Abstract
Introduction
The High Frequency Airway Oscillating device (HFAO) was developed to help patients with COPD feel less breathless through flow resistive respiratory muscle training and fixed rate oscillations. Previous work has demonstrated that this device can improve inspiratory muscle strength over and above a sham device. Both groups improved their breathlessness and preserved clinical benefits though there were no statistically significant differences seen over and above the sham device. It is important to understand patient perceptions of using a device and how this may influence their treatment and therefore a qualitative analysis was conducted to understand participant experiences of a HFAO device.
Methods
This was an exploratory qualitative analysis involving participants recruited to the Training to Improve Dyspnoea (TIDe) study. Participants completed a satisfaction survey and were invited to take part in a focus group. Focus groups were conducted by a researcher independent to the randomised controlled trial. Data was analysed independently by two researchers using inductive thematic analysis, and themes/sub-themes were agreed jointly. Data is presented in themes and sub themes and triangulated with survey response data.
Results
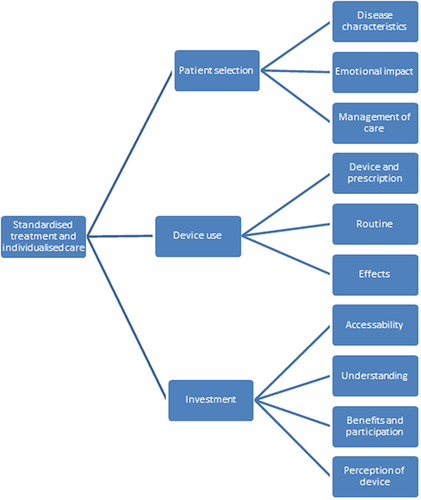
Fourteen participants were recruited to two focus groups (71% male, mean [SD] age 64[9] years). The key themes were patient selection, device use, and investment. Patient selection explores the disease characteristics, emotional impact and management of care. Device use explores the device prescription and usage, routine and lifestyle and effectiveness. Investment covers accessibility, understanding, benefits vs participation and overall perceptions of the device.
Conclusion
This research demonstrates the complexity of device interventions and that key considerations should be given to patient selection, the device use itself and, the time and cost investment required for participants to successfully implement the device into daily life.
Keywords:
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a disease of the small airways that leads to debilitating breathlessness, which can impact on function and quality of life.Citation1 Other symptoms such as excessive sputum and cough can co-exist in patients with COPD.Citation2 Breathlessness is a multifactorial symptom with various pathophysiologies such as inspiratory muscle weakness and inefficiency. Changes in breathing mechanics and an increased work of breathing results in an inability to meet the respiratory demands leading to intolerable breathlessness.Citation3 The inspiratory muscles are often placed on a stretch due to hyperinflation and have a reduced capacity; this can cause or contribute to breathlessness for some patients. Respiratory muscle training has been used to reduce breathlessness in some groups of patients, particularly those that have a baseline maximal inspiratory pressure of ≤60cmH2O.Citation3,Citation4 There are a variety of different inspiratory muscle training devices that have differing benefits and research has not yet identified which patients have the greatest clinical benefit.Citation4 Expiratory muscle training or combined respiratory muscle training has been shown to improve breathlessness compared to a control group, however, there is less supporting evidence.Citation5 Devices have also shown promise in managing excessive sputum and cough through oscillatory positive expiratory pressure, demonstrating significant improvements in sputum volume, cough and quality of life.Citation6,Citation7
The High Frequency Airway Oscillations (HFAO) (Aerosure ©) device utilises for inspiratory and expiratory muscle training to target breathlessness, and airway oscillations that may improve sputum and quality of life. The HFAO device has demonstrated improvements in respiratory muscle strength and breathlessness in healthy volunteers through flow resistance.Citation8 The use of HFAO in patients with COPD (the TIDe study) did not demonstrate clinical benefits in breathlessness above a sham, however both groups did improve their breathlessness above established Minimal Clinical Important Differences. The HFAO device demonstrated improves inspiratory muscle strength above the sham group, however, this was not translated into significant benefits in breathlessness.Citation9,Citation10 Though some participants demonstrated benefits, it was not possible to determine characteristics that predict clinical benefit. There is a large amount of literature for respiratory muscle training, which inconsistently results in benefits to breathlessness though the reason for this is poorly understood.
There are a number of factors that determine whether the treatment is perceived as beneficial and worthwhile for patients. Schroeder et al explored the use of inhaled medications in patients with COPD and found that favourable attributes to usage were ease of use, frequency and efficacy.Citation11 Additionally, factors that influence patients’ self-management behaviours are motivation, personal life, and knowledge and understanding.Citation12 Little is known on the patient experience or perception of using respiratory muscle training devices, and this is particularly important as devices have demonstrated varying benefits. Therefore, this study aims to explore in-depth patient experiences and perceptions of using a HFAO device to help manage breathlessness in patients recruited to the TIDe study.Citation10,Citation13 The objectives were as follows: (1) to explore if and how the device was used; (2) to understand the acceptability of using the device; (3) barriers and facilitators to using the device and recommendations for use and improvement of the device.
Methods
This is an in-depth exploratory qualitative analysis of participant experiences of the TIDe study. Training to Improve Dyspnoea (TIDe) double blinded, randomised controlled trial conducted at the University Hospitals of Leicester exploring the use of a High Frequency Airway Oscillating (HFAO) device compared to a sham. Details of this protocol have been previously published,Citation10 and from the trial registry ISRCTN45695543. This trial was approved by the East Midlands-Leicester South Research Ethics Committee and Health Research Authority (reference 17/EM/0156). Patients were eligible for this trial if they had a diagnosis of COPD, confirmed by spirometry, and a Medical Research Council (MRC) Dyspnoea score of two or more. Informed consent was gained from participants prior to participation, including consent to include anonymised responses as quotes, and this research was performed in line with the declaration of Helsinki. Patients were instructed to use a HFAO device or a sham, three times per day for five minutes at a time, for a total of eight weeks. Participants were followed up after the intervention, at eight weeks, and offered to retain the HFAO at this visit if they had been in the sham group, or to keep the device if they had been in the intervention group.
Setting
Participants were recruited from the TIDe study. During consent for the TIDe study, patients indicated whether they would wish to be contacted for focus groups, and only those that expressed an interest were invited. Participants that completed the primary endpoint (eight weeks) were invited to participate in the focus group, regardless of their intervention group and compliance. All participants had access to the active HFAO for at least six weeks prior to the focus groups. Focus groups were conducted in August 2019 after trial recruitment was completed. Focus groups were conducted in a neutral setting in a room unrelated to the trial conduct and by researchers not involved in the initial study.
Sample
Purposive sampling was used to recruit participants who had completed the primary outcome. At least six participants were required for the focus groups and a maximum of ten to allow for in-depth discussion, and in line with best practice recommendations.Citation14 Informed consent was obtained prior to the commencement of the focus groups. The sample was limited to the participants in the TIDe study, all participants were invited to share their views.
Procedures
Preliminary information on the participant’s medical diagnosis and age was obtained in earlier stages of the TIDe randomised control trial. Semi-structured, face to face focus groups were conducted by researcher’s independent from the randomised controlled trials (AB, CB, AJ). The focus groups were conducted by a primary researcher who followed an interview guide (Appendix 1) and moderated by a secondary researcher. Each focus group lasted between 60 and 90 minutes. Participants were provided with refreshments during the focus group. Prior to, during and following the study interviews, a process of reflexivity was conducted to identify any possible avenues for bias that may arise throughout the interaction with study participants. As recommended in qualitative research, a reflective log was used to capture these reflections.Citation15
Additionally, and as part of the TIDe study, participants completed a survey response indicating their use of the device and if they felt it were beneficial. These survey responses were used to triangulate the data.
Data Analysis
The focus groups were recorded on an encrypted Dictaphone, securely transferred to a third party transcription service to transpose into written format and returned securely to the research site for data analysis. Data was analysed inductively using Braun and Clarkes Thematic AnalysisCitation16 which followed the following principles: (1) familiarisation with the data, (2) generating initial codes, (3) generating themes, (4) reviewing themes, (5) defining and naming themes. Analysis was performed by two researchers (ED, LHW). Initial coding was conducted independently and then researchers collaborated to discuss and generate the themes and sub themes. These were fed back and discussed with the study team to reach consensus on the finalised themes.
Results
Demographics
53 (out of 104 TIDe study participants) consented to be contacted for the focus groups and were invited to take part. 14 agreed and were recruited to take part in this study across two focus groups (7 per group). 13 (93%) participants were originally assigned to the HFAO group, and one participant received the device after the initial trial intervention period. The mean [SD] age was 64[9] years, and 71% (n = 10) were male. The mean [SD] FEV1% predicted was 40[15]%. Patients were representative of the trial population.
There were three key themes generated from the focus group data; patient selection, device use and investment. There was some overlap between themes that have been explored and highlighted in the . Each theme is presented below alongside sub themes and supporting quotes. There was an overarching concept of standardised treatment and individualised care that runs throughout all themes.
Theme 1 Patient Selection
The theme of patient selection explored the importance of selecting the right patient for the device. Participants felt that some patients would improve from using the device, and those should be targeted when offering this treatment. This theme is made up of three sub-themes: disease characteristics, emotional burden, and management of care.
Disease Characteristics
Disease characteristics were felt to contribute to patient selection, where severity was a key part of this discussion. Some patients felt they were too severe to benefit from the intervention, whereas others felt they were too mild and did not yet need to use the device. Those that felt they were too mild felt it was helpful to have the device now for when their disease progressed. The perception of disease severity was made predominantly on symptom severity and level of treatment input (i.e regular review by a respiratory consultant, requiring oxygen, number of medications) rather than lung function (FEV1). The level of breathlessness and breathing distress contributed to who they felt would benefit from the device, and similarly to the severity, they felt some people were too breathless to use the device and others did not have “enough” breathlessness to feel they needed the device. Other confounding factors were discussed, including co-existence of other respiratory diseases.
I think it came a bit too late for me, had it been a couple of years ago I probably would have benefited.
M3 (Male, aged 79)
“I think it depends on the severity of your COPD” F2 (Female, aged 65)
Now obviously if people are struggling with breathing they’re not going to have the energy to do that… Whether it’s a machine just for people with the onset of COPD to improve rather than someone with full blown [COPD].
M7 (Male, aged 57)
Emotional Burden
The emotional burden of the disease was a crucial topic area and contributed to whether they would use the device. Some participants reported they felt embarrassed and frustrated by their breathlessness, cough and sputum and therefore felt if the device could help their symptoms this could help them to feel less conscious or frustrated by their symptoms. Some participants felt blame, in that they caused their disease by smoking and they felt they deserved the consequences. This negatively impacted their impression of the device, in that they felt they were serving a sentence and that nothing would undo the damage they had caused. Participants also described experiences of anxiety related to their breathlessness and as a result around using the device, which may induce breathlessness. Some participants felt anxious to participate in using the device, for fear it would induce breathlessness. The induced breathlessness could raise associated experiences of breathlessness to times where they felt they were in breathing distress and this could further contribute to breathlessness related anxiety. This discussion highlighted the impact of mind-set and how emotional burden can affect the response to an intervention and was discussed predominantly in those with severe disease as reported by the individual symptoms, rather than physiological measures such as lung function.
Well I’ve got nobody to blame but myself- I smoked for 50 years.
M8 (Male, aged 67)
You don’t think about breathing until you can’t breathe and then you start thinking about it and that’s the killer.
M2 (Male, aged 72)
I get up and I am panicking because I can’t get my breath.
M7 (Male, aged 57)
You’ll try any mortal thing to help you breathe a bit better.
M3 (Male, aged 79)
Management of Care
Lastly participants felt the management of care needed to be individualised. They felt this treatment should be offered to those that may benefit and that decision could largely be based on disease characteristics. Participants felt that continuity of care was important and seeing the same healthcare professional was beneficial. Participants were in favour of treatments they felt they could use independently and focussed on self-management, however, they also felt they needed support from a healthcare professional to ensure correct intervention administration.
Rather than go to my Ventolin I’ve gone for five minutes on that and its made a difference.
F3 (Female, aged 73)
The combination of inhalers and the oscillator could well be [important].
M5 (Male, aged 73)
I think the results show themselves. If you’re getting some benefit from it then you’re using it correctly I guess.
M5 (male, aged 73)
Theme 2 Device Use
The practicalities and effects of using the device were a point of discussion. The discussion mainly surrounded three sub-themes: the physical device and its prescription, daily routine, and device effects.
Device and Prescription
Most participants felt that the device was easy to use, robust and ergonomic, though one participant was unclear on the different settings. Some participants found the device difficult to use whereas others found it easy. There was an emphasis on hygiene and participants were satisfied with instructions of how and why they needed to clean the device. Participants felt the timing of the intervention around their medications was beneficial; however, the duration and intensity should differ for individuals and they felt that this should be prescribed per participant. Participants were clear on the function of the device, but there was little clarity on how the device would help.
It’s easy to understand.
M5 (Male, aged 73)
For me it was heavy labour on my lungs, I wanted to do it for a shorter time.
F2 (Female, aged 65)
Daily Routine
For the device to be successfully utilised and have continued use, participants felt it important to fit into participants' daily routines and lifestyles. Lifestyle was an important element for this theme mostly around how to use the device around your daily tasks. There was a strong focus on medications and they were perceived to be more important. Therefore, using a device needed to fit alongside their current medical management and not be too burdensome. Participants discussed the order of treatment, and found medications prior to using the device induced less breathlessness. Participants were concerned with how the device would interact with their current medical management and there were discussions around interventional load and this contributed to compliance. There was a perceived order of importance for treatment and those that fell lower down the list were less likely to be utilised. Participants felt their healthcare professionals should consider their treatment burden when prescribing or offering additional treatment. Those that had more difficulty fitting the use of the device into their daily routine used the device on a more adhoc basis.
Sometimes I use it five or six times. But I use it every day anyhow before I go to bed.
M1 (Male, aged 71)
I take my medication and then I give myself at least 5 minutes on the oscillator [device]
M5 (Male, aged 73)
Effects of Device
Participants discussed the effects of using the device, which links to benefits and participation (theme 3, sub theme 3). Some participants felt improvements in strength and stamina, whereas others felt using the device was too much and described discomfort when using or shortly after using the device, which hindered device use. Using the device required concentration and was difficult for some participants. This increased concentration brought awareness to the sensation of breathlessness that sometimes worsened their symptoms in the short term. Whilst using the device was effortful for some, they felt the benefit after using it and felt it was complimentary to their current management. Participants discussed some mild side effects, such as dry throat after using, or on the contrary, having increased saliva production, but these side effects were easily rectified and subsided immediately after using the device.
Nice bit of kit to add to the armoury of other things you can use.
M7 (Male, aged 57)
It made me feels stronger.
F1 (Female, aged 69)
I think my stamina’s improved.
M5 (Male, aged 73)
Because your conscious of it [breathing] you are taking more deeper breaths.
M8 (Male, aged 67)
Theme 3 Investment
The final theme surrounded the investment of the device which explored accessibility, understanding of device use, benefits and participation, and overall perceptions of the device. Overall the benefits had to outweigh financial and time costs in order for it to be seen as a valuable investment.
Accessibility
There was a discussion around accessing the device, particularly regarding the cost of the device. In some cases, this impacted negatively on their experience, though there were discussions comparing the price to other common treatments, and the participants felt they had little understanding of the cost of medical investigations and interventions. The price and affordability of the device was discussed and for some it was felt to be too expensive, whereas others felt it was worth the price if they could guarantee they would feel less breathless as a result. They felt their symptoms were severe, and so they were desperate to try anything and would be willing to pay the cost. The participants agreed that it was an investment and should be based on their response to the intervention and the financial and time costs associated.
But then you’re going to push it away from people that can’t afford it.
M1 (Male, aged 71)
The only thing I could say if that product had worked for me I would say yeah it’s worth the money.
M2 (Male, aged 72)
As a COPD sufferer you would look and think oh yeah, if that’s going to help me I’ll get one. And then you look at the bottom and you see how much it is. No thanks!
M7 (Male, aged 57)
Benefits and Participation
Those that felt their symptoms improved and found it helpful were continuing to use the device. Those that found it unhelpful thought using the device was burdensome and therefore discontinued the device after the trial. Generally, there was a reliance on medication, where participants described it is low in time cost and highly beneficial. Additionally, medications are available on the National Health Service British National Formulary and there are less cost implications than the HFAO device which is not available on the Formulary. The group acknowledged that if you experienced a benefit, then it would be worth using and therefore could acknowledge this was a suitable investment. Some participants perceived a therapeutic benefit, and those participants were more likely to engage in exercise in addition, to help with their management.
If somebody come to me and said look with got a cure for the common cold, they’re £10 a packet, everybody would buy them. But they wouldn’t necessarily work on everybody would they.
M2 (Male, aged 72)
I can walk up it [hill] now and have a conversation whereas before I couldn’t
M5 (Male, aged 73)
I wouldn’t want to be without it [the device]
F3 (Female, aged 73)
[But] when you look at the real cost of medicine and the stuff you’re getting on repeat prescriptions…this is good value.
M1 (Male, aged 71)
Understanding
Some participants felt a desire to understand how the device worked and this influenced whether they would invest in using the device. The understanding of the mechanism of action contributed to the efficacy of the device and quality of the intervention and felt it was helpful to their therapeutic management. Whereas, others felt the mechanism was less important as long as it was effective.
I’ve had a look how it works, being an engineer.
It doesn’t matter as long as it works.
F1 (Female, aged 69)
Overall Perception of Device
The overall perception of the device was important in dictating a response to the intervention, and the perceived benefit contributed to the perception of the device. Some participants felt no difference in symptoms, however, an obligation to continue to use the device during the trial period. Others who felt no improvements in symptoms continued to use the device as it was considered easy and low burden so felt there was no harm in continuing. Others who noticed a benefit, continued to use the device in a way they felt was appropriate for them, with some changes to frequency and intensity.
If you’re positive like this gentleman, you’ll do well, but if you get down you might not…
M3 (Male, aged 79)
I saw no benefit but I don’t know if it’s psychosomatic.
M5 (Male, aged 73)
Interaction of Themes
Standardisation of treatment and individualisation of care was a key topic throughout all three themes. Participants felt that everyone should have access to the same treatment options but that it should be individualised to the patient. They acknowledged that some patients would benefit from using the HFAO device and those patients should have access to it. It was felt that those who may benefit could be determined by disease severity/ symptom severity where there are some that felt too severe to use the device, and others that felt they were too mild so did not need to use the device yet. Additionally, the frequency and duration of device use should be tailored to the individual. And lastly, those that experience benefit from the device felt it was worthy of their investment, but this decision should be made on an individual basis ().
But like I say we’re all different.
M6 (Male, aged 71)
But everybody’s individual, so they’ve now decided, oh well that’s better for you and that’s better for you and that’s better for you.
M2 (Male, aged 72)
I suppose that’s for a lot of tablets and all medicines, they don’t all work for everybody do they.
M2 (Male, aged 72)
Triangulation with Survey and Study Data
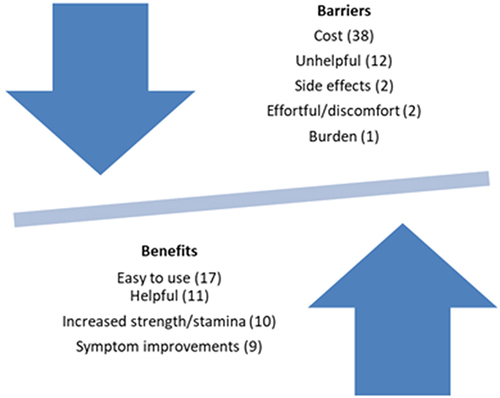
During the discussion there was a mix of patients that found the device helpful and those that did not. This is supported by the survey data, which was embedded in the trialCitation8 In the postal survey 23 out of 42 respondents (55%) were still using the device. 22 (58%) reported they found the device helpful and “helpful” was coded 11 times in the two focus groups, and “unhelpful” was coded 12 times (). 11 (69%) patients in the focus groups reported that they were using the device and 5 (31%) were no longer using it at all. Further analysis of the survey results are reported in the main trial paper.Citation8
Discussion
This was an exploration of device use following a previously reported randomised controlled trial using focus groups of participants in the study.Citation10 These focus groups were conducted in the context of a double blinded, sham, randomised controlled trial where there were no statistically significant differences seen in measures of breathlessness and health related quality of life of exercise capacity between groups, however, the results of this study were not known at the time of conducting the focus groups. Though, there were statistical significant improvements within group for breathlessness and respiratory muscle strength. Overall, three key themes illustrated participant experiences of using a HFAO to manage symptoms associated with COPD. These were: patient selection, device use and investment. A common interconnection between themes emphasised the need for individualised care. Largely, if participants felt the benefit outweighed the financial and time cost then it was worth the investment. It was felt that this treatment may not benefit everyone; therefore, disease characteristics and anxiety-induced breathlessness should be considered when selecting the patients that may benefit. Participants recommended individualised prescription of the device, with attention given to interventional load, to support uptake and routine generation.
The results of this study suggest some participants experienced a perceived benefit irrespective of device allocation and this is supported by the findings from the randomised controlled trial, which did not demonstrate improvements in breathlessness over the sham group, despite benefits in inspiratory muscle strength. It is likely that participants benefited from a placebo effect, and the mechanism for improved breathlessness was not an increase in respiratory muscle strength. Respiratory muscle strength inconsistently translates into improvements in breathlessness and this may be explained by the complexities of the breathlessness symptoms that are multifactorial in nature. These focus groups highlight the importance of patient perceptions on treatments, and how this influences their response. It is likely that this device may have improved breathlessness through other mechanisms such as influencing psychological contributions to breathlessness.
There is little qualitative literature to understand the patient experience of using devices though there is some evidence in medical treatment preferences for patients with COPD. Our findings corroborate those of Schroder et al (2021) who identified that ease of use, efficacy and frequency were important factors, and these were identified as themes within our exploration in devices.Citation11 Importantly there was an emphasis on medications among our participants, which is reflective of the emphasis placed on medical management in clinical practice. The use of devices is often complimentary to inhalers and has been identified in these focus groups that it may reduce the use of reliever inhalers. Research on self-management in COPD identified patient factors, practitioner factors and organisational factors, many of these topics were not explored directly in these focus groups, but there was overlap in themes around emotional impact, knowledge and understanding and a focus on the biomedical model.Citation12 This research and comparisons in the literature highlight the importance of a biopsychosocial model of management and how patient selection and interventional load is important in the adherence and compliance to treatment.Citation12
The focus groups invited everyone that had participated in the trial across both arms and there are strengths in that participants who were both positive and negative about the device participated. This is supported by the survey results that demonstrate a similar percentage of patients that were using the device though the focus groups had a slightly higher representation of those using the device, despite several of these participants were only using on an adhoc basis. However, the nature of this methodology may exclude those who have indifference around the device as they may feel they have little to add, and therefore it is likely to present extreme views only. Additionally, participants that dropped out were not invited to the focus groups and this may exclude more participants not using the device, however, this study did demonstrate a low dropout rate of 8% (n = 7). Despite this, all willing participants were invited to participate. As the questions were related to the HFAO device, the results are not generalisable to the entire COPD population or any other device that may be used. However, there is overlap with current literature and there are key findings that can inform the development and implementation of future device trials.
In conclusion, this research demonstrates the complexity of device interventions and that key considerations should be given to patient selection, the device use itself and, the investment required for participants. The need for individualised care and understanding the patient groups that might benefit from treatments is key to this device’s implementation.
Data Sharing Statement
De-identified individual data transcripts, and/or data codes can be made available upon reasonable request to the corresponding author. This data is available for five years after the date of publication. Original recordings will not be available.
Author Contributions
ED, NJG, SJS were involved in the development of the study protocol. ED, LHW and ACB developed the interview schedule. Interviews were conducted by ACB and ED. Analysis and interpret the results were performed by ACB, LHW and ED. ED wrote the manuscript all authors reviewed the manuscript. All authors contributed significantly to the revision of the manuscript. All authors agreed on the final submitted manuscript and journal of submission. All authors take responsibility and accountability for the contents of this manuscript.
Disclosure
Enya Daynes was awarded an educational grant from Actegy LTD for this study. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors would like to acknowledge Claire Bourne and Amy Jones for their assistance in moderating the focus groups. We would also like to acknowledge the patients and public who were crucial to the study. We dedicate this work to those who participated but have sadly lost their battle to COPD.
Additional information
Funding
References
- Global Strategy For The Diagnosis, Management And Prevention Of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease; 2006.
- Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(3):228. doi:10.1164/rccm.201210-1843CI
- Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Europ resp J. 2011;37(2):416–425. doi:10.1183/09031936.00031810
- Beaumont M, Forget P, Couturaud F, Reychler G. Effects of inspiratory muscle training in COPD patients: a systematic review and meta‐analysis. Clin Respir J. 2018;12(7):2178–2188. doi:10.1111/crj.12905
- Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest. 2003;124(4):1357–1364.
- Alghamdi SM, Alsulayyim AS, Alasmari AM, et al. Oscillatory positive expiratory pressure therapy in COPD (O-COPD): a randomised controlled trial. Thorax. 2023;78(2):136–143. PMID: 35948418. doi:10.1136/thorax-2022-219077
- Daynes E, Jones AW, Greening NJ, Singh SJ. The use of airway clearance devices in the management of chronic obstructive pulmonary disease. A systematic review and meta-analysis of randomized controlled trials. Ann Am Thorac Soc. 2021;18(2):308–320. PMID: 32783774. doi:10.1513/AnnalsATS.202005-482OC
- Morris T, Sumners DP, Green DA. Inspiratory high frequency airway oscillation attenuates resistive loaded dyspnea and modulates respiratory function in young healthy individuals. PLoS One. 2014;9(3):e91291. doi:10.1371/journal.pone.0091291
- Daynes E, Greening NJ, Harvey-Dunstan TC, Singh SJ. High-frequency airway oscillating device for respiratory muscle training in subjects with COPD. Respir Care. 2018;63(4):584–590. doi:10.4187/respcare.05837
- Daynes E, Greening N, Siddiqui S, Singh S. A randomised controlled trial to investigate the use of high-frequency airway oscillations as training to improve dyspnoea in COPD. ERJ Open Research. 2019;5(3):00064–2019. doi:10.1183/23120541.00064-2019
- Schroeder M, Hall K, Eliasson L, et al. Treatment Preferences of Patients with Chronic Obstructive Pulmonary Disease: Results from Qualitative Interviews and Focus Groups in the United Kingdom, United States, and Germany. COPD Foundation; 2021.
- Ogunbayo OJ, Russell S, Newham JJ, et al. Understanding the factors affecting self-management of COPD from the perspectives of healthcare practitioners: a qualitative study. NPJ Pri Care Respir Med. 2017;27(1):54–59. doi:10.1038/s41533-017-0054-6
- Daynes E, Greening N, Singh SJ. Randomised controlled trial to investigate the use of high-frequency airway oscillations as training to improve dyspnoea (TIDe) in COPD Thorax Published Online First. Thorax. 2021. doi:10.1136/thoraxjnl-2021-217072
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi:10.1191/1478088706qp063oa
- Finlay L, Gough B. Reflexivity: A Practical Guide for Researchers in Health Hand Social Sciences. 1 ed. Cornwall: Blackwell Science; 2008.
- Braun V, Clarke V. Conceptual and design thinking for thematic analysis. Qual Psychol. 2021;9(1):3.