Abstract
Purpose
The long-acting inhaled anticholinergic agent, tiotropium, is recommended as first-line maintenance therapy for moderate to very severe Chronic Obstructive Pulmonary Disease (COPD) to improve symptoms, exercise tolerance, health status, and to reduce exacerbations. Few studies have evaluated the therapeutic efficacy of tiotropium in patients in routine clinical conditions. The current study was designed to investigate the therapeutic efficacy of tiotropium delivered via the HandiHaler® device on the health status of patients with COPD with Global initiative for chronic Obstructive Lung Disease (GOLD) disease classification 2–4 in six central and eastern European countries in a real-life clinical setting.
Methods
The study was an open-label, prospective, uncontrolled, and single-arm surveillance study with three clinic visits during a 6-month observation period (baseline, and months 3 and 6). Health status was measured using the disease-specific St George’s Respiratory Questionnaire (SGRQ). The primary efficacy endpoint was the mean change from baseline in SGRQ total score at the end of the 6-month observational period.
Results
Patients treated with tiotropium 18 μg once daily showed statistically significant and clinically meaningful reduction (improvement) of 21.7 units in the SGRQ total score, regardless of smoking status or cardiac comorbidities at enrollment (P < 0.0001). The analysis also showed that age, treatment compliance, and GOLD disease classification were significant factors that impact the health status of patients with COPD differently.
Conclusion
These results provide further support for the use of the tiotropium HandiHaler® as first-line maintenance treatment of patients with COPD with a clinician-assessed disease.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a respiratory disease characterized by persistent and progressive airflow limitation and is a major cause of morbidity and mortality.Citation1 In Europe, COPD mortality ranges between 200,000 and 300,000 each year, with an annual treatment burden estimated at €102 billion.Citation2,Citation3
The clinical manifestations of COPD include dyspnea and impaired activity levels, which impact negatively on health-related quality of life (HRQoL).Citation4 Improving HRQoL is, therefore, an important treatment goal in COPD, and health status measurements now form an established assessment of treatment efficacy, especially as health status is often poorly correlated with forced expiratory volume in 1 second (FEV1). HRQoL can be measured using disease-specific questionnaires such as the St George’s Respiratory Questionnaire (SGRQ), a validated and widely accepted instrument that aims to provide an overall measure of impairment, and one that has often been used in recent COPD randomized clinical trials.Citation5,Citation6 The SGRQ score ranges from 0–100, and a reduction of ≥4 units is considered to be a clinically relevant improvement in health status.Citation7
The 2006 Global initiative for chronic Obstructive Lung Disease (GOLD) treatment guidelines recommend the use of long-acting inhaled anticholinergics as a first-choice maintenance therapy for patients with stage 2 COPD (FEV1/forced vital capacity [FVC] < 0.70; 50% ≤ FEV1 < 80% predicted), stage 3 (FEV1/FVC < 0.70; 30% ≤ FEV1 < 50% predicted), and stage 4 (FEV1/FVC < 0.70; FEV1 < 30% predicted [or as defined in the 2011 GOLD guidelines, risk groups B, C, and D]), to improve symptoms, exercise tolerance, and health status, as well as to reduce exacerbations.Citation8,Citation9 Using data compiled from 6 months to 4 years of treatment, clinical trials of patients with COPD have demonstrated that the long-acting inhaled anticholinergic agent, tiotropium (Spiriva®; Boehringer Ingelheim Pharma and Co, KG, Ingelheim, Germany), significantly improves HRQoL compared with either placebo,Citation7,Citation9–Citation11 or with the long-acting β2-agonist (LABA), salmeterol.Citation12 The effect of tiotropium on HRQoL has been confirmed in the 4-year Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT®) study, which showed long-term, tiotropium-mediated improvements in HRQoL.Citation10 In these studies, patients were often recruited from secondary care centers. However, the effect of tiotropium on HRQoL has not been investigated under routine clinical conditions in the community respiratory and primary care centers within Central and Eastern European countries (CEE). In this region, many COPD patients seen in routine clinical practice have severe or very severe disease,Citation13 yet management of their COPD might not always be in accordance with international guideline recommendations because the community pneumologists and primary care physicians may have differing interpretations of guidelines or a limited understanding of COPD.Citation14
Suboptimal treatment is likely to worsen the already significant impact of COPD on HRQoL, particularly if a patient’s lung function is poor. It is challenging to assess HRQoL in COPD patients from CEE countries, as there is no established procedure for its objective evaluation in daily practice, and the use of the SGRQ is almost exclusively reserved for clinical trials.
The SPIRIVA® Observational Study measuring SGRQ in Routine Medical Practice in Central and Eastern European Region (SOSPES) was conducted to assess the therapeutic effect of tiotropium administered with the HandiHaler® device on the HRQoL of patients with COPD. The study was conducted in six CEE countries within a real-life clinical setting. We also sought to evaluate the real-world applicability of the evidence of tiotropium’s efficacy in COPD, derived largely through randomized COPD trials, to study patients and conditions not typically included or studied in these trials.
Methods
Study objective
The objective of the SOSPES observational study was to evaluate the therapeutic effect of tiotropium 18 μg once daily given via the HandiHaler® device on the HRQoL of patients with COPD in CEE countries in a real-life clinical setting over 6 months. The study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonisation/Good Clinical Practice standards. It was approved by the relevant local ethics board and/or Institutional Review Board in accordance with the local regulations in each country. Written informed consent was obtained from subjects prior to initiation of the studies, if required by the national regulations.
Study design
SOSPES was an open-label, prospective, uncontrolled and single-arm, postmarketing, surveillance study conducted with the involvement of 897 study centers based in Croatia, Poland, Romania, Russian Federation, Slovakia, and Slovenia. The study consisted of three clinic visits during a 6-month observation period (baseline, month 3, and month 6). Only patients who had undergone regular medical assessments and previously discussed the introduction of the HandiHaler® as part of their treatment regimen during regular visits to their physicians were considered eligible to take part in the study. Inclusion criterion was adult outpatients aged ≥40 years with a clinical diagnosis of COPD (GOLD stage 2–4; FEV1/FVC < 0.7 and FEV1 < 80% predicted, as defined in the 2006 GOLD guidelines), who were attending a participating physician’s office for routine care. Patients were required to: have stable disease (no COPD exacerbation for ≥1 month before the study); have had no prior treatment with tiotropium within the previous 4 weeks; be suitable for this therapy as described in the product label; be fluent in the language of the questionnaire; and to have the cognitive and functional abilities required to complete it without help. There were no restrictions on concomitant, rescue, and additional medications required by study participants. However, all treatment decisions for regimen remained with the patient’s physician. Patients were excluded from participation if they were considered unlikely to be able to cooperate with the requirements of the study, had any condition that might decrease the chance of obtaining satisfactory data, were ineligible for treatment with tiotropium HandiHaler® according to the country-specific approval of this agent, and/or were currently enrolled in another clinical trial that required a change in medication for respiratory problems. Due to the noninterventional character of the study, violations in the planned schedule of the study were not considered as important protocol deviations and these patients were included into analyses as well as those patients with COPD GOLD stage 1 (FEV1/FVC < 0.7 and FEV1 > 80% predicted, as defined in the 2006 GOLD guidelines).Citation1
The primary efficacy endpoint was the mean change from baseline in SGRQ total score at the end of the 6-month observational period. Secondary endpoints included mean change in SGRQ total score by domains; the use of concomitant COPD therapy; and compliance with tiotropium HandiHaler® (<10 capsules’ difference between the number of days, and the actual number of capsules taken during these days, assessed between study visits).
Statistical analysis
Data were analyzed descriptively with calculation of 95% confidence intervals (CIs). The statistical significance of SGRQ score changes was assessed using the nonparametric signed-rank test and Kruskal–Wallis test for post hoc analysis comparing GOLD stages. The analysis was undertaken using SAS® Version 9.2 (SAS Institute, Inc, Cary, NC, USA). In addition to the treated set (TS) (all patients with documented evidence of tiotropium treatment during the observation period), the full analysis set (FAS) (all patients with documented SGRQ scores at months 3 and 6), the per-protocol set (PPS) (all patients from the FAS without important protocol violations), and a subgroup of PPS with compliance between 80% and 120% (inclusive) were defined. The primary and secondary endpoints have been presented for the FAS population. No attempt was made to impute missing data. Values for which no clarification with the investigator was possible were excluded from analyses.
A series of post hoc analyses were undertaken to explore changes in SGRQ scores among clinically relevant subgroups of patients. In addition, adjusted changes in SGRQ scores were calculated using a mixed-effect model, the standard technique for analysis of longitudinal data,Citation15 with patients nested within country as random effect and gender, age, stage of COPD, occurrence of cardiac comorbidities, compliance, and smoking status as fixed effect (ie, covariates). Further, the proportion of patients with a minimal clinically important difference (MCID) of 4 units in SGRQ score was analyzed.Citation16 Patients were classified as improved, worsened and unchanged if the difference from baseline in SGRQ score was <4 units, >4 units, and within range ±4 units, respectively.
The association between improvement by 4 units of the SGRQ score and patients’ characteristics was analyzed using generalized estimating equations for the logistic model (implemented in PROC GENMOD), which enabled calculation of robust estimates controlling within-country correlations.Citation17 Relative improvement (improvement ratio, similar to a risk ratio) and its 95% CIs based on multivariate analysis are presented. The characteristics of patients (gender, age, weight, stage of COPD disease, compliance, occurrence of cardiac comorbidities and smoking status) were included in the model as covariates.
Adverse events (AEs) were evaluated in the TS and are presented descriptively. An AE was defined as any untoward medical occurrence in a patient administered the study drug regardless of whether or not it was considered to be related to the study drug. An AE was considered serious if it was either immediately life threatening or resulted in persistent or significant disability, prolonged hospitalization, or death.
Results
In all, 4854 COPD patients participated in SOSPES and were evaluated from October 2009 to June 2011. Of these, 4852 were included in the TS, 4435 were included in the FAS, 4280 were included in the PPS, and 3891 were included in a subgroup of the PPS (). The majority of the patients in the FAS were male (70.9%), current or exsmokers (85.8%), with a mean age of 62.6 (standard deviation: 9.8) years (). Almost all patients were classified by their physicians as being GOLD stage 2 or 3 (89%) with 50% of the population classified as having severe (stage 3) or very severe (stage 4) disease. At baseline, 67.4% of patients in the TS were receiving COPD medication, the most common of which were LABAs (24.5%), SABAs (22.9%), theophylline (20.1%), LABA/inhaled corticosteroid (ICS) combinations (20.0%), and ICS (15.4%). Most patients had at least one concomitant disease (75.1%), the most common of which either were cardiac (41.6%) or peripheral vascular and cerebrovascular (37.4%) in nature.
Table 1 Patient baseline demography and disease characteristics (FAS population; N = 4435)
Figure 1 Disposition of study patients.
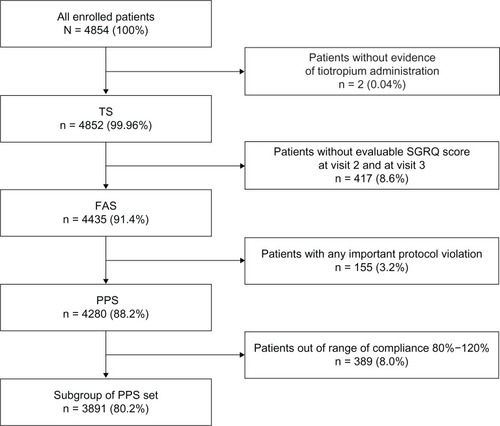
Change in SGRQ score
Patients in the FAS population treated with tiotropium 18 μg once daily via the HandiHaler® device showed a statistically significant and clinically relevant improvement from baseline in SGRQ total score, with a reduction of 21.7 units (95% CI: 22.3–21.2; P < 0.0001) after 6 months’ observation (). A statistically significant and clinically relevant reduction in SGRQ total score of 14.6 units (95% CI: 15.1–14.2; P < 0.0001) was also observed after 3 months’ treatment. The greatest improvement in SGRQ score after 6 months was seen in the symptoms domain (reduction of 27.2 units; 95% CI: 27.9–26.5), followed by the impact domain score (reduction of 22.0 units; 95% CI: 22.6–21.3) and the activity domain score (reduction of 18.2 units; 95% CI: 18.8–17.6) (all P < 0.0001 versus baseline) ().
Figure 2 Summary change from baseline in SGRQ total scores, activity domain, impact domain, and symptom domain (FAS population).
Abbreviations: FAS, full analysis set; SGRQ, St George’s Respiratory Questionnaire.
Mean change in SGRQ total score by country
Statistically significant reductions from baseline to month 6 in the mean total SGRQ score was observed for the FAS population in each of the six participating countries (P < 0.0001 for each country). The mean reduction in SGRQ total score among the six CEE countries ranged from 13.7–32.7 units, with the smallest change observed in patients from Slovenia and the largest change observed in patients from Romania ().
Figure 3 Summary change from baseline in SGRQ total scores among COPD patients.
Abbreviations: COPD, chronic obstructive pulmonary disease; CR, Croatia; PL, Poland; RO, Romania; RU, Russian Federation; SD, standard deviation; SGRQ, St George’s Respiratory Questionnaire; SI, Slovenia; SK, Slovakia.
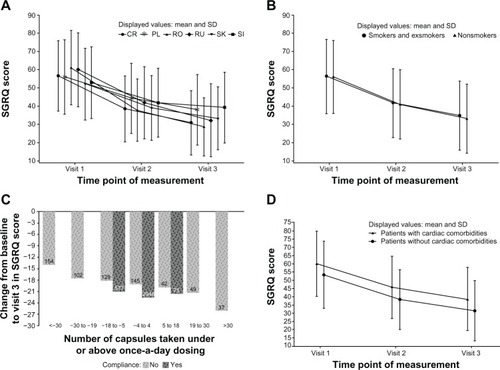
Mean change in SGRQ total score by smoking status
Statistically significant reductions from baseline to month 6 were noted, regardless of smoking status. Among current or exsmokers, the mean reduction in the SGRQ total score was 21.6 units (95% CI: 22.2–21.0; P < 0.0001); among never-smokers, the mean reduction was 23.1 units (95% CI: 24.7–21.5; P < 0.0001) (). The difference in mean reduction in SGRQ total score between current or exsmokers and nonsmokers was not significant (P = 0.2).
Mean change in SGRQ total score by age
There were statistically significant reductions in SGRQ total score from baseline to month 6, regardless of age (either ≤65 or >65 years). Among patients who were aged ≤65 years, the mean reduction was 22.3 units (95% CI: 23.1–21.6; P < 0.0001); among patients aged >65 years, the mean reduction was 20.6 units (95% CI: 21.5–19.7; P < 0.0001) (). The difference in mean reduction in SGRQ total score between patients aged ≤65 years or >65 years was statistically significant (P = 0.002).
Mean change in SGRQ total score by the presence of cardiac comorbidities
Statistically significant reductions in SGRQ total score from baseline to month 6 were noted, regardless of the presence of cardiac comorbidities. There was a mean reduction of 21.7 units (95% CI: 22.6–20.8; P < 0.0001) and 21.7 units (95% CI: 22.5–21.0; P < 0.0001) () for patients with and without recorded comorbid cardiac conditions, respectively, so there was no difference between these two groups of patients (P = 0.9693).
Mean change in SGRQ total score by documented compliance
The mean reduction from baseline to month 6 in the mean SGRQ total score was greater among patients classed as compliant during the observation period (22.4 units; 95% CI: 23.0–21.8; P < 0.0001) than among those patients receiving more than or less than once-daily dosing, classed as noncompliant (18.3 units; 95% CI: 19.7–17.0; P < 0.0001) (). For those patients classed as noncompliant because of receiving more than or less than once-daily dosing, the mean reduction in SGRQ total score was 20.3 units (95% CI: 17.7–22.9; P < 0.0001) and 17.5 units (95% CI: 16.0–19.1; P < 0.0001), respectively.
There is a greater mean reduction in the SGRQ total score between patients who were compliant with the prescribed treatment regimen and those who were classed as noncompliant. However, this difference was statistically significant (P < 0.0001) for all noncompliant patients and for noncompliant patients on less than once-daily dosing, but not statistically significant for those on more than once-daily dosing (P = 0.08).
Mean change in SGRQ total score by GOLD stage 1–4
The mean reduction from baseline to month 6 in the mean SGRQ total score was independent of GOLD disease classification. Patients having GOLD stage 1, 2, 3, or 4 disease classification (as defined by the 2006 GOLD guidelines) had mean reductions in SGRQ total score of 17.4 units (95% CI: 21.8–13.1), 20.3 units (95% CI: 21.1–19.5), 23.2 units (95% CI: 24.1–22.3), and 22.8 units (95% CI: 25.0–20.6), respectively. The mean reduction in SGRQ total score from baseline to visit 3 was statistically significant (P < 0.0001) for patients with GOLD stage 1, 2, 3, or 4 disease. Between GOLD stages, significant differences in the mean reduction in SGRQ total score from baseline to visit 3 were observed between GOLD stage 2 and stage 3 (P < 0.0001) only.
Minimum clinically important difference (MCID) in SGRQ scores overall and by patient characteristics
The majority of patients enrolled in SOSPES showed improvement in their SGRQ score within 3 months. This improvement persisted for the study duration. After 6 months, 83.5% of patients showed an improvement in their SGRQ score, whereas 12.5% and 4% of patients showed either no change or worsening in their SGRQ score, respectively.
Older patients with COPD enrolled in SOSPES were significantly less likely to achieve a clinically important change. An increment of 5 years of age decreased the probability of the improvement by 1% (). Furthermore, patient compliance increased the probability of improvement by 9%. Other factors examined such as weight, body mass index, disease severity, presence of cardiac comorbidities, and smoking status did not have a significant effect on the likelihood of the patient showing a relative improvement in their SGRQ score ().
Table 2 Summary of patients SGRQ status and the association between characteristic of patients and relative improvement in SGRQ score within 6 months – FAS
Requirement for additional COPD medications
Of the 4435 patients in the FAS population, 3289 (74.2%) did not require COPD medication in addition to that prescribed at the baseline visit. Among those patients who did require additional medication, 293 were treated with combination LABA/ICS and 363 required treatment with SABAs.
Adherence to treatment regimen during the observation period
In the FAS patient population, 3636 (82.0%) were compliant with the treatment regimen (treatment compliance of ≥80% and ≤120%). Overall, noncompliance among the FAS patient population was 18%. There were 537 patients (12%) who were noncompliant due to undertreatment (noncompliant patients receiving less than once-daily dosing) and 262 patients (6%) were noncompliant due to overtreatment (noncompliant patients receiving more than once-daily dosing). The median number of inhaled capsules used during the 6 months of the SOSPES study was 180 (interquartile range, 178–182).
Exposure to study treatment, safety, and tolerability
The mean exposure to tiotropium was 25.7 weeks and the majority of patients (4208 [86.7%]) were exposed between ≥25 and 30 weeks. In all, 306 AEs were reported by 182 patients from the TS population during the 6-month study (). Of the reported AEs, 90 were determined by the investigator to be related to the study drug. The most common AE considered by physicians to be related to the study drug was dry mouth (51 cases). In all, 14 patients (0.3%) reported a total of 23 AEs and discontinued treatment (). The most common AEs leading to treatment discontinuation were dry mouth (four patients reporting four cases), cough (two patients reporting two cases), and urinary retention (two patients reporting two cases).
Table 3 Summary of reported AEs (TS population; N = 4852)
In all, 15 patients (0.3%) reported 26 serious AEs (SAEs) (). Four patients reported 10 SAEs and discontinued treatment. However, all of the SAEs were determined to be unrelated to the study drug by the investigators and sponsor. During the 6-month observation period, there were seven SAEs with fatal outcomes reported. None of these deaths were considered to be related to the study drug.
Discussion
The SOSPES study recruited a population of patients who were treated with tiotropium as per the usual, day-to-day practice in CEE. In the SOSPES countries, the recruited patient population was treated routinely in day-to-day clinical practice: there were no strict enrollment criteria imposed. This differs from controlled clinical trials where disease severity and comorbidities within the study population are strictly determined.Citation18 Observational studies such as SOSPES tend to have broader inclusion criteria, fewer exclusion criteria, and are able to capture patients across the full spectrum of COPD disease severity. These patients are therefore more representative of day-to-day practice. Since tiotropium is recommended as the first-line maintenance treatment of choice in COPD patients with disease classification GOLD stages 2–4, or risk groups B, C, and D (as defined in the 2011 GOLD guidelines),Citation8 the inclusion of COPD patients of different GOLD stages/groups and the prescription of tiotropium was acceptable to physicians involved in SOSPES.
The hypothesis that our study included patients with suboptimal management of the disease at enrollment is supported by baseline observations; these showed that the patient population in SOSPES had a worse mean SGRQ total score at baseline (56.3) compared with COPD patients in the clinical trials that assessed this endpoint, such as the UPLIFT® (score of 45.7)Citation9 and the Tiotropium: Influence sur la Perception de l’amélioration des activites Habituelles Objectivée par une echelle Numerique (TIPHON) trial (score of 45.8).Citation7
We have shown that, in a population of patients with COPD treated in day-to-day clinical practice within six countries of the CEE region, tiotropium HandiHaler® maintenance therapy was associated with a statistically significant and clinically relevant improvement in HRQoL over a 6-month period, with statistically significant improvements observed across all three domains of the SGRQ (symptoms, impact, and activity). The magnitude of improvement was greatest in the symptoms domain (27.2 units) and smallest in the activity domain (18.2 units). However, two studies by Casaburi et alCitation19 and Kesten et alCitation20 have suggested that tiotropium, together with encouraging patients to get involved in a regular rehabilitation or activity plan, may increase the impact on the activity domain by enhancing exercise endurance. Patients who enrolled in the SOSPES study achieved a statistically significant reduction of 21.7 units (95% CI: 22.3–21.2; P < 0.0001) in SGRQ total score after 6 months of maintenance treatment with tiotropium. Statistically significant improvements in the SGRQ total score were noted at the month 3 assessment, with patients continuing to experience improvements through to the month 6 assessment.
Post hoc analyses of the change in SGRQ total score among clinically relevant subgroups of patients showed that patients experienced improvements in HRQoL, regardless of smoking status (current and exsmoker, and those who have never smoked) and presence of comorbidities. Age (≤65 years and >65 years) and disease severity (GOLD stages 1–4, as defined in the 2006 GOLD guidelines) were significant factors in the magnitude of the improvement in SGRQ total score. Patients aged ≤65 years achieved better improvement in health status compared to those patients aged >65 years. This is most likely because patients who are aged ≤65 years are more physically active and more likely to present with fewer comorbidities. Furthermore, patients who complied with their treatment regimen showed statistically significant improvement in health status as well as a significantly higher probability to achieve clinically important improvement in comparison with patients who did not use, or used less than, the recommended treatment. The change in SGRQ score over time was not reduced by controlling gender, age, stage of COPD, occurrence of cardiac comorbidities, compliance, smoking status, and within-country correlation effect; this provides evidence that the robust treatment effect observed with tiotropium was independent of patient characteristics.
Marked differences in the mean SGRQ improvement were noted between the patient populations from the six participating countries. This suggests that country-specific differences in the approach to treating COPD might impact clinical outcomes resulting from treatment with tiotropium. What these country-specific differences might be requires further investigation. Regardless, the mean SGRQ total score improved significantly from baseline in the patient populations for each country. This latter observation suggests that considerable undertreatment of COPD patients at entry in this observational study was common to all six participating countries. It is unknown whether this undertreatment is the result of underdosing, lack of compliance with treatment, differences in the interpretation of international clinical guidelines, or use of inappropriate medication. Regardless, our results show that many of the patients recruited in SOSPES would have benefited from earlier intervention with tiotropium. One-third of COPD patients included in the SOSPES analysis commenced tiotropium treatment at the initiation of the study; this also raises the possibility that the effect of being regularly monitored within this observational trial, in addition to the effect of the study drug, might have contributed to improved measures of HRQoL in these patients.
The improvement in the mean SGRQ total score reported here was greater than that reported in the Towards a Revolution in COPD Health (TORCH) study,Citation21 in which a change in SGRQ total score of −2.2 was recorded for patients from Eastern Europe treated with a salmeterol/fluticasone propionate combination. The magnitude of our result was also unexpected; most clinical trials with active treatment struggled to achieve the minimum clinically important difference in SGRQ score of four units compared to placebo.Citation7,Citation10 In the TIPHON study,Citation7 for example, a clinically important improvement in SGRQ score of up to 8.5 units versus baseline was achieved, but that was still significantly less than that achieved in SOSPES. Considering the absolute scores achieved in this study, compared to the 6-month data from the UPLIFT® studyCitation9 and the 9-month data from the TIPHON study,Citation7 the mean SGRQ scores of 39.4 and 37.3, respectively, were comparable to the SGRQ score of 34.6 achieved in this study at month 6. Not only is this SGRQ score comparable to the data from other clinical trials, it was consistent across all six participating countries.
Compliance with the once-daily tiotropium HandiHaler® was high and suggests that when patients with COPD saw major improvements in their disease condition with good tolerability, they were highly motivated to continue with their tiotropium HandiHaler®. Similarly, it may be that patient preference for a particular device might be a motivating factor in compliance.
Reported AEs and their frequency were in accordance with the known safety profile of the tiotropium HandiHaler®, although the overall number of reported deaths or AEs was lower than that seen in randomized clinical trials;Citation7,Citation10 this is consistent with the real-life setting and with the reporting of these incidents by primary care physicians in everyday practice.Citation18 The most frequently reported AE (dry mouth) is a known class effect and other frequently reported AEs (such as dyspnea and cough) are common within COPD patient groups.
When reviewing the results presented here, it is important to consider the limitations inherent in the design of an observational study such as the present one. As the study did not utilize a randomized, double-blind, placebo-controlled design, potential bias could have been introduced as a result of country-specific climate conditions during the 6-month follow-up period. However, since the period from first patient in to last patient out was around 1 year for all the SOSPES countries, any potential seasonal impact would be minimal. Additionally, no attempt was made to impute missing data.
Real-life studies such as SOSPES provide valuable information about patient populations encountered in clinical practice and their response to interventions outside the clinical trial setting. The high percentage of never-smokers (14%) in the study population is also worthy of note because it raises the possibility that a proportion of patients may not have been appropriately diagnosed with COPD and instead may have been more appropriately diagnosed with bronchiolitis or asthma with remodeling. Among the patient population who were smokers or exsmokers with ≥10 pack/years, the SOSPES study population is similar to the patient population in randomized clinical trials. The burden of nonsmoking COPD is also much higher than previously thought.Citation22
In addition, disease severity (GOLD stage) was not formally defined in terms of lung function and relied on physician assessment only. However, a recent report of a cross-sectional study among primary care physicians managing patients in five European countries showed that they were successfully able to grade COPD severity clinically and that their rating of disease severity was closely related to the patients’ HRQoL.Citation23 This observation is mirrored in the updated GOLD 2011 recommendations, which emphasize assessment of COPD based on the patient’s level of symptoms, future risk of exacerbations, the severity of the spirometry abnormality, and identification of comorbidities instead of spirometry alone.Citation8
Conclusion
Despite the limitations discussed previously, the results of this observational, real-world trial show that patients with COPD experience considerable and clinically relevant improvements in HRQoL, improving the SGRQ total score and all three domains, during extended treatment with tiotropium delivered via the HandiHaler® device. The results reported here provide further support for the use of the tiotropium for first-line maintenance treatment of patients with COPD with a clinician-assessed disease severity ranging from Groups B, C, and D (as defined in the 2011 GOLD guidelines). The reported AEs and their frequency were in accordance to the known safety profile of tiotropium HandiHaler®.
Acknowledgments
The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Godfrey Lisk, of PAREXEL International, during the preparation of this manuscript. The authors express their thanks to the Data Monitoring Committee. Clinical trial number: NCT1006135.
Disclosure
Matjaž Fležar has received funds from Boehringer Ingelheim, Novartis, AstraZeneca, Takeda, and GlaxoSmithKline for preparing and giving lectures regarding COPD and asthma in Slovenia and as a member of the Boehringer Ingelheim Advisory Board. He is also involved as investigator in clinical trials sponsored by the aforementioned companies.
Karina Jahnz-Różyk has received research funding in the last 2 years from Boehringer Ingelheim and has no other financial relationship with commercial activity that is of interest to the subject of this manuscript.
Tatiana Martynenko has no conflict of interest to declare.
Peter Kristufek is an occasional consultant and lecturer of Novartis, Boehringer Ingelheim, Takeda, GlaxoSmithKline and Pfizer. He has no commercial activity with regard to the subject of this manuscript.
Sanda Škrinjarić-Cincar has received honoraria for a lecture from Boehringer Ingelheim and has no other financial relationship or commercial activity with regard to the subject of this manuscript.
Gloria Enache and Pavla Kadlecová have no conflicts of interest to declare.
Dr Goran Martinovic is an employee of Boehringer Ingelheim RCV GmbH and Co KG. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease2013 Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease2013 Available at http://www.gold-copd.org/guidelines-global-strategy-for-diagnosis-management.htmlAccessed June 21, 2013
- European Lung Foundation[homepage on the Internet] Available at http://www.european-lung-foundation.org/63-european-lung-foundation-elf-burden-in-europe.htmAccessed June 21, 2013
- Organisation for Economic Cooperation and Development (OECD)Health at a GlanceEurope2012 Available at http://www.oecd.org/els/health-systems/HealthAtAGlanceEurope2012pdfAccessed June 21, 2013
- ShavroSAEzhilarasuPAugustineJBechtelJJChristopherDJCorrelation of health-related quality of life with other disease severity indices in Indian chronic obstructive pulmonary disease patientsInt J Chron Obstruct Pulmon Dis2012729129622615528
- JonesPWQuality of life measurement for patients with diseases of the airwaysThorax19914696766821835178
- JonesPWHealth status measurement in chronic obstructive pulmonary diseaseThorax2001561188088711641515
- TonnelABPerezTGrosboisJMVerkindreCBravoMLBrunMTIPHON study groupEffect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPDInt J Chron Obstruct Pulmon Dis20083230131018686739
- Global Initiative for Chronic Obstructive Lung Disease2011. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease2011 Available at http://www.goldcopd.org/uploads/users/files/GOLD2011_Summary.pdfAccessed June 21, 2013
- DecramerMCelliBKestenSLystigTMehraSTashkinDPUPLIFT investigatorsEffect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trialLancet200937496961171117819716598
- TashkinDPCelliBSennSKestenSMenjogeSDecramerMUPLIFT Study InvestigatorsA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- TroostersTCelliBLystigTUplift InvestigatorsTiotropium as a first maintenance drug in COPD: secondary analysis of the UPLIFT trialEur Respir J2010361657320185426
- BrusascoVHodderRMiravitllesMKorduckiLTowseLKestenSHealth outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax200358539940412728159
- StepanianIEKhmel’kovaNGBelin-AtaracBThe efficacy of tiotropium bromide (spiriva) in the treatment of patients with chronic obstructive pulmonary disease of varying severity: results of the Russian trialTer Arkh201082104651 Russian21341464
- AisanovZBaiCBauerleOPrimary care physician perceptions on the diagnosis and management of chronic obstructive pulmonary disease in diverse regions of the worldInt J Chron Obstruct Pulmon Dis2012727128222563246
- VerbekeGMolenberghsGLinear Mixed Models for Longitudinal DataNew York, NYSpringer2009
- JonesPWSt George’s Respiratory Questionnaire: MCIDCOPD200521757917136966
- CarterREZhangXWoolsonRFApfelCCStatistical analysis of correlated relative risksJ Data Sci20097397407
- FreemanDLeeAPriceDEfficacy and safety of tiotropium in COPD patients in primary care – the SPiRiva Usual CarE (SPRUCE) studyRespir Res200784517605774
- CasaburiRKukafkaDCooperCBWitekTJJrKestenSImprovement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPDChest2005127380981715764761
- KestenSCasaburiRKukafkaDCooperCBImprovement in self-reported exercise participation with the combination of tiotropium and rehabilitative exercise training in COPD patientsInt J Chron Obstruct Pulmon Dis20083112713618488436
- JonesPWAndersonJACalverleyPMfor TORCH investigatorsHealth status in the TORCH study of COPD: treatment efficacy and other determinants of changeRespir Res2011127121627828
- SalviSSBarnesPJChronic obstructive pulmonary disease in non-smokersLancet2009374969173374319716966
- JonesPWBrusselleGNegroRWPatient-centred assessment of COPD in primary care: experience from a cross-sectional study of health-related quality of life in EuropePrim Care Respir J201221332933622885563