,Citation17 as has the fact that smoking cessation, provided it is achieved sufficiently early, reduces the rate of decline, though does not restore the FEV1 lost.Citation18–Citation21
However, information gained on the disease from measurement of FEV1 does have limitations. FEV1 does not correlate well with other markers of disease progression that are also prognostic of mortality,Citation22 which question whether decline in FEV1 is the best marker of disease progression.Citation23,Citation24 Additionally, little is understood about the course through which FEV1 declines, which is unlikely to be linear,Citation25 but could be graduated and variable depending on COPD exacerbations and comorbidities.Citation26 Varying standards of spirometry and inter-patient variability adds to the challenge of comparing treatment groups with respect to the rate of decline in FEV1.
Past studies that evaluated FEV1 decline
Numerous specifically designed trials have failed to show an effect of pharmacotherapy on the rate of decline in FEV1, including short-acting anticholinergic (ipratropium),Citation18 an antioxidant (N-acetylcysteine),Citation27 and inhaled corticosteroids.Citation28–Citation31 Two meta-analyses on the effects of inhaled corticosteroids (ICS) have contradicted each other,Citation32,Citation33 and a pooled analysis suggests that ICS do not have efficacy in reducing the rate of decline in FEV1.Citation34
In addition, regular physical activity has been suggested to reduce the rate of decline in FEV1 in smokers from a population study,Citation35 however, this needs to be investigated in a prospective, controlled trial.
Rationale for current disease modification studies (TORCH and UPLIFT®)
A post-hoc analysis of 1-year data from two, double-blind, randomized, placebo-controlled trials, indicated that the long-acting anticholinergic drug, tiotropium, may reduce the rate of decline in FEV1 in COPD patients.Citation36 In addition, two retrospective analyses (representing over 9700 COPD patients) have suggested that use of ICS, either as monotherapy or in combination with a long-acting β2-agonist (LABA), may reduce all-cause mortality in patients with COPD.Citation37,Citation38 Longer-term trials were required to further investigate these effects: specifically an effect of LABA/ICS on all-cause mortality and of tiotropium on the rate of decline in FEV1.
The Towards a Revolution in COPD Health (TORCH) trial was a 3-year, double-blind, parallel-group, placebo-controlled study of 6184 COPD patients randomized to salmeterol and fluticasone propionate, either as monotherapy or in combination. Primary outcome was all-cause mortality over 3 years,Citation39 with a post-hoc analysis on the rate of decline in FEV1.Citation40
The Understanding Potential Long-term Impacts on Function and Tiotropium (UPLIFT®) trial was a 4-year, randomized, double-blind, placebo-controlled, parallel-group study involving 5993 patients with moderate-to-severe COPD randomized to receive either tiotropium or placebo. These patients continued to receive their otherwise usual bronchodilator therapy. The primary outcome of the UPLIFT® study was rate of decline in FEV1 over 4 years.Citation41
Summary of key findings from TORCH and UPLIFT®
TORCH
TORCH did not achieve a significant decrease in mortality among patients treated with LABA–ICS combination therapy versus short-acting bronchodilators (placebo) (hazard ratio [HR] 0.825, 95% confidence interval [CI], 0.681–1.002; p = 0.052).Citation42 However, active treatments significantly reduced the annual rate of exacerbations compared with placebo (p < 0.001) and exacerbations requiring hospital admission were reduced with the combination therapy and salmeterol alone compared with placebo (p ≤ 0.03). The combination therapy also improved average HRQL compared with placebo and monotherapies over the 3-year trial period. Adverse event data from TORCH indicated an increased incidence of pneumonia among patients receiving ICS treatment, both as a combination treatment and as monotherapy. The treatment arms containing ICS had 439 deaths compared with 436 deaths in the non-ICS-containing arms.
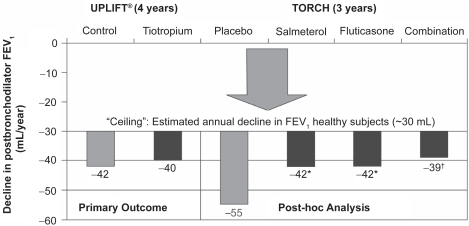
Sustained increase in lung function was observed in all active groups compared with placebo.Citation42 A post-hoc analysis (stated as being planned before unblinding) on rate of decline in postbronchodilator FEV1 showed an effect of the active treatment groups compared with placebo.Citation40 The rate of FEV1 decline was 55 mL/year in the placebo (short-acting bronchodilators) group. In comparison, the rates of decline in the active treatment groups were significantly less (p ≤ 0.03) at 39 mL/year for combined therapy and 42 mL/year for ICS and LABA monotherapy. The rates of decline were similar between the active treatment groups, with no significant benefit of the combination over the individual components.
UPLIFT®
UPLIFT® reported on moderate to severe COPD patients that received usual treatment (including LABA, ICS alone or in combination), and were randomized to tiotropium or placebo (control). These data did not show significant differences in the rate of decline in lung function or HRQL score between the tiotropium and control groups, but achieved a sustained increase in lung function and HRQL over 4 years (p < 0.001).Citation43 Rate of postbronchodilator FEV1 decline was 40 mL/year for tiotropium and 42 mL/year for the control group. At the end of the 4-year treatment period, the tiotropium group had not yet reached the level of impaired HRQL documented at baseline. Further, statistical significant increase in the proportion of patients achieving the reported minimal clinically significant difference of at least 4 units (p < 0.001) occurred in the tiotropium group compared with the control group.
UPLIFT® demonstrated a significant reduction in the risk of having an exacerbation and an exacerbation leading to a hospitalization in the tiotropium group. Survival was significantly increased while patients received tiotropium and when including the follow-up of prematurely discontinued patients for the protocol-defined treatment period. However, the improvement in survival lost statistical significance when the 30-day, protocol-defined washout period was included. Additionally, overall cardiac and respiratory morbidity was reduced.
Comparison of designs
As expected for trials with different primary objectives, considerable differences exist between the TORCH and UPLIFT® trial designs and entry criteria (). Aside from the different primary and secondary outcomes and treatment durations, a key difference is the medications that were permitted during the trials, other than the study drugs.Citation39,Citation41 TORCH permitted maintenance use of short-acting bronchodilators and short courses (eg, 10 days) of oral corticosteroids for the treatment of exacerbations.Citation42 Excluded were long-term use of oral corticosteroids, maintenance use of tiotropium (which was unavailable at the onset of the trial), and LABA and ICS, other than the study drugs.Citation39,Citation42 Hence, patients in the placebo (maintenance with short-acting bronchodilators) group of TORCH did not receive appropriate maintenance treatment according to the GOLD guidelines,Citation44 which should include long-acting bronchodilators. This treatment group cannot, therefore, be considered as “standard” or “usual” care. Indeed, in part due to the positive outcomes of TORCH, this type of long-term, inappropriately-treated “placebo” comparison would no longer be considered ethical.
Table 1 Study designs
The UPLIFT® study permitted use of ICS, LABA, and their combination, but excluded inhaled anticholinergics. No restrictions were imposed for medications prescribed to treat exacerbations. Hence, UPLIFT® closely represented “usual” COPD care as underlying therapy, other than inhaled anticholinergics, regardless of whether patients were randomized to receive tiotropium or placebo.
Although UPLIFT® is a placebo-controlled trial, in comparison to TORCH, the placebo group is better described as the control group. At baseline, 60% of patients in UPLIFT were being treated with ICS or LABA (either alone or in combination) and evidence suggests that more patients may have been prescribed these drugs during the trial.Citation43 Hence, many patients in the control group in UPLIFT® could have been receiving similar medication to that of the active groups in TORCH.
Another general difference that should be highlighted is the disparity in patient numbers in the treatment groups. UPLIFT® was a two-arm study with approximately 3000 patients in each treatment arm;Citation43 TORCH was a four-arm study with approximately 1500 patients in each treatment arm.Citation42 Both trials were designed to be sufficiently powered for the primary outcome; however, the difference in patient numbers could be a general consideration when comparing some secondary and subanalyses.
Unlike UPLIFT®, the TORCH study excluded patients based on acute reversibility to short-acting bronchodilators exceeding 10% of predicted ().Citation39,Citation41 Reversibility to short-acting bronchodilators is frequently used as a means for excluding asthmatic patients from COPD studies; Citation44 however, several studies (including data from UPLIFT®) indicate that such practice could also exclude patients with COPD.Citation45–Citation47 Repeat testing of the same patients for reversibility to bronchodilators shows considerable variability in magnitude of response. This difference in exclusion, along with the less severe postbronchodilator FEV1 inclusion criterion in UPLIFT®, may have accounted for the high proportion of GOLD stage II patients in the UPLIFT® study. Additionally, acute response to short-acting β2-agonist identify a subgroup of COPD patients more likely to respond to ICS with a significant increase in FEV1.Citation48 Therefore, excluding reversible patients in TORCH may have selected a population less likely to present positive outcomes with LABA and ICS therapy.
With respect to postbronchodilator FEV1 measurements during the trials, UPLIFT® included use of the anticholinergic, ipratropium, as well as the short-acting β2-agonist (SABA), salbutamol (albuterol), that was also used in TORCH. This was to insure maximal bronchodilation and, thereby, minimize the influence of bronchomotor tone on measurement of the rate of decline in FEV1. This difference between the trials may have little relevance beyond assessment in rate of decline except in making the actual magnitude of postbronchodilator FEV1 less comparable between the trials.
Despite the differences in the primary outcome (and hence, statistical power) and study designs, both UPLIFT® and TORCH are of sufficient size and duration to provide important insights into the natural course of COPD and the effect of pharmacologic treatment.
Comparison of patients
Demographic characteristics were similar between the two study populations. In both trials, the mean age of patients was 65 years and 75% of patients were male, which is typical of the demographic of treated patients with COPD. Mean body mass index (BMI) for both studies was also similar (25 and 26 for TORCH and UPLIFT®, respectively). Mean duration of COPD was approximately 10 years in the UPLIFT® study; however, similar statistics were not provided for the TORCH trial.
There were fewer current smokers in UPLIFT® (approximately 30%) compared with 43% in TORCH. Entry criteria were the same with respect to ≥10 pack-years and mean pack-years were similar between the two studies (around 48 pack-years). However, smoking cessation programs were offered to all patients in UPLIFT® prior to randomization and smoking status was balanced between the two groups following randomization.Citation41,Citation43 In contrast, patients were stratified at randomization according to smoking status in TORCH.Citation39,Citation42 Since smoking cessation is known to influence the rate of decline in lung function, this difference could influence the relative rate of decline observed between the two studies.
Mean prebronchodilator FEV1 was similar between the two trials (approximately 1.1 L). Mean baseline postbronchodilator FEV1 (absolute and percent predicted) was higher in UPLIFT® (around 47% of predicted) than in TORCH (around 44% of predicted). In contrast, the mean baseline FEV1/forced vital capacity (FVC) was lower in UPLIFT® (around 43% of predicted) compared with TORCH (around 48% of predicted). However, these differences between the trials may in part be due to the different short-acting bronchodilator regimen used (see above), which makes comparisons difficult.
In both studies, HRQL was measured by total score on the St George’s Respiratory Questionnaire (SGRQ). Baseline SGRQ score differed between the two trials by approximately 3 units, with UPLIFT® having the lower (less severe) score. While this may not be considered as clinically relevant (ie, ≥4 units), it does numerically support the suggestion from the lung function data that patients in the UPLIFT® trial were skewed towards less severe compared with patients in TORCH. Indeed, 46% of patients in UPLIFT® were reported to have GOLD stage II (moderate) COPD (three patients with GOLD stage I were enrolled, recognized as a protocol violation, but data were included).Citation43 Similar statistics were not reported in TORCH.
Comparison of the TORCH and UPLIFT® decline in lung function
Rate of decline in FEV1
The data from the post-hoc analysis of TORCH suggest that all three active groups reduce the rate of decline in postbronchodilator FEV1, the only spirometric parameter reported.Citation40 Adjusted rates of decline in FEV1 were −42 ± 3 mL/year for salmeterol alone and fluticasone propionate alone, −39 ± 3 mL/year for salmeterol/fluticasone combination, and −55 ± 3 mL/year for placebo (short-acting bronchodilators) (); this corresponded to reductions in the rate of FEV1 decline versus placebo by 13 ± 4 mL/year each (95% CI, 5–22, p = 0.003) for salmeterol alone and fluticasone alone, and 16 ± 4 mL/year (95% CI, 7–25; p < 0.001) for the salmeterol/fluticasone combination. No significant differences exist between the combination and individual drugs alone. Nearly 18% of patients randomized to the placebo group in TORCH withdrew before contributing an FEV1 value. The TORCH authors commented that this could have underestimated the decline in lung function in the placebo group, with those patients who withdrew having a steeper rate of decline. However, in an editorial, Suissa suggested that the patients who withdrew from the study could have had the lowest FEV1 values at the beginning of the study and, therefore, the “regression to the mean” could have exaggerated the rate of decline since these were the patients with the slowest decline in FEV1.Citation49
Figure 1 Change in rate of decline in FEV1 in UPLIFT® and TORCH, including ceiling effect (possible rate of decline in healthy individuals). Data from the individual trials have been placed on the same axes for illustrative purposes only and do not represent directly comparable data between the trials.
Notes: *p = 0.003 vs placebo; †p < 0.001 vs placebo.
The data from the TORCH post-hoc analysis is valuable, but adds to the ambiguity of understanding. Inhaled Steroids in Obstructive Lung Disease in Europe (ISOLDE) study was specifically designed to investigate the effect of fluticasone priopionate on the rate of decline in FEV1 but suggested there was no additional effect above that achieved with placebo (rates of decline in FEV1 in ISOLDE were −50 ± 4 vs −59 ± 4 mL/year for the active vs short-acting bronchodilators [placebo] groups; p = 0.16).Citation31 The effect of ICS on the rate of decline in FEV1 were reported from two meta-analyses of randomized placebo-controlled trials ≥1 year in length.Citation32,Citation33 The majority of the same trials were included in both meta-analyses. Sutherland etal reported significantly lower rates of decline in FEV1 with ICS than placebo (−7.7 mL/year vs placebo; p = 0.02)Citation32 whereas Highland etal reported no significant difference (−5 mL/year vs placebo; p = 0.11).Citation33 Results from a pooled analysis of seven studies (total N = 3911) have also suggested that ICS do not affect the rate of decline in FEV1 (–0.01% vs placebo from months 6–36, p = 0.86).Citation34 As rate of FEV1 decline was a tertiary endpoint in TORCH, it is sensible to view the data from this trial as hypothesis generating only; they continue to suggest the hypothesis that FEV1 decline in COPD can be reduced by pharmacotherapy.
Additional support for this hypothesis comes from a post-hoc, retrospective analysis of two 1-year trials with tiotropium. These double-blind, placebo-controlled, randomized trials comparing tiotropium (total N = 971) with placebo showed promising improvement in the rate of decline of FEV1. The mean decline in trough (premedication) FEV1 was 46 mL/year lower between Days 8 and 344 (p = 0.005) and 40 mL/year lower between Days 50 and 344 (p = 0.036) versus short-acting bronchodilators (placebo).Citation36 These data, along with the TORCH post-hoc data, suggest that FEV1 decline can be reduced by effective treatment with maintenance long-acting bronchodilator therapy alone.
UPLIFT® was specifically designed to prospectively test the reduction in decline in pre- and post-bronchodilator FEV1 with tiotropium in a controlled, 4-year study. UPLIFT® showed no difference between tiotropium and the control group in terms of rate of decline in FEV1 (calculated from Day 30 until end of study) for both components of the primary outcome: differences were 2 mL/year when measured postbronchodilator (−40 ± 1 vs −42 ± 1 mL/year; p = 0.21; ) and 0 mL/year when measured prebronchodilator (−30 ± 1 mL/year for each group; p = 0.95).Citation43 Similar data were also reported for tiotropium versus control for the secondary endpoints of pre- and postbronchodilator rates of decline in FVC (−43 ± 3 vs −39 ± 3, p = 0.30 and −61 ± 3 vs −61 ± 3, p = 0.84, respectively) and slow vital capacity (SVC) (−47 ± 3 vs −41 ± 3 mL/year, p = 0.11 and −66 ± 3 vs −65 ± 3, p = 0.79, respectively). In isolation, and since this was a specifically designed trial, these data would seemingly suggest that pharmacotherapy cannot reduce the rate of decline in lung function; however, this may not be the case.
Considering the UPLIFT® and TORCH data together, and in the context of other studies, may indicate an important insight into the decline in FEV1. The rate of decline observed in the control arm of UPLIFT® (–42 mL/year) is similar to that for the active monotherapy groups in TORCH (−42 mL/year). The active group in UPLIFT® produced a similar rate of decline to the TORCH salmeterol/fluticasone combination group (−40 vs −39 mL/year). As the TORCH investigators indicated, the TORCH placebo group was similar to the placebo groups reported in previous trials.Citation19,Citation20,Citation27,Citation28,Citation30,Citation31,Citation34 However, the UPLIFT® control group included patients treated with LABA and ICS (72%, 74%, and 46% received LABA, ICS, or LABA/ICS combination, respectively), therefore, could be considered a more “active” control group than the TORCH placebo. It is possible that tiotropium was unable to further reduce this decline due to a ceiling effect (ie, medications can only reduce the rate of decline to a certain amount since there is a basal rate of decline in FEV1 in normal individuals) ().
The UPLIFT® authors suggest some preliminary evidence to support the above hypothesis of a ceiling effect. Subgroup analysis of the 1554 patients not receiving ICS or LABA at baseline showed a significantly lower postbronchodilator rate of decline in FEV1 for tiotropium versus control (40 ± 3 vs 47 ± 3 mL/year; p = 0.046).Citation43 However, this needs further investigation, since interpretation is currently difficult. As such, the greater effect size seen in TORCH compared with UPLIFT® may be driven by the less active treatment received by the placebo group in the TORCH study versus the control group in the UPLIFT® study.
In previous studies, the rate of decline in FEV1 seen in both active and placebo groups has been higher than those seen in UPLIFT® and TORCH. For example, in the European Respiratory Society Study on Chronic Obstructive Pulmonary Disease (EUROSCOP),Citation28 Bronchitis Randomized on NAC Cost-Utility Study (BRONCUS),Citation27 and ISOLDE study,Citation31 declines in postbronchodilator FEV1 were between 44 and 57 mL/year and 47 and 69 mL/year in the active and placebo (short-acting bronchodilators) groups, respectively. Therefore, results from TORCH and UPLIFT® suggest that all treatments, including maintenance therapy with long-acting bronchodilators, can reduce the rate of decline in FEV1, with the impact on this decline being dependent on the type of agent or combination of agents received.
Subgroup analyses of UPLIFT® and TORCH also provide us with some insight into patient characteristics that may affect the rate of FEV1 decline (). There are some consistencies in the results seen in the studies. For instance, seemingly contrary to previous models of decline in lung function in COPD,Citation17 decline is more rapid in younger patients (aged < 55 years) than older patients. BMI was associated with the rate of FEV1 decline, with a higher BMI seemingly being beneficial in both trials. Low BMI and fat-free mass are known independent predictors of disease severity and mortality; Citation50,Citation51 however, this association with rate of FEV1 decline is a novel finding. Although the rate of FEV1 decline appears to be more rapid in men than women, percentage changes in rate of decline in FEV1 were similar between the sexes, suggesting that this may be associated with airway size rather than a true difference in rate of disease progression. Geographical region was also associated with the rate of decline; however, with the exception of a lower rate of decline in Asia, this was not consistent between the studies and, therefore, could also be an artefact of airway size. In UPLIFT®, the rate of FEV1 decline was more rapid in earlier stages of COPD (GOLD stage II vs stages III and IV); importantly, there is some suggestion that tiotropium may positively affect the rate of decline in these earlier stage patients (p = 0.02), although further supportive evidence is required to confirm this observation.
Table 2 Mean (SE) rate of decline (mL/year) in postbrochodilator FEV1 by subgroup
Absolute changes in FEV1
Both trials show that improvements in FEV1 are sustained over a considerable period after initiation of treatment. In UPLIFT®, lung function was significantly better with tiotropium than control at all measured time points throughout the trial (difference in mean FEV1 values ranged between 87 and 103 mL, and 47 and 65 mL for pre- and postbronchodilator measurements, respectively; p < 0.001). In the tiotropium group, mean FEV1 values returned to baseline level after around 24 months (postbronchodilator) and 48 months (prebronchodilator), whereas mean FEV1 values in the control group returned to baseline after only around 12 and 10 months, respectively. Similar patterns for postbronchodilator FEV1 values were observed in the TORCH study, with a reported difference in mean change from baseline over the 3-year trial period compared with placebo of 93 mL with the combination, 42 mL with salmeterol monotherapy and 47 mL with fluticasone monotherapy (all p < 0.001, as was the comparison between the combination and monotherapies). Mean postbronchodilator FEV1 values returned to baseline level after around 30 months in the combination group, compared with approximately 18 months in the monotherapy groups and approximately 6 months in the placebo group. Prebronchodilator mean FEV1 values were not reported. In the ISOLDE study, the mean FEV1 (postbronchodilator) was also significantly higher in the ICS group than the placebo group (by 76 mL and 100 mL at the 3- and 36-month time points, respectively; p ≤ 0.001).Citation31
An interesting phenomenon in UPLIFT® was the improvement in postbronchodilator spirometry values. The expected “maximal bronchodilation” produced by the high-dose salbutamol and ipratropium may have been expected to cause patients in the two treatment groups to be equal in terms of bronchodilation capacity postbronchodilator. However, postbronchodilator FEV1 and FVC values were higher with tiotropium than control at all time points. This finding is difficult to interpret given the complexity from the use of three bronchodilators (two of which were anticholinergics) and factors other than airway diameter that could affect spirometry.
Overall, evidence gained from UPLIFT®, TORCH, and previous, smaller studies indicate that, while it is difficult to conclude that long-acting bronchodilators significantly reduce the rate of decline in lung function, these agents certainly improve lung function. Therefore, they may delay disease progression even if they do not affect the disease course itself. This is contrary to smoking cessation, which, provided it occurs sufficiently early in the course of COPD, can reduce the rate of decline in FEV1 but does not restore lung function that has been lost.Citation17,Citation21 As pharmacotherapy in COPD can improve lung function, there is an argument for administering pharmacotherapy at a similarly early stage in COPD.
Comparison of exacerbation data
Exacerbations are part of the natural course of COPDCitation52 and are responsible for the morbidity and mortality of this disease.Citation26,Citation53–Citation55 Exacerbations are associated with reduced quality of lifeCitation56–Citation58 and increased mortality.Citation59 They are also the main driver of costs in COPD.Citation60 Unsurprisingly, therefore, reducing exacerbations is a key goal of COPD treatment.Citation44
There is inconsistency in how exacerbations are defined and analyzed, which makes it difficult to compare data on exacerbations between trials.Citation61 In UPLIFT®, an exacerbation was defined as “an increase or new onset of more than one of the following respiratory symptoms (cough, sputum, sputum purulence, wheezing, dyspnea) with a duration of 3 or more days requiring treatment with an antibiotic and/or systemic (oral, intramuscular or intravenous) steroid”.Citation41 Exacerbations were categorized as mild (treated at home without seeing a healthcare provider), moderate (visit with healthcare provider, at home or as outpatient), or severe (requiring hospitalization for >24 hours). In TORCH, exacerbations were defined as symptomatic deterioration requiring treatment with systemic corticosteroids and/or antibiotics (moderate exacerbation) or hospitalization (severe exacerbation).Citation39,Citation42
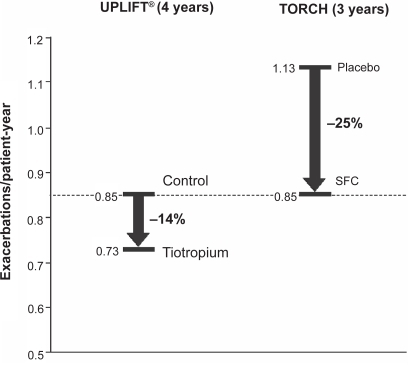
In UPLIFT®, compared with control, tiotropium significantly delayed time-to-first exacerbation (16.7 vs 12.5 months) and time-to-first hospitalization for exacerbations (lower risk of hospitalization; HR, 0.86 [95% CI, 0.78–0.95]; p = 0.002) ().Citation43 Exacerbations requiring hospitalization were infrequent (0.15 vs 0.16 per patient-year), which may explain why any difference between tiotropium and placebo groups was not statistically significant (p = 0.34). Tiotropium also reduced the mean number of exacerbations by 14% (rate per patient-year, 0.73 vs 0.85; HR, 0.86 [95% CI, 0.81–0.91]; p < 0.001), and reduced the number of days with exacerbations (13.64 vs 12.11; HR, 0.89 [95% CI, 0.83–0.95]; p = 0.001) compared with control. These results were consistent with those from other shorter duration studies in which tiotropium has been shown to reduce the number of exacerbations by 20% to 50%,Citation62–Citation66 number of exacerbation days by 31% to 50%,Citation63,Citation65,Citation66 number of hospitalizations due to exacerbations by 20% to 30%Citation67 and time-to-first exacerbation.Citation62,Citation63,Citation65 Compared with short-acting bronchodilators, associated healthcare resource utlilization was also consistently reduced with tiotropium in these earlier studies.Citation63,Citation65,Citation67
Table 3 Exacerbations
Rates of moderate or severe exacerbations per patient-year were reduced in all active groups in the TORCH study (0.85, 0.93, 0.97, and 1.13 for combination, fluticasone only, salmeterol only, and placebo groups, respectively; p < 0.001 vs placebo); significantly lower rates were also seen with the combination group vs salmeterol (p = 0.002) and fluticasone (p = 0.02) monotherapy groups ().Citation42 Both the combination and salmeterol groups reduced, by the same magnitude, the rate of exacerbations requiring hospitalization versus placebo (0.16 and 0.16 vs 0.19 per patient-year; p = 0.03 and p = 0.02, respectively).Citation42 No data on time-to-first exacerbation, number of days with exacerbations, or number of days in hospital in TORCH were published. These data confirm those from a previous 1-year study in which salmeterol/fluticasone combination and the single agents have reduced the frequency of exacerbations by 25% and 19% to 20%, respectively.Citation68
It is possible to draw some comparisons between the UPLIFT® and TORCH findings despite the difference in counting and analyses. There were significant improvements in multiple measures of exacerbations with the active groups in both trials. In terms of rates of exacerbations per patient-year, these were slightly lower with tiotropium compared with the active treatment groups in TORCH (0.73 vs 0.85–0.97). Similar to the results for lung function decline, the exacerbation rate (0.85 per patient-year) in the placebo group in UPLIFT® was closer to the rates seen in the active treatment groups in TORCH than the TORCH placebo group (exacerbation rate of 1.13 per patient-year). Again, the fact that the UPLIFT® placebo group included patients treated with LABA and ICS, and the postulated “ceiling effect” described above, may explain why there was a relatively low rate of exacerbation in this group compared with the TORCH placebo group and, therefore, why the extent of the improvement beyond this (14%) with tiotropium was lower than the 25% improvement seen in TORCH (). This may also suggest a benefit from a triple combination of tiotropium, LABA and ICS.
Figure 2 Change in exacerbation rates by the active treatment groups in UPLIFT® and TORCH. Data from the individual trials have been placed on the same axes for illustrative purposes only and do not represent directly comparable data between the trials.
Reduction in exacerbations and exacerbations leading to hospitalization is one of the mechanisms that may explain a reduction in mortality in both trials. The mechanism of the link between exacerbations and mortality could be associated with hyperinflation, which is also related to mortality.Citation69 Hyperinflation is likely to worsen during exacerbations of COPD. Tiotropium, salmeterol, and the combination of salmeterol and fluticasone are effective in reducing hyper-inflation and improving exercise capacity.Citation70–Citation74
Comparison of HRQL data
Both trials evaluated HRQL using SGRQ. This is one of the most widely used instruments for measuring HRQL in respiratory patients, and has been used extensively in therapeutic evaluation studies.
Comparing the results of UPLIFT® and TORCH (), all treatments failed to achieve a mean clinical significance at the end of the trials, ie, a mean reduction of 4 or more units on the SGRQ total score. However, all treatments achieved statistical significance. Statistically significant differences in mean change in SGRQ total score were observed at all time points throughout the UPLIFT® trial, in favor of tiotropium. In TORCH, time point data were not reported; instead, mean changes in SGRQ total score were averaged over 3 years, which demonstrated a statistically significant improvement in the combination therapy group compared with the placebo and monotherapy groups.
Table 4 SGRQ total score
In addition, a significantly higher proportion of patients in the tiotropium group than the control group in UPLIFT® had an improvement of 4 or more units from baseline at 1 year (49% vs 41%), 2 years (48% vs 39%), 3 years (46% vs 37%), and 4 years (45% vs 36%; p < 0.001 for all comparisons). These data compare favorably with a previous long-term study of tiotropium in which the percentages of patients with a clinically meaningful response after 1 year were 49% and 30% in the tiotropium and placebo groups, respectively.Citation75 The proportion of patients in TORCH achieving a change of at least 4 units on the SGRQ total score was not reported, preventing further comparison between the two studies.
Similar to the lung function results, no between-group differences were observed in UPLIFT® in the rate of decline in SRGQ scores (). The permitted use of ICS, LABA, and their combinations as rescue medication, together with the unrestricted use of medications to treat exacerbations in UPLIFT®, may have narrowed any between-treatment improvements in HRQL. As previously stated, maintenance treatment with inhaled fluticasone alone affected the rate of deterioration in SGRQ in the ISOLDE trial.Citation76 Rate of decline in SRGQ scores has not yet been reported from TORCH.
Comparison of mortality data
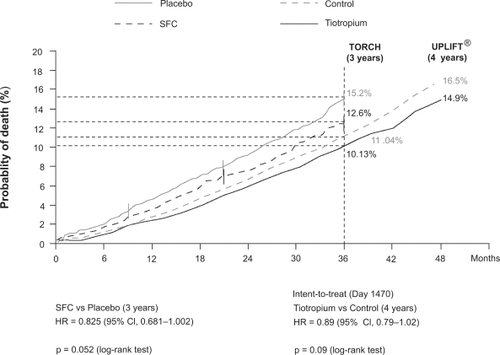
A key goal of COPD therapy is to reduce mortality.Citation44 In UPLIFT®, mortality data have been reported for two intent-to-treat 4-year “vital status” analyses for which at least 45 months follow-up was available, including patients who had discontinued, and for patients “on treatment”.Citation43 These analyses were a) from Days 1–1440 (planned 4 years of study treatment), b) the protocol-defined on-treatment period of 1470 days (1440 days planned treatment plus 30 days follow-up), and c) the first to actual last day of treatment plus 30 days follow-up. The difference in 4-year all-cause mortality between the tiotropium and placebo groups was not statistically significant for the protocol-defined 1470-day vital status analysis (p = 0.09) (); a significant difference between groups was observed, however, according to both the 1440-day analysis and the on-treatment analysis. A possible reason for the difference between the 1470-day and the other analyses is that data were received on only 75% of patients for the former compared with, for example, 95% of patients for the 1440-day analysis. A recent meta-analysis of safety with tiotropium has addressed a composite variable of cardiovascular death, non-fatal myocardial infarction or non-fatal stroke.Citation77 The results of this meta-analysis is discussed later in the safety and tolerability section.
Table 5 Mortality
In TORCH, mortality data was on an intent-to-treat basis and was analyzed from Day 1 to the end of the treatment period (3 years). Unlike UPLIFT®, the follow-up period (15 days in TORCH; 30 days in UPLIFT®) was not included. There was no statistically significant difference in the 3-year all-cause mortality rate between the salmeterol/fluticasone combination group and the placebo group (p = 0.052) (). A slightly higher mortality rate was observed in the salmeterol-only arm (13.5%) than the combination arm (difference not significant vs combination arm [12.6%] or placebo [15.2%]). In the fluticasone-only arm, the mortality rate (16%) was actually higher numerically than placebo and significantly higher than in the combination arm (p = 0.007).
Although the UPLIFT® and TORCH studies are not directly comparable, provides a comparison similar to . The results of UPLIFT® showed that a long-acting bronchodilator (tiotropium) appeared to have an impact on mortality. Consistent with this, after factorial analysis, some authors suggested that the effect on mortality observed in the combination arm in TORCH is entirely due to the long-acting bronchodilator, ie, the LABA (salmeterol).Citation49,Citation78 Indeed, a higher mortality rate was seen in the fluticasone-only arm compared with the combination arm and with placebo (difference vs placebo was not significant). The role of ICS in long-term treatment in COPD is still under debate.Citation79,Citation80
Figure 3 Comparison of selected mortality data presented in the TORCH and UPLIFT ® primary publications.Citation40,Citation43 Data from the individual trials have been placed on the same axes for illustrative purposes only and do not represent directly comparable data between the trials.
Abbreviations: CI, confidence interval; HR, hazard ratio; SFC, salmeterol and fluticasone in combination.
Overall, the results from UPLIFT® and TORCH suggest that both tiotropium and the salmeterol/fluticasone combination may reduce the risk of mortality.
Comparison of safety and tolerability data
The proportions of patients experiencing adverse events (AEs) were similar between UPLIFT® and TORCH (ranges across groups and trials were 89% to 93%, 40% to 52%, and 18% to 25% for AEs, serious AEs [SAEs], and events leading to withdrawal, respectively).Citation42,Citation43 Side effects were generally those expected from the class of drugs used. In UPLIFT®, tiotropium reduced the rate of cardiac (including congestive heart failure and myocardial infarction) and lower respiratory (including respiratory failure and dyspnea) SAEs compared with control (p < 0.05); there was no significant difference in the incidence of stroke between tiotropium and control. This finding contrasts with the suggestion from a recent meta-analysis that anticholinergics are associated with an increase in risk of cardiovascular events,Citation77 though the results of UPLIFT® are consistent with a pooled analysis of patient-level data from 19 other trials with tiotropium.Citation81 The most frequently occurring AE in all groups of both trials was COPD exacerbations (). Fluticasone-containing treatment was associated with an increased probability of having pneumonia in TORCH. Incidences of pneumonia were 19.6%, 18.3%, 13.3%, and 12.3% in the combination, fluticasone-only, salmeterol-only, and placebo arms, respectively, with significant differences between the combination and fluticasone-only arms versus placebo (p < 0.001) and combination versus salmeterol-only arms (p < 0.001). There were no significant ocular or bone-related safety signals observed with active TORCH treatments. In UPLIFT®, dry mouth and constipation were observed, two side effects that are consistent with the known safety profile for tiotropium.
Table 6 The most frequently occurring adverse events categorized by ranges of incidence rate per year
Overall, the results from UPLIFT® confirm the favorable safety profile with tiotropium.Citation81 No strong safety signals were seen with salmeterol monotherapy in TORCH; the increased risk of pneumonia with fluticasone-containing regimens mirrors previous studies.Citation82,Citation83
Conclusions
Although neither UPLIFT® nor TORCH reached their primary endpoints, these trials have revealed the impact of long-term bronchodilators in the treatment of COPD. Long-acting bronchodilators in the form of tiotropium and salmeterol (in combination with fluticasone propionate) can actually improve lung function and may delay progression of COPD, thus positively affecting disease prognosis. Mortality may also be reduced and HRQL improved. Despite international and national guidelines recommending long-acting bronchodilators for COPD, these agents are currently under-prescribed. UPLIFT® and TORCH results support an urgent change in prescribing practices.
Acknowledgements
Acknowledgments and disclosures
The authors are grateful for the editorial support from PAREXEL MMS in assisting in the drafting of this article. PAREXEL MMS was funded jointly by Boehringer Ingelheim and Pfizer. The authors are exclusively responsible for direction with respect to the text.
References
- BuistASMcBurnieMAVollmerWMGillespieSBurneyPManninoDMInternational variation in the prevalence of COPD (the BOLD Study): a population-based prevalence studyLancet200737074175017765523
- HalbertRJNatoliJLGanoABadamgaravEBuistASManninoDMGlobal burden of COPD: systematic review and meta-analysisEur Respir J20062852353216611654
- PenaVSMiravitllesMGabrielRJimenez-RuizCAVillasanteCMasaJFGeographic variations in prevalence and underdiagnosis of COPD: results of the IBERPOC multicentre epidemiological studyChest200011898198911035667
- LopezADShibuyaKRaoCMathersCDHansellALHeldLSChronic obstructive pulmonary disease: current burden and future projectionsEur Respir J20062739741216452599
- CelliBRMacNeeWcommittee membersStandards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J20042393294615219010
- JemalAWardEHaoYThunMTrends in the leading causes of death in the United States, 1970–2002JAMA20052941255125916160134
- World Health Organization Chronic obstructive pulmonary disease (COPD) fact sheet No. 315;2008.
- MiravitllesMMurioCGuerreroTGisbertRCosts of chronic bronchitis and COPD: a 1-year follow-up studyChest200312378479112628879
- Serra-BatllesJPlazaVMorejonEComellaABruguesJCosts of asthma according to the degree of severityEur Respir J199812132213269877485
- PauwelsRARabeKFBurden and clinical features of chronic obstructive pulmonary disease (COPD)Lancet200436461362015313363
- CooperCBThe connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and functionAm J Med2006119213116996896
- Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2008 Update. www.goldcopd.org. 2–11–2008
- TraverGAClineMGBurrowsBPredictors of mortality in chronic obstructive pulmonary disease. A 15-year follow-up studyAm Rev Respir Dis1979119895902453709
- SchunemannHJDornJGrantBJWinkelsteinWJrTrevisanMPulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health StudyChest200011865666410988186
- YoungRPHopkinsREatonTEForced expiratory volume in one second: not just a lung function test but a marker of premature death from all causesEur Respir J20073061662217906084
- ManninoDMDavisKJLung function decline and outcomes in an elderly populationThorax20066147247716517577
- FletcherCPetoRThe natural history of chronic airflow obstructionBMJ1977116451648871704
- AnthonisenNRConnettJEKileyJPAltoseMDBaileyWCBuistASEffects of smoking intervention and the use of an inhaled anti-cholinergic bronchodilator on the rate of decline of FEV1. The Lung Health StudyJAMA1994272149715057966841
- BurchfielCMMarcusEBCurbJDMacLeanCJVollmerWMJohnsonLREffects of smoking and smoking cessation on longitudinal decline in pulmonary functionAm J Respir Crit Care Med1995151177817857767520
- ScanlonPDConnettJEWallerLAAltoseMDBaileyWCBuistASSmoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health StudyAm J Respir Crit Care Med200016138139010673175
- AnthonisenNRConnettJEMurrayRPSmoking and lung function of Lung Health Study participants after 11 yearsAm J Respir Crit Care Med200216667567912204864
- CazzolaMMacNeeWMartinezFJRabeKFFranciosiLGBarnesPJOutcomes for COPD pharmacological trials: from lung function to biomarkersEur Respir J20083141646918238951
- CelliBRCoteCGLareauSCMeekPMPredictors of Survival in COPD: more than just the FEV1Respir Med2008102Suppl 1S27S3518582794
- CooperCBDransfieldMPrimary care of the patient with chronic obstructive pulmonary disease-part 4: understanding the clinical manifestations of a progressive diseaseAm J Med2008121S33S4518558106
- KerstjensHARijckenBSchoutenJPPostmaDSDecline of FEV1 by age and smoking status: facts, figures, and fallaciesThorax1997528208279371217
- DonaldsonGCSeemungalTARBhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax20025784785212324669
- DecramerMRutten-van MolkenMDekhuijzenPNTroostersTvan HerwaardenCPellegrinoREffects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trialLancet20053651552156015866309
- PauwelsRALofdahlCGLaitinenLASchoutenJPPostmaDSPrideNBLong-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smokingN Engl J Med19993401948195310379018
- VestboJSorensenTLangePBrixATorrePViskumKLong-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trialLancet19993531819182310359405
- The Lung Health Study Research GroupEffect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary diseaseN Engl J Med20003431902190911136260
- BurgePSCalverleyPMAJonesPWSpencerSAndersonJAMaslenTKRandomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trialBMJ20003201297130310807619
- SutherlandERAllmersHAyasNTVennAJMartinRJInhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysisThorax20035893794114586043
- HighlandKBStrangeCHeffnerJELong-term effects of inhaled in patients with chronic obstructive pulmonary corticosteroids on FEV1 disease. A meta-analysisAnn Intern Med200313896997312809453
- SorianoJBSinDDZhangXCampPGAndersonJAAnthonisenNRA pooled analysis of FEV 1 decline in COPD patients randomized to inhaled corticosteroids or placeboChest200713168268917356080
- Garcia-AymerichJLangePBenetMSchnohrPAntoJMRegular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort studyAm J Respir Crit Care Med200717545846317158282
- AnzuetoATashkinDMenjogeSKestenSOne-year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropiumPulm Pharmacol Ther200518758115649848
- SorianoJBVestboJPrideNBKiriVMadenCMaierWCSurvival in COPD patients after regular use of fluticasone propionate and salmeterol in general practiceEur Respir J20022081982512412670
- SinDDWuLAndersonJAAnthonisenNRBuistASBurgePSInhaled corticosteroids and mortality in chronic obstructive pulmonary diseaseThorax20056099299716227327
- VestboJThe TORCH (towards a revolution in COPD health) survival study protocolEur Respir J20042420621015332386
- CelliBRThomasNEAndersonJAFergusonGTJenkinsCRJonesPWEffect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH studyAm J Respir Crit Care Med200817833233818511702
- DecramerMCelliBTashkinDPPauwelsRABurkhartDCassinoCClinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the UPLIFT trialCOPD2004130331217136995
- CalverleyPMAAndersonJACelliBFergusonGTJenkinsCJonesPWSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med200735677578917314337
- TashkinDPCelliBSennSBurkhartDKestenSMenjogeSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med20083591543155418836213
- RabeKFHurdSAnzuetoABarnesPJBuistSACalverleyPGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med200717653255517507545
- AnthonisenNRBronchodilator response in chronic obstructive pulmonary diseaseAm Rev Respir Dis19861338148193706890
- DorinskyPMReisnerCFergusonGTMenjogeSSSerbyCWWitekTJJrThe combination of ipratropium and albuterol optimizes pulmonary function reversibility testing in patients with COPDChest199911596697110208193
- TashkinDPCelliBDecramerMLiuDBurkhartDCassinoCBronchodilator responsiveness in patients with COPDEur Respir J20083174275018256071
- BleeckerEREmmettACraterGKnobilKKalbergCLung function and symptom improvement with fluticasone propionate/salmeterol and ipratropium bromide/albuterol in COPD: response by beta-agonist reversibilityPulm Pharmacol Ther20082168268818541448
- SuissaSMedications to modify lung function decline in chronic obstructive pulmonary disease: some hopeful signsAm J Respir Crit Care Med200817832232318676961
- BoltonCEIonescuAAShielsKMPettitRJEdwardsPHStoneMDAssociated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20041701286129315374843
- ScholsAMWJSlangenJVolovicsLWoutersEFMWeight loss is a reversible factor in the prognosis of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1998157179117979620907
- AnzuetoAMiravitllesMModifying the clinical course of COPDHot Topics in Respiratory Medicine20087719
- CrooksSWBayleyDLHillSLStockleyRABronchial inflammation in acute bacterial exacerbations of chronic bronchitis: the role of leukotriene B4Eur Respir J20001527428010706491
- StanescuDSannaAVeriterCKostianevSCalcagniPGFabbriLMAirways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophilsThorax1996512672718779129
- GompertzSBayleyDLHillSLStockleyRARelationship between airway inflammation and the frequency of exacerbations in patients with smoking related COPDThorax200156364111120902
- SeemungalTARDonaldsonGCPaulEABestallJCJeffriesDJWedzichaJAEffect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1998157141814229603117
- MiravitllesMFerrerMPontAZalacainRAlvarez-SalaJLMasaFEffect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up studyThorax20045938739515115864
- DollHMiravitllesMHealth-related QOL in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease: a review of the literaturePharmacoeconomics20052334536315853435
- Soler-CataluñaJJMartínez-GarcíaMÁRománSánchez PSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax20056092593116055622
- MiravitllesMMurioCGuerreroTGisbertRPharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPDChest20021211449145512006427
- AaronSDFergussonDMarksGBSuissaSVandemheenKLDoucetteSCounting, analysing and reporting exacerbations of COPD in randomised controlled trialsThorax20086312212817702790
- VinckenWvan NoordJAGreefhorstAPMBantjeThAKestenSKorduckiLImproved health outcomes in patients with COPD during 1 yr’s treatment with tiotropiumEur Respir J20021920921611871363
- BrusascoVHodderRMiravitllesMKorduckiLTowseLKestenSHealth outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax20035839940412728159
- BarrRGBourbeauJCamargoCARamFSInhaled tiotropium for stable chronic obstructive pulmonary diseaseCochrane Database Syst Rev2005CD00287615846642
- DusserDBravoMLIaconoPThe effect of tiotropium on exacerbations and airflow in patients with COPDEur Respir J20062754755516507855
- PowrieDJWilkinsonTMDonaldsonGCJonesPScrineKVielKEffect of tiotropium on sputum and serum inflammatory markers and exacerbations in chronic obstructive pulmonary diseaseEur Respir J20073047247817504798
- NiewoehnerDERiceKCoteCPaulsonDCooperJAJrKorduckiLPrevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trialAnn Intern Med200514331732616144890
- CalverleyPPauwelsRVestboJJonesPPrideNGulsvikACombined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trialLancet200336144945612583942
- CasanovaCCoteCde TorresJPAguirre-JaimeAMarinJMPinto-PlataVThe Inspiratory to total lung capacity ratio predicts mortality in patients with COPDAm J Respir Crit Care Med200517159159715591470
- CelliBZuWallackRWangSKestenSImprovement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumesChest20031241743174814605043
- O’DonnellDEFlügeTGerkenFHamiltonAWebbKAguilaniuBEffects of tiotropium on lung hyperinflation, dyspnea and exercise tolerance in patients with COPDEur Respir J20042383284015218994
- CasaburiRKukafkaDCooperCBWitekTJJrKestenSImprovement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPDChest200512780981715764761
- MaltaisFHamiltonAMarciniukDHernandezPSciurbaFCRichterKImprovements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPDChest20051281168117816162703
- O’DonnellDESciurbaFCelliBMahlerDAWebbKAKalbergCJEffect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPDChest200613064765616963658
- CasaburiRMahlerDAJonesPWWannerASan PedroGZuWallackRLA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J20021921722411866001
- SpencerSCalverleyPMABurgePSJonesPWHealth status deterioration in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200116312212811208636
- SinghSLokeYKFurbergCDInhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysisJAMA20083001439145018812535
- La VecchiaCFabbriLMPrevention of death in COPDN Engl J Med20073562211221217526088
- CalverleyPMInhaled corticosteroids are beneficial in chronic obstructive diseaseAm J Respir Crit Care Med200016134134210673165
- BarnesPJInhaled corticosteroids are not beneficial in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200016134234410673166
- KestenSJaraMWentworthCLanesSPooled clinical trial analysis of tiotropium safetyChest20061301695170317166984
- ErnstPGonzalezAVBrassardPSuissaSInhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumoniaAm J Respir Crit Care Med200717616216617400730
- WedzichaJACalverleyPMSeemungalTAHaganGAnsariZStockleyRAThe prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromideAm J Respir Crit Care Med2008177192617916806
- QuanjerPHStandardised lung function testing of the European Community for Coal and SteelBull Eur Physiopathol Respir1983197106850151