Abstract
Objective
Sarcopenia is a common complication of COPD associated with an age-related reduction in skeletal muscle mass associated with decreased muscle strength and / or reduced mobility. The incidence of sarcopenia in patients with COPD is twice that of non-COPD patients and is associated with poor prognosis, this study aimed to investigate the influencing factors of sarcopenia in COPD patients.
Methods
Selected studies from PubMed, Embase, Web of Science, Cochrane Library, Wanfang, Wanfang, CNKI, CBM, and Wanfang databases as of November 12023. Patients aged 18 were selected; data were then independently extracted by two reviewers using a standard data collection form.
Results
In total, 17 articles reporting on 5408 patients were included. Age (OR = 1.083; 95% CI, 1.024–1.145), ALB (OR = 0.752; 95% CI, 0.724–0.780), BMI(OR = 0.701; 95% CI, 0.586–0.838), smoking (OR = 1.859; 95% CI, 1.037–3.334), diabetes (OR = 1.361; 95% CI, 1.095–1.692), qi deficiency (OR = 9.883; 95% CI, 2.052, 47.593), GOLD C (OR =2.232; 95% CI, 1.866, 2.670) and GOLD D (OR = 2.195; 95% CI, 1.826–2.637) were factors affecting muscle loss in COPD patients.
Conclusion
Sarcopenia is more prevalent in patients with COPD. Age, body mass index, smoking, diabetes mellitus, qi deficiency, ALB, and GOLD grade were the contributing factors for sarcopenia in patients with chronic obstructive pulmonary disease. In the future, medical staff should not only pay attention to the early screening of sarcopenia in high-risk groups, but also provide relevant prevention information.
Introduction
Chronic obstructive pulmonary disease (COPD) is a treatable and preventable chronic respiratory disease characterised by persistent respiratory symptoms and incomplete reversible airflow limitation.Citation1 The European Working Group on Sarcopenia in the Elderly (EWGSOP) defines it as a syndrome characterised by progressive and generalised loss of skeletal muscle mass and strength with risk of adverse outcomes,Citation2 characterised by a decline in muscle mass and function, which can increase the risk of falls, fractures, physical disability, and death.Citation3 The identification of sarcopenia referred to the Asian Working Group for Sarcopenia (AWGS) guideline following the criteria:Citation4 low muscle mass [bioelectrical impedance (M: <7.0 kg/m2, F: <5.7 kg/m2)] and low muscle strength [handgrip strength (M: <28 kg, F: <18 kg)] and/or poor physical performance (five-time chair stand test: ≥12 s). Obesity was defined as body mass index (BMI) ≥ 25.0 kg·m− 2. Sarcopenia is a common comorbidity of COPD, specifically a geriatric syndrome of age-related loss of skeletal muscle mass accompanied by decreased muscle strength and/or reduced mobility.Citation5 Byun et alCitation6 showed that the prevalence of sarcopenia in COPD patients was 25%, with the elderly, low body mass index, comorbid cardiovascular disease, and high inflammation levels being risk factors for the complication of sarcopenia in COPD patients. Benz et alCitation7 in a systematic evaluation and Meta-analysis of COPD and sarcopenia noted that patients with COPD are highly susceptible to the complication of sarcopenia, with prevalence rates of 21.6% in clinical studies and up to 63.0% in nursing homes. The prevalence of muscle loss in Chinese COPD patients is 28.1%, and the risk factors include low body mass index in COPD patients, long duration of disease, advanced age, long-term smoking.Citation8 According to the data, the prevalence of sarcopenia in patients with COPD is twice as high as in the healthy population, and increases gradually with the severity of COPD, with a higher prevalence in patients with acute exacerbations of COPD.Citation7,Citation9–11 The study also found that sarcopenia resulted in poorer lung function and exercise capacity, more severe airflow limitation and longer hospital stays in COPD patients.Citation12 It has been shown that sarcopenia has a sustained negative impact on a range of clinical outcomes associated with COPD, eg sarcopenia is an independent risk factor for respiratory failure in patients with acute exacerbations of COPD, and comorbid sarcopenia in patients with COPD predicts a higher risk of death.Citation13 However, there is a lack of discussion and summary of risk factors for sarcopenia in COPD patients, and there is a lack of data from Chinese COPD patients with sarcopenia. Early screening for sarcopenia can help to implement interventions to prevent progression of sarcopenia and improve the quality of life of COPD patients. Therefore, the aim of this study was to identify the risk factors for myasthenia gravis in COPD patients, and to provide strategic support for the prevention and treatment of myasthenia gravis in COPD patients.
Materials and Methods
Databases and Search Strategy
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses 2020 statement (PRISMA 2020) 18 principles were followed in this systematic review. A computer-based search of relevant literature publicly available from CNKI, Wanfang Data, SinoMed, VIP, PubMed, Medline, Web of Science, The Cochrane Library, and Embase databases built up to 1 November 2023 was performed. A search strategy was developed with a combination of subject terms and free.We used the following text words: “Chronic Obstructive Lung Disease/Chronic Obstructive Pulmonary Diseases/COAD/COPD/Chronic Obstructive Airway Disease/Chronic Obstructive Pulmonary Disease/Airflow Obstruction, Chronic/Airflow Obstructions”, Chronic/Chronic Airflow Obstructions/Chronic Airflow Obstruction, “Sarcopenia/Muscle/Thin tissue/muscle wasting/muscle atrophy/muscle mass/muscle strength/Grip strength”, “Incidence/Prevalence/Epidemiology/Frequency”, “risk factor/influencing factor/related factor/predictive factor/potential factor/risk/associated factors/dangerous factor”. In the Chinese database, the corresponding Chinese terms were used for searching.Citation14
Inclusion/Exclusion Criteria
The inclusion criteria were as follows: (1) the study population was COPD patients (GOLD: Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung DiseaseCitation15); (2)assessment of sarcopenia had to be implemented according to the sarcopenia consensus criteria; (3) providing odds ratio (OR) values and 95% confidence interval (CI) or providing raw data that can be converted into the above data; and (4) the study design was a cross-sectional, case-control or cohort study. The exclusion criteria were as follows: (1)full text not available; (2) inclusion of only the most detailed studies in the same sample; (3)inability to extract or transform raw data. (4) studies that were not published in Chinese or English.
Literature Selection and Data Extraction
Results of Searching Various Databases were imported into Endnote software for literature screening and management, and two researchers independently followed the inclusion and exclusion criteria to read the titles and abstracts for initial screening. Two researchers independently followed the inclusion and exclusion criteria to read the titles and abstracts for initial screening, and then read the full text for re-screening to determine the final inclusion. Two researchers independently followed the inclusion and exclusion criteria to read the title and abstract for initial screening, then read the full text for re-screening to determine the final inclusion of literature. In case of disagreement between the two were not in agreement, they were negotiated or third-party advice was sought. The data were extracted using A standardized form template was used to extract the data, including first author, year of publication, country of publication, country of study, study design, type of study, sample size, percentage of males in the sample, diagnosis of sarcopenia, prevalence of sarcopenia, and associated risk factors.
Literature Quality Evaluation
Two investigators independently evaluated the quality of the final included literature, and the cross-sections were rated using the cross-sectional study evaluation criteria recommended by the Agency for Health care Research and Quality (AHRQ),Citation16 containing 11 entries, with scores of 0 to 3, 4 to 7, and 8 to 11 points were evaluated as low, medium, and high quality, respectively. Case-control, cohort studies were assessed using the Newcastle-Ottawa Scale (NOS),Citation17 which consists of 8 entries, with a score of ≥6 indicating moderate to high quality literature.
Statistical Analysis
Stata 16.0 software was used for statistical analysis. In the quantitative analysis of effects, the OR value was chosen as the main statistical index and the corresponding 95% confidence interval (95% CI) was provided. Heterogeneity was assessed by using the χ2 test (test level α=0.1) in combination with the I2 test, and the fixed-effects model was used if the I2 ≤ 50% or P>0.1 suggested that the heterogeneity among the studies was small; the random-effects model was used if the I2 >50% or P ≤ 0.1. In addition, sensitivity analysis was performed to test the stability of the results. Funnel plots were drawn and publication bias was tested using the Egger test. A difference of P<0.05 was considered statistically significant.
Ethical Approval Statement
Institutional review board approval was not required because the analysis was based on the secondary processing of data from previously published studies.
Results
Literature Search and Screening Results
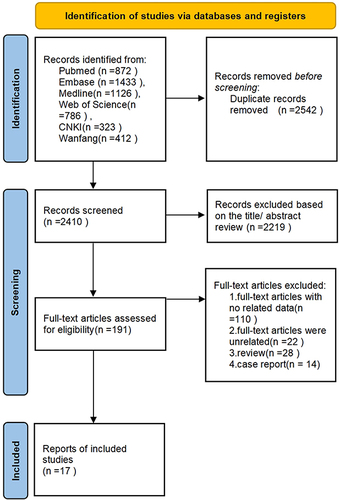
A flowchart of the literature selection process is presented in . Through database searches of PubMed, Embase, Medline, Web of Science, CNKI and Wanfang, 4952 articles were selected for subsequent filtering. Of these, 2542 duplicate studies were excluded. After checking the title and abstract of each paper and excluding inconsistent literature types, 191 studies were found to be pertinent to the research topic. Among them, 110 articles were excluded due to inaccessible data, 22 articles were excluded after a detailed review of the full text, 28 were review studies, and 14 were case reports. Finally, 17 original studies were included in the meta-analysis.
Summary of Studies
shows the characteristics of the 17 included studiesCitation6,Citation9,Citation10,Citation18–28, with a total of 5408 participants. 2 included studiesCitation10,Citation29 were cohort studies, 10 included studiesCitation6,Citation9,Citation18,Citation19,Citation21,Citation23,Citation25,Citation27,Citation30,Citation31 were cross-sectional studies and 4 included studiesCitation20,Citation22,Citation24,Citation26 were case control study. In the 17 included studies, 3 different definitions of sarcopenia were adopted, including AWGS (8 studiesCitation18–21,Citation25,Citation29–31) and EWGSOP (8 studiesCitation6,Citation9,Citation10,Citation22–24,Citation26–28). Literature basic characteristics and quality evaluation results, see .
Table 1 Characteristics of the Studies Included in This Review
Meta-Analysis Results
Influence Factors
The results of the meta-analysis showed that age (11 studies;Citation6,Citation10,Citation18–20,Citation22–24,Citation26,Citation27,Citation31 odds ratio [OR] = 1.083; 95% CI, 1.024–1.145) was a risk factor for sarcopenia in patients with COPD. BMI (8 studies;Citation6,Citation18–20,Citation24,Citation26,Citation29,Citation31 OR = 0.701; 95% CI, 0.586–0.838) and ALB(2 studies;Citation19,Citation24 OR = 0.752; 95% CI, 0.724–0.780), however, was significantly associated with a decreased risk of sarcopenia in patients with COPD. The results of the meta-analysis showed that smoke (6 studiesCitation18,Citation19,Citation22,Citation26–28; OR = 1.859; 95% CI,1.037–3.334), diabetes (3 studies;Citation6,Citation19,Citation20 OR = 1.361; 95% CI, 1.095–1.692), Qi deficiency (2 studies;Citation19,Citation30 OR =9.883; 95% CI, 2.052,47.593),GOLD spirometric classification C (4 studies;Citation9,Citation21,Citation25,Citation30 OR =2.232; 95% CI, 1.866,2.670), GOLD spirometric classification D (2 studies;Citation9,Citation21 OR = 2.195; 95% CI, 1.826–2.637) increased the likelihood of sarcopenia in patients with COPD. The results of the risk factors analysis can be found in
Table 2 Analyses of the Risk Factors of Sarcopenia in Patients with COPD
Publication Bias and Sensitivity Analysis
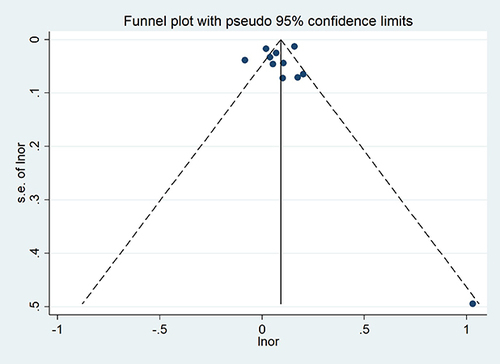
In this study, the funnel plots and Egger test for individual influencing factors with 10 or more included articles were evaluated for publication bias. The results showed that the funnel plot assessed the risk of publication bias showed symmetry, and Egger’s test results were t = 2.30 and p=0.787, indicating that there was no publication bias including the studies ().
Discussion
Age
The results of this study show that age is a risk factor for the development of sarcopenia in COPD patients. Age itself is associated with muscle loss, fibrosis, reduced mitochondrial efficiency and decreased neuromuscular junction function, and in COPD patients >50 years of age, there is a 1% to 2% muscle loss per year.Citation32
Bmi
Low BMI was strongly associated with the development of sarcopenia (OR=0.701, 95% CI: 0.586 to 0.838). Low BMI reduces mitochondrial energy conversion in muscle fibres and increases the risk of negative nitrogen balance, leading to accelerated muscle loss.Citation33 Higher BMI levels may mean that the body has more fat reserves, which help provide energy and nutrients and reduce the likelihood of muscle breakdown, thus maintaining muscle mass.Citation34 Therefore, while avoiding obesity, adequate nutritional intake and sufficient physical activity should be maintained to maintain muscle mass, strength and function.
Smoke
The results of this study showed that smoking habits is a risk factor for the development of sarcopenia in patients with COPD, and meta-analysis showedCitation35 that smoking is an independent risk factor for sarcopenia, and that smoking reduces the oxygen transporting capacity of the blood, which leads to a decrease in the supply of oxygen to the mitochondria of myocytes, which ultimately affects the functioning of skeletal muscles.Citation36 However, Maria Tsekoura et alCitation37 showed no statistically significant difference of smoking on combined sarcopenia in COPD patients. It is suggested that in the future, the sample size can be expanded and high quality studies can be conducted to further investigate whether smoking is an influential factor on comorbid sarcopenia in patients with COPD.
Diabetes
The results of this study show that diabetes is a risk factor for the development of sarcopenia in COPD patients. Sugimoto et al showed that the prevalence of sarcopenia increased linearly with increasing HbA1c concentration increase in the prevalence of sarcopenia.Citation38 Yoon et al also found that muscle function declined and the prevalence of sarcopenia increased significantly when glycated haemoglobin exceeded 8.5%.Citation39 Poor glycaemic control in diabetic patients can exacerbate insulin resistance, and diabetes mellitus.The development of sarcopenia in patients with diabetes mellitus may be associated with a number of causes: increased levels of reactive oxygen clusters can damage the structure and function of skeletal muscle cells.Citation40 Insulin resistance and insufficient secretion, inflammatory factors, hyperglycaemic state can cause muscle loss, diabetic nephropathy due to the loss of large amounts of proteinuria leads to a decline in muscle mass, there are also studies confirming that a variety of diabetic complications, such as retinopathy, neuropathy, etc., can lead to the decline in tissue and organ function can further lead to the occurrence of hypomyelitis.Citation41,Citation42
Qi Deficiency
The results of this study show that qi deficiency is a risk factor for the development of sarcopenia in COPD patients. Modern research has shown that qi deficiency is the most common symptomatic element of lung distension.Citation43 Qi refers to the vital energy of the body in traditional Chinese medicine(TCM). It maintains blood circulation, warms the body and fights diseases. When qi is deficient, all physiological functions of the body are reduced.Citation44
Alb
Low ALB was strongly associated with the development of sarcopenia (OR=0.752, 95% CI: 0.724to 0.780), Same results as Anna Picca’s study.Citation45.The association between higher albumin levels and the absence of sarcopenia in the elderly can be explained by the fact that the major nutrient deficiency in this population is protein.Citation46 Thus, in the absence of inflammation, albumin is a suitable biomarker of the protein status of the body’s viscera, and since albumin is the major protein synthesised by the liver, it may reflect the balance between protein intake and protein consumption. However, the long half-life of albumin of 18–21 days does not reflect acute changes in nutritional status.Citation47,Citation48
GOLD Classification
GOLD classification is a risk factor for sarcopenia in COPD patients, and GOLD class C and D are more likely to develop sarcopenia than GOLD class A. Lung function classification is a clinical indicator for assessing the degree of airflow limitation in COPD patients, and it is a clinical indicator for assessing the degree of airflow limitation in COPD patients. The GOLD classification is a clinical indicator for assessing the degree of airflow limitation in COPD patients.COPD patients with longstanding disease are affected by long-term glucocorticoid use and the reduction of protein synthesising hormones in the elderly, both of which affect the synthesis of muscle proteins, accelerating muscle catabolism and depletion.Citation49,Citation50 With the aggravation of the disease in patients with COPD, the level of oxidative stress is enhanced in the body, which, through the inhibition of PGC-1β, leads to the development of sarcopenia in skeletal muscle satellite cells. This leads to abnormal mitochondrial homeostasis in skeletal muscle satellite cells, impaired myogenic differentiation, inhibition of myofibre repair and direct inhibition of myosin ATPase activity, leading to a decrease in skeletal muscle contractility.Citation51
Limitations
The sample size of individual studies included in this study is limited, and the reliability and stability of the conclusions are limited; only Chinese and English literature are included, and the tracking of non-Chinese and English literature is lacking, which may be selection bias; some influencing factors cannot be meta-analyzed due to the insufficient number of literature, and large sample and multi-center studies need to be verified in the future.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Sharing Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Additional information
Funding
References
- Global strategy for prevention. Diagnosis and management of COPD:2022 report[EB/OL]. Available from: https://goldcopd.org/2022-gold-reports-2/. Accessed January 12, 2022.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi:10.1093/ageing/afq034
- Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29(2):154–159. doi:10.1016/j.clnu.2009.12.004
- Chen N, Zhou L, Zhang Z, Xu J, Wan Z, Qin L. Resistin induces lipolysis and suppresses adiponectin secretion in cultured human visceral adipose tissue. Regul Pept. 2014;194–195:49–54. doi:10.1016/j.regpep.2014.10.001
- Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi:10.1016/j.jamda.2019.12.012
- Byun MK, Cho EN, Chang J, Ahn CM, Kim HJ. Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:669–675. doi:10.2147/COPD.S130790
- Benz E, Trajanoska K, Lahousse L, et al. Sarcopenia in COPD: a systematic review and meta-analysis. Eur Respir Rev. 2019;28(154):190049. doi:10.1183/16000617.0049-2019
- Lian J, Pan D, An X, et al. Study on the influencing factors of body composition changes and combined sarcopenia in patients with chronic obstructive pulmonary disease. Chin General Med. 2017;20(28):3504–3508. doi:10.3969/j.issn.1007-9572.2017.28.011
- Demircioğlu H, Cihan FG, Kutlu R, Yosunkaya Ş, Zamani A. Frequency of sarcopenia and associated outcomes in patients with chronic obstructive pulmonary disease. Turk J Med Sci. 2020;50(5):1270–1279. doi:10.3906/sag-1909-36
- de Araújo B E, Teixeira PP, Valduga K, da Silva Fink J, Silva FM. Prevalence, associated factors, and prognostic value of sarcopenia in patients with acute exacerbated chronic obstructive pulmonary disease: a cohort study. Clin Nutr ESPEN. 2021;42:188–194. doi:10.1016/j.clnesp.2021.01.042
- Kaluźniak-Szymanowska A, Krzymińska-Siemaszko R, Deskur-śmielecka E, Lewandowicz M, Kaczmarek B, Malnutrition W-TK. Sarcopenia, and malnutrition-sarcopenia syndrome in older adults with COPD. Nutrients. 2021;14(1):44. doi:10.3390/nu14010044
- Dávalos-Yerovi V, Marco E, Sánchez-Rodríguez D, et al. Sarcopenia according to the revised European consensus on definition and diagnosis (EWGSOP2) criteria predicts hospitalizations and long-term mortality in rehabilitation patients with stable chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2019;20(8):1047–1049. doi:10.1016/j.jamda.2019.03.019
- Zhao X, Su R, Hu R, et al. Sarcopenia index as a predictor of clinical outcomes among older adult patients with acute exacerbation of chronic obstructive pulmonary disease: a cross-sectional study. BMC Geriatr. 2023;23(1):89. doi:10.1186/s12877-023-03784-7
- Feng L, Gao Q, Hu K, et al. Prevalence and risk factors of sarcopenia in patients with diabetes: a meta-analysis. J Clin Endocrinol Metab. 2022;107(5):1470–1483. doi:10.1210/clinem/dgab884
- Global Strategy for the Diagnosis. Management and prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2017.
- Zeng XT, Liu H, Chen X, et al. Meta-analysis series IV: a quality assessment tool for observational studies. Chin J Evid Based Cardiovasc Med. 2012;4(4):297–299. doi:10.3969/j.1674-4055.2012.04.004
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi:10.1007/s10654-010-9491-z
- Tang Rao. Survey on the prevalence of chronic obstructive pulmonary disease combined with sarcopenia in Chongqing community and analysis of related factors. Chongqing Medical University; 2022.
- Jiyou Z, Yanxin S, Xiaoyan C, et al. Analysis of muscle attenuation status and influencing factors in elderly patients with chronic obstructive pulmonary disease. ChinJ Integr Med West Med. 2021;41(9):1042–1048.
- Lei B. Analysis of risk factors of concurrent sarcopenia and osteoporosis in hospitalised elderly COPD patients. Jishou University. 2021. doi:10.27750/d.cnki.gjsdx.2021.000064
- Deng Y, Li L, Zhang H, Pei H. Current status of the development of muscle wasting syndrome in COPD patients and construction of risk assessment model. J Nurs. 2020;35(22):19–24.
- Yan C. The effect and correlation analysis of myasthenia gravis on lung function and blood gas analysis indexes of COPD patients. Jilin University; 2020. doi:10.27162/d.cnki.gjlin.2020.002972
- Lage VKDS, de Paula FA, Dos Santos JM, et al. Are oxidative stress biomarkers and respiratory muscles strength associated with COPD-related sarcopenia in older adults? Exp Gerontol. 2022;157:111630. doi:10.1016/j.exger.2021.111630
- Baiyang LIN. Relationship between serum IL-6 and IL-10 levels and sarcopenia in elderly COPD patients. Shanxi Medical University; 2019.
- Limpawattana P, Inthasuwan P, Putraveephong S, Boonsawat W, Theerakulpisut D, Sawanyawisuth K. Sarcopenia in chronic obstructive pulmonary disease: a study of prevalence and associated factors in the Southeast Asian population. Chron Respir Dis. 2018;15(3):250–257. doi:10.1177/1479972317743759
- Jie LIAN, Dianzhu PAN, Xiaoqin AN, et al. Study on factors affecting body composition changes and combined sarcopenia in patients with chronic obstructive pulmonary disease. Chin General Med. 2017;20(28):3504–3508+3514.
- Costa TM, Costa FM, Moreira CA, Rabelo LM, Boguszewski CL, Borba VZ. Sarcopenia in COPD: relationship with COPD severity and prognosis. J Bras Pneumol. 2015;41(5):415–421. doi:10.1590/S1806-37132015000000040
- Tasar PT, Sahin S, Karaman E, et al. Prevalence and risk factors of sarcopenia in elderly nursing home residents. Eur Geriatr Med. 2015;6(3):214–219. doi:10.1016/j.eurger.2015.03.002
- Wu JF, Jia J, Chen P, et al. Sarcopenia and its clinical correlation in elderly chronic obstructive pulmonary disease: a prospective cohort study. Eur Rev Med Pharmacol Sci. 2023;27(20):9762–9772. doi:10.26355/eurrev_202310_34150
- Hou X, Kong H, Ren S. Exploration of independent risk factors and establishment of risk prediction model for COPD stable stage combined with sarcopenia. Liaon Univer Tradit Chin Med. 2023;18(1). doi:10.27213/d.cnki.glnzc.2023.000566
- Wang Z, Zhou X, Deng M, et al. Clinical impacts of sarcopenic obesity on chronic obstructive pulmonary disease: a cross-sectional study. BMC Pulm Med. 2023;23(1):394. doi:10.1186/s12890-023-02702-2
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(1):53–59. doi:10.1093/ajcn.82.1.53
- Volkert D. The role of nutrition in the prevention of sarcopenia. Wien Med Wochenschr. 2011;161(17–18):409–415. doi:10.1007/s10354-011-0910-x
- Zamboni M, Rubele S, Rossi AP. Sarcopenia and obesity. Curr Opin Clin Nutr Metab Care. 2019;22(1):13–19. doi:10.1097/MCO.0000000000000519
- Zou H, Liu Y, Jiang DX, et al. Meta-analysis of factors affecting myasthenia gravis. Chin J Prev Med. 2021;22(2):86–92. doi:10.16506/j.1009-6639.2021.02.002
- Yun N, Linlin P, Xufan G, et al. Chronic obstructive pulmonary disease combined with sarcopenia. Chin J Pract Internal Med. 2022;42(08):626–630. doi:10.19538/j.nk2022080104
- Tsekoura M, Tsepis E, Billis E, Gliatis J. Sarcopenia in patients with chronic obstructive pulmonary disease: a study of prevalence and associated factors in Western Greek population. Lung India. 2020;37(6):479–484. doi:10.4103/lungindia.lungindia_143_20
- Sugimoto K, Tabara Y, Ikegami H, et al. Hyperglycemia in non-obese patients with type 2 diabetes is associated with low muscle mass: the multicenter study for clarifying evidence for sarcopenia in patients with diabetes mellitus. J Diabetes Investig. 2019;10(6):1471–1479. doi:10.1111/jdi.13070
- Yoon JW, Jang HC. Response: hyperglycemia is associated with impaired muscle quality in older men with diabetes: the Korean longitudinal study on health and aging (diabetes metab j 2016;40:140-6). Diabetes Metab J. 2016;40(3):250–251. doi:10.4093/dmj.2016.40.3.250
- Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993–1997. doi:10.2337/dc09-0264
- Zhan Y, Luo L, Zhang Y, et al. Research progress of diabetes-related sarcopenia. Chin J Gerontol. 2019;39(23):5892–5896. doi:10.3969/j.issn.1005-9202.2019.23.073
- Hoffmann MR, Senior PA, Jackson ST, Jindal K, Mager DR. Vitamin D status, body composition and glycemic control in an ambulatory population with diabetes and chronic kidney disease. Eur J Clin Nutr. 2016;70(6):743–749. doi:10.1038/ejcn.2015.185
- Wei-Liang WANG, Xing-Hua LIN, Jia-Rong YE, et al. A study on the correlation between body quality and evidence type in chronic obstructive pulmonary disease in Chinese medicine. J Modern Integr Med. 2020;29(13):1369–1372+1378.
- Liao YC, Chou CY, Chang CT, et al. Qi deficiency is associated with depression in chronic hemodialysis patients. Complement Ther Med. 2017;30:102–106. doi:10.1016/j.ctim.2016.12.008
- Picca A, Coelho-Junior HJ, Calvani R, Marzetti E, Vetrano DL. Biomarkers shared by frailty and sarcopenia in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2022;73:101530. doi:10.1016/j.arr.2021.101530
- Valenzuela RE, Ponce JA, Morales-Figueroa GG, Muro KA, Carreón VR, Alemán-Mateo H. Insufficient amounts and inadequate distribution of dietary protein intake in apparently healthy older adults in a developing country: implications for dietary strategies to prevent sarcopenia. Clin Interv Aging. 2013;8:1143–1148. doi:10.2147/CIA.S49810
- Kragh-Hansen U, Otagiri M, Chuang V. Albumin in Medicine. In: Human serum albumin: A multifunctional protein. Singapore: Springer; 2016:1–24
- Silva-Fhon JR, Rojas-Huayta VM, Aparco-Balboa JP, Céspedes-Panduro B, Partezani-Rodrigues RA. Sarcopenia and blood albumin: a systematic review with meta-analysis. Sarcopenia y albúmina sanguínea: revisión sistemática con metaanálisis. Biomedica. 2021;41(3):590–603. doi:10.7705/biomedica.5765
- Sanders KJ, Kneppers AE, van de Bool C, Langen RC, Schols AM. Cachexia in chronic obstructive pulmonary disease: new insights and therapeutic perspective. J Cachexia Sarcopenia Muscle. 2016;7(1):5–22. doi:10.1002/jcsm.12062
- Schakman O, Kalista S, Bertrand L, et al. Role of Akt/GSK-3beta/beta-catenin transduction pathway in the muscle anti-atrophy action of insulin-like growth factor-I in glucocorticoid-treated rats. Endocrinology. 2008;149(8):3900–3908. doi:10.1210/en.2008-0439
- Kunzke T, Buck A, Prade VM, et al. Derangements of amino acids in cachectic skeletal muscle are caused by mitochondrial dysfunction. J Cachexia Sarcopenia Muscle. 2020;11(1):226–240. doi:10.1002/jcsm.12498