Abstract
Background
The increase in forced expiratory volume in one second (FEV1) effected by a bronchodilator is routinely assessed when patients undertake pulmonary function testing (PFT). Several drug classes can theoretically affect the magnitude of the increase in FEV1. Withholding periods are advised for many but not all such drugs. Anecdotally, many subjects presenting for PFT are found to have taken drugs that might affect the test. We did an audit of patients presenting for PFT to assess the frequency with which FEV1 reversibility might be affected by drugs.
Methods
One hundred subjects presenting to the laboratory for PFT were questioned about recent drug consumption by an independent pharmacy intern. Reversibility of FEV1 was assumed to have been affected if drugs of interest were consumed within defined withholding periods or two half-lives for drugs without such data.
Results
Sixty-three subjects were prescribed drugs likely to affect FEV1 reversibility. Thirty-six subjects consumed at least one such drug within the withholding period. Half (18) of these patients consumed β-blockers with or without β-agonists. Sixty-five subjects did not recall receiving any advice about withholding drugs prior to the test and only 10 recalled receiving advice from their clinician or pulmonary function technician.
Conclusion
Subjects presenting for PFT are infrequently advised to withhold drugs that may affect FEV1 reversibility, and consequently, often take such drugs close to the time of the test. Therefore, it is likely that the increase in FEV1 is frequently affected by interference from drugs and this might impact on diagnosis and/or treatment options.
Introduction
Pulmonary function testing (PFT) usually includes an assessment of the forced expiratory volume in one second (FEV1) before and after inhalation of a bronchodilator. The resulting increase in FEV1 (Δ FEV1) is valuable in both the diagnosis and management of reversible airways disease. Authoritative guidelines on how PFTs should be conducted have been published by a combined task force of the American Thoracic Society and the European Respiratory Society.Citation1 When the PFT is performed to demonstrate whether airflow reversibility is present, the guidelines recommend a baseline test be performed when the patient is “not taking any drugs prior to the test”. This advice is presumably given to reduce the likelihood that any of the metrics will be affected by prior drug use. Drugs likely to affect the caliber of the airways include beta-adrenergic agonists (β-agonists) and antagonists (β-blockers) and other bronchodilators.
The likelihood that any particular drug will affect any of the PFT metrics will depend on the number of molecules in the vicinity of the receptor at the time of the test, which is dependent upon the half-life of the drug. The guidelines recommend a four-hour withholding period for short-acting β-agonists (SABA) and ipratropium, and a 12-hour withholding period for long-acting β-agonists (LABA) and aminophylline/theophylline. There are no recommended withholding periods for the long-acting anticholinergic bronchodilator, tiotropium, ultralong-acting β-agonists (eg, indacaterol) or β-blockers.
Historically, it has been unlikely that patients taking β-blockers would have presented for PFT because these drugs were seen as relatively contraindicated in patients with airways disease. However, the magnitude of benefit afforded by β-blockers in heart failure and myocardial infarction is such that some advocate their use even when patients have reversible airways disease, although many advocate careful patient selection and/or PFT.Citation2–Citation9 While there are several studies on β-blocker use in patients with airways disease in the literature, they are typically limited by short duration of therapy, patient selection, or retrospective study design. A meta-analysis of 19 single-dose and 10 “continued treatment” (three days to four weeks) studies of cardioselective β-blocker use in patients with reactive airways disease concluded that these agents “…should not be withheld in patients with reactive airways disease or COPD”.Citation3 The analysis revealed a decrease in FEV1 of 7.5% in the single-dose studies (baseline FEV1 2.4 L) and of only 0.4% in the “continued treatment studies” (baseline FEV1 1.8 L). However, there were many limitations in the studies analyzed (noted by the authors), including methodological concerns, too few participants, and only moderate disease severity. In addition, the longest period of treatment was four weeks, which is too short to reliably capture events that might trigger airways reactivity, including upper respiratory tract infections and seasonal allergies.
Anecdotal evidence suggests that patients were presenting for PFT soon after consuming drugs that might affect ΔFEV1 and that, on occasion, a “negative” result (ie, one where the increase in FEV1 was minimal) was interpreted as being safe to prescribe β-blockers. Therefore, we undertook an audit of 100 consecutive patients to assess the frequency with which drugs that might affect ΔFEV1 were consumed within a defined withholding period.
Materials and methods
Patients presenting to our hospital pulmonary function laboratory, which is accredited by the Thoracic Society of Australia and New Zealand, were interviewed by a pharmacy intern either immediately prior to or after their planned PFT. Laboratory staff were only informed that a student counseling project was being conducted so as to not affect usual practice of providing advice on withholding drugs of interest prior to PFT. Subjects were questioned about all drugs consumed (prescribed over-the-counter, cigarettes, alternative drugs), when they were taken, what advice was provided about withholding drugs prior to the PFT, and by whom the advice was provided.
Our laboratory practice is to perform a baseline FEV1 and to repeat the test five minutes after inhaling four puffs of salbutamol 100 μg via a large-volume (750 mL) spacer. We assumed that the increase in FEV1 would be affected by drugs if taken within the recommended withholding times (advised by the American Thoracic Society/European Respiratory Society guidelines). For tiotropium (no guidelines available), we adopted a 24-hour withholding period. For β-blockers, we adopted a withholding period equal to twice the reported half-life in normal subjects, ie, 12 hours for metoprolol and carvedilol, 20 hours for atenolol, and 24 hours for bisoprolol and sotalol.
Results
One hundred “consecutive” patients (ie, convenience sampling) were interviewed. Fifty-one subjects were referred from respirologists and 10 from cardiologists. Twenty-six subjects were inpatients at the time of their PFT. Sixty-three subjects were taking drugs likely to affect PFT. Thirteen subjects were current smokers, four of whom had smoked within the recommended withholding period (one hour) prior to PFT.
Thirty-six subjects had taken at least one drug likely to affect ΔFEV1 within the withholding period (). Of these, 18 were taking β-blockers () and of these 18, six were also taking β-agonists (three combination inhaled corticosteroid/LABA, five SABA) and four were also prescribed anticholinergic bronchodilators. Because of the long half-life involved and the usual habit of taking drugs in the morning, most subjects taking β-blockers consumed them within our defined withholding period (). Four of the 10 patients (40%) referred by cardiologists and seven of the 51 (13.7%) patients referred by respirologists were taking β-blockers.
Table 1 Demographics for 100 patients audited regarding drugs consumed prior to pulmonary function testing
Table 2 Drugs affecting pulmonary function testing
Sixty-five subjects did not recall receiving any advice about withholding drugs prior to their PFT, 23 recalled seeing generic advice on the appointment card only, six recalled verbal advice being given by their doctor, and four recalled receiving verbal advice from PFT laboratory staff.
Of the 26 inpatients referred for PFT, five were prescribed nebulized salbutamol and three PFTs were considered to be affected. One of the others did not have the nebulizer within four hours of the PFT (ie, accidental withholding) and the remaining subject had the nebulizer withheld by the requestor of the test because of the PFT (ie, deliberate withholding).
Discussion
Almost two thirds of patients were prescribed at least one drug that might affect ΔFEV1. Given the authoritative guidelines recommending withholding such drugs, we were surprised that almost 60% did not withhold them. Our accredited laboratory conducts more than 1000 PFTs annually and is typical of many public hospital PFT laboratories in Australia. Advice on withholding drugs was clearly suboptimal and warrants greater attention.
Of the 18 patients prescribed β-blockers, six were also prescribed bronchodilators (three LABA, five SABA, two tiotropium, and two ipratropium). Coprescription of β-blockers and β-agonists has been observed before, and while we did not investigate the reasons for the coprescription, this might be as a consequence of “compartmentalization” of respiratory and cardiac problems.Citation5,Citation8,Citation10
Because we did not want to affect normal laboratory practice of providing advice on withholding drugs likely to affect ΔFEV1, we were unable to repeat the PFT in the same laboratory on a proximate day when such drugs were withheld. Therefore we can only speculate about the effect on the magnitude of ΔFEV1 caused by prior use of these drugs.
Superficially it might be assumed that prior β-blocker consumption would lessen ΔFEV1 (via competition with agonist salbutamol) while prior β-agonist consumption might enhance ΔFEV1 (by increasing the number of agonist molecules). The potential effect is more complex and would depend on the numbers of agonist/antagonist molecules in the vicinity of the β-receptor, the numbers of β-receptors available, and the relative affinities of these molecules for the receptors. If salbutamol 400 μg maximally dilated the bronchioles, then post-bronchodilator FEV1 could not be increased by prior β-agonist use, but baseline FEV1 would likely be elevated (and hence ΔFEV1 would be reduced). However, if salbutamol 400 μg was insufficient to dilate the bronchioles maximally, then prior β-agonist use might elevate both prebronchodilator and post-bronchodilator FEV1, and if the magnitude of the effect on both was similar, ΔFEV1 would be unaffected.
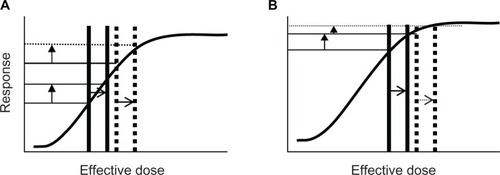
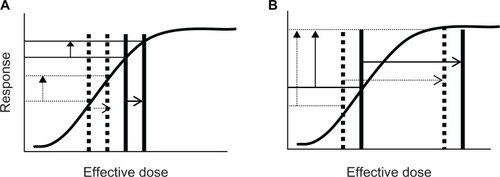
The effect of prior use of β-blockers would also depend upon the numbers of agonist and antagonist molecules (and their respective affinities) in the vicinity of β-receptors. If there were few endogenous agonist molecules present, baseline FEV1 would likely not be affected by prior β-blocker use, and if salbutamol 400 μg produced just maximal or submaximal bronchodilatation, then β-blockers would likely reduce post-bronchodilator FEV1. In this scenario, the ΔFEV1 would be reduced. However, if there were endogenous β-agonist molecules present, baseline FEV1 would likely be reduced, and if the post-bronchodilator FEV1 was similarly reduced ΔFEV1 might be unaffected. A final scenario would be if salbutamol 400 μg greatly exceeded the dose required for maximal bronchodilatation, then prior β-blocker use may not reduce post-bronchodilator FEV1 and unless baseline FEV1 was reduced ΔFEV1 would be unaffected. This can be explained diagrammatically via “dose-response” curves (–) where the “effective dose” (horizontal axis) represents the net effect. This net effect consists of the sum of both endogenous and exogenous β-agonist molecules minus exogenous β-blocker antagonist molecules.
Figure 1 Effect of prior β-agonist use on magnitude of FEV1 increase (vertical arrows) if salbutamol 400 μg produces less-than-maximal bronchodilatation (A) or maximal bronchodilatation (B). Solid lines represent the situation (pre and post salbutamol 400 μg) that would have occurred without prior use of β-agonist and dotted lines represent the situation (pre and post salbutamol 400 μg) that would have occurred in the presence of the β-agonist. (A) Prior use of β-agonist moves the “effective dose” to the right (more agonist molecules present) but the response (magnitude of increase in FEV1) is unaffected because the shift occurs on the linear portion of the curve. (B) Prior use of a β-agonist moves the “effective dose” to the right but the response (magnitude of increase in FEV1) is greatly reduced because the shift occurs on the nonlinear portion of the curve.
Published data suggest that salbutamol 400 μg is insufficient to produce maximal/supramaximal bronchodilatation and hence the scenarios in , , and are unlikely. In a study of 24 subjects with chronic obstructive pulmonary disease, the greatest relative increase in FEV1 was achieved by increasing the salbutamol dose from20 μg to 50 μg, but an increase in FEV1 was observed over the entire salbutamol range of 20–800 μg, with an additional 2.6% being attained by increasing the dose from 400 μg to 800 μg.Citation11 Another study that examined the dose-response effect on FEV1 in healthy subjects and mild/moderate asthmatics showed a continued increase in FEV1 over the range of 10–800 μg.Citation12
Figure 2 Effect of prior use of β-blocker on magnitude of FEV1 increase (vertical arrows) if salbutamol 400 μg produces just-maximal bronchodilatation (A) or is more than sufficient to produce maximal bronchodilatation (B) If baseline FEV1 is unaffected by β-blocker. Solid lines represent the situation (pre and post salbutamol 400 μg) that would have occurred without prior use of β-blocker and the dotted line represents the situation (post salbutamol 400 μg) that would have occurred in the presence of β-blocker. (A) Baseline FEV1 unaffected by a β-agonist (no endogenous agonist molecules present) but the response (magnitude of increase in FEV1) is greatly reduced due to competition for β-receptors by agonist and antagonist molecules (an effect equivalent to reducing the “effective dose”). (B) Baseline FEV1 unaffected by the β-agonist, and the response (magnitude of increase in FEV1) is unaffected because excess β-agonist molecules effectively compete for β-receptors and while the “effective dose” is reduced, the response remains maximal.
Abbreviation: FEV1, forced expiratory volume in one second.
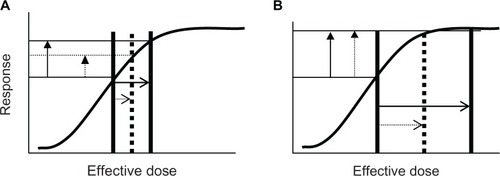
Figure 3 Effect of prior use of β-blockers on magnitude of FEV1 increase (vertical arrows) if salbutamol 400 μg produces less than maximal bronchodilatation (A) or is more than sufficient to produce maximal bronchodilatation (B) if baseline FEV1 is affected by prior β-blocker use. Solid lines represent the situation (pre and post salbutamol 400 μg) that would have occurred without prior use of β-blocker and dotted lines represent the situation (pre and post salbutamol 400 μg) that would have occurred in the presence of β-blocker. (A) Prior use of a β-agonist moves “effective dose” to the left (access to β-receptors by salbutamol is reduced) but the response (magnitude of increase in FEV1) is unaffected because the shift is on the linear part of the curve. (B) Prior use of a β-agonist moves the “effective dose” to the left (access to β-receptors by salbutamol is reduced) but the response (magnitude of increase in FEV1) is increased because the shift occurs on the nonlinear portion of the curve. Whether the response would be an increase, no change or a decrease in response would depend upon the relative effects of the β-agonist on baseline and post-bronchodilator FEV1 and where these values sit on the dose-response curve.
The American Thoracic Society/European Respiratory Society guidelines advise that the post-bronchodilator FEV1 should be measured 10–15 minutes after the last of four puffs of salbutamol, but our laboratory uses an interval of only 5 minutes.Citation1 This might lower the frequency with which airways reversibility is diagnosed by our laboratory. These guidelines also allow the test requestor to choose the bronchodilator (and dose). In our laboratory it is rare for the test requestor to specify either the bronchodilator drug or dose, and none of the patients in this study had such a request.
Eleven of the 18 patients prescribed tiotropium took it within the withholding period. Whether prior use of this anticholinergic bronchodilator would affect ΔFEV1 would depend on the relative effect of tiotropium on both baseline and post-salbutamol FEV1 values. Published data suggest anticholinergic agents provide bronchodilatation additional to salbutamol. In a study of 20 patients with chronic obstructive pulmonary disease, 5 mg of nebulized salbutamol produced an increase in FEV1 similar to that of ipratropium 500 μg and this effect was additive.Citation13 In the recently published UPLIFT study, participants received ipratropium 80 μg followed by salbutamol 400 μg, and post-bronchodilator FEV1 was determined 30 minutes later.Citation14 Although the participants in UPLIFT were diagnosed with chronic obstructive pulmonary disease (on the basis of age, smoking history, and PFT results), 54% experienced a AFEV1 of ≥12% and ≥200 mL, ie, consistent with an acute bronchodilator response.Citation15 The additional bronchodilator effect can serve to blur the laboratory distinction between asthma and COPD.Citation16
Some of the PFT requests in our study might have been ordered to assess whether additional benefit might be afforded by escalating therapy. In such cases, the test requestor might require the PFT to be performed without withholding β-agonists or other drugs and this would be factored into the interpretation of results. However, it is also likely that some PFTs were requested to assess patient suitability for β-blocker therapy (especially requests from cardiologists). Seventeen of the 18 subjects prescribed β-blockers in this study took them within the withholding period. If ΔFEV1 was modest (as a result of β-blocker consumption, ie, the scenario in ), this might have been interpreted as being safe to prescribe β-blockers. However, in reality, these patients might be at risk of bronchoconstriction. A caveat should be emphasized on the PFT report when a “negative” result (ie, ΔFEV1 fails to reach 12% and 200 mL) is observed, and this might be as a consequence of prior drug consumption, that it does not exclude the possibility of reversible airways disease.
We chose a withholding period twice that of the published half-life for β-blockers, but this may be too conservative, especially for nonselective β-blockers and for those drugs for which the half-life might be prolonged. Longer half-lives are likely for sotalol, bisoprolol, and atenolol because they are eliminated mainly via the renal route and many patients will have impaired renal function, if only as a consequence of ageing. The very long half-life of ultra-LABAs (eg, indacaterol, half life ≥40 hours) would require long periods of abstinence (more than three days) if an effect on ΔFEV1 was to be minimized.
Because we did not want to affect normal laboratory practice, we were unable to repeat the PFT in the same laboratory on a proximate day when drugs likely to affect ΔFEV1 were withheld, so we can only speculate about the actual effect on increase in FEV1 caused by these drugs.
Disclosure
This work was presented in poster form at the Thoracic Society of Australia and New Zealand National Scientific Conference, Brisbane, Australia, March 30–April 2, 2010. Otherwise, the authors report no conflicts of interest in this work.
References
- MillerMHankinsonJBrusascoVStandardisation of spirometryEur Resp J200526319338
- EverlyMHeatonPCCluxtonRJβ-blocker underuse in secondary prevention of myocardial infarctionAnn Pharmacother20043828629314742768
- SalpeterSROrmistonTMSalpeterEECardioselective β-blockers in patients with reactive airway disease: a meta-analysisAnn Intern Med200213771572512416945
- GottliebSSMcCarterRJVogelRAEffect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarctionN Engl J Med19983394894979709041
- ChenJRadfordMJWangYMarciniakTAKrumholzHMEffectiveness of beta-blocker therapy after acute myocardial infarction in elderly patients with chronic obstructive pulmonary disease or asthmaJAMA20013719501956
- EgredMShawSMohammedBWaittPRodriguesEUnder-use of beta-blockers in patients with ischaemic heart disease and concomitant chronic obstructive pulmonary diseaseQJM20059849349715955798
- OlenchockBAFonarowGGPanWCurrent use of beta blockers in patients with reactive airways disease who are hospitalised with acute coronary syndromesAm J Cardiol200910329530019166678
- ShortPMLipworthSIWElderDHJSchembriSLipworthBJEffect of β blockers in treatment of chronic obstructive disease: a retrospective cohort studyBMJ2001342
- RuttenFHZuithoffNPAHakEβ-blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary diseaseArch Intern Med201017088088720498416
- LingYSaleemWSheeCDConcomitant use of β-blockers and β2-agonistsEur Respir J2008490590618378787
- BorrillZLHoughtonCMWoodcockAAVestboJSinghDMeasuring bronchodilation in COPD clinical trialsBr J Clin Pharmacol20045937938415801931
- HoughtonCMWoodcockAASinghDA comparison of lung function methods for assessing dose-response effects of salbutamolBr J Clin Pharmacol20045813414115255795
- HadcroftJCalverleyPMAAlternative methods for assessing bronchodilator reversibility in chronic obstructive diseaseThorax20015671372011514693
- TashkinDPCelliBSennSA 4-yr trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med20083591543155418836213
- TashkinDPCelliBDecramerMBronchodilator responsiveness in patients with COPDEur Respir J20083174275018256071
- SorianoJBManninoDMReversing concepts on COPD irreversibilityEur J Resp J200831695696