Abstract
Purpose
The exact link between cognitive impairment (CI) and chronic obstructive pulmonary disease (COPD) is still limited. Thus, we aim to find the relationship and interaction of quantitative CT (QCT), lung function, HIF-1α, and clinical factors with the development of CI among COPD patients.
Patients and Methods
A cross-sectional multicentre study was conducted from January 2022 to December 2023. We collected clinical data, spirometry, CT images, and venous blood samples from 114 COPD participants. Cognitive impairment assessment using the Montreal Cognitive Assessment Indonesian version (MoCA-Ina) with a cutoff value 26. The QCT analysis consists of lung density, airway wall thickness, pulmonary artery-to-aorta ratio (PA:A), and pectoralis muscles using 3D Slicer software. Serum HIF-1α analysis was performed using ELISA.
Results
We found significant differences between %LAA−950, age, COPD duration, BMI, FEV1 pp, and FEV1/FVC among GOLD grades I–IV. Only education duration was found to correlate with CI (r = 0.40; p < 0.001). We found no significant difference in HIF-1α among GOLD grades (p = 0.149) and no correlation between HIF-1α and CI (p = 0.105). From multiple linear regression, we observed that the MoCA-Ina score was influenced mainly by %LAA−950 (p = 0.02) and education duration (p = 0.01). The path analysis model showed both %LAA and education duration directly and indirectly through FEV1 pp contributing to CI.
Conclusion
We conclude that the utilization of QCT parameters is beneficial as it can identify abnormalities and contribute to the development of CI, indicating its potential utility in clinical decision-making. The MoCA-Ina score in COPD is mainly affected by %LAA−950 and education duration. Contrary to expectations, this study concludes that HIF-1α does not affect CI among COPD patients.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is marked by persistent and progressive respiratory symptoms and airflow obstruction, which are associated with intrapulmonary and extrapulmonary complications such as cognitive impairment (CI).Citation1 There is a wide range of CI prevalence among COPD, ranging from 4% to 61%, and a recent study indicates that 18.4% of individuals with mild CI (MCI) may develop dementia within a year.Citation2,Citation3 The presence of CI in COPD has substantial consequences, such as poor medication adherence, smoking cessation, and functional dependency, that can affect the self-management of COPD patients. Thus, early detection of those with MCI is needed to prevent early deterioration and intervention.Citation2
With a deeper insight into the pathomechanism and conducting thorough evaluations beyond spirometry, early prevention, and intervention can be done to avoid CI and its progression to dementia that occurs in many COPD patients. Up until now, the exact links between COPD and CI are unknown, but hypoxia, inflammation, and vascular abnormalities in COPD are thought to be the cause of neuronal damage that leads to CI. These conditions can be assessed by clinical factors or examination, such as pulmonary function test (PFT), radiological imaging, or serological analyses. Particularly in hypoxia, increased expression of HIF-1ɑ has been observed in correlation with worsening COPD due to its function as an oxygen homeostasis regulator.Citation4 Making the association between HIF-1ɑ and COPD severity a favoured study topic.Citation5–7
The standard for diagnosing and classifying COPD severity is a pulmonary function test (PFT) using spirometry. However, high-resolution CT scan (HRCT) is becoming a phenomenon among COPD cases due to its ability to identify abnormalities not detected by pulmonary function tests, phenotyping COPD cases into emphysema-dominant or airway-dominant, and further quantify the number of abnormalities such as parenchymal destruction and bronchial thickening.Citation8,Citation9 Common intrapulmonary quantitative CT (QCT) parameters that can precisely and objectively evaluate COPD phenotypes are the percentage of low attenuation area (%LAA−950) during inspiration with a threshold <−950 Hounsfield Units (HU) for emphysema and percentage wall area (%WA) for chronic bronchitis. However, previous studies have also found intrathoracic extrapulmonary QCT parameters to be well correlated with COPD diagnosis and severity, such as pulmonary artery-to-aorta ratio (PA:A), pectoralis muscle area (PMA), and pectoralis muscle density (PMD).Citation10–14 HRCT, together with the benefit of QCT, may lead to earlier identification and treatment. The importance of early detection of COPD, especially in early and moderate stages, may have a favourable impact on disease progression and clinical outcome, including mitigating future complications such as CI.
The primary aim of this study is to find the relationship and interaction of various quantitative CT (QCT) parameters, lung function, HIF-1α, and clinical factors with the development of CI among COPD patients. This study is the first to assess the role of QCT parameters in the pathomechanism of CI in patients with COPD. With a deeper insight into the pathomechanism and conducting thorough evaluations beyond spirometry, early prevention, and intervention can be done to avoid CI and its progression to dementia that occurs in many COPD patients.
Material and Methods
Subject Recruitment
This cross-sectional study is a multi-center study focusing on the role of clinical and QCT characteristics of CI in COPD. This study received two ethical approvals, that is, Ethical Committee of Persahabatan Hospital (no: 02/KEPK-RSUPP/01/2022), which allow us to recruit participants from Persahabatan Hospital from January 2022. Another ethical approval released by the Faculty of Medicine, University of Indonesia (no: KET-133/UN2.F1/ETIK/PPM.00.02/2022), which allowed us to recruit participants from three other hospitals (Cipto Mangunkusumo Hospital, Gatot Soebroto Army Hospital, and Jakarta Islamic Hospital Cempaka Putih) from February 2022. The overall study recruitment starts from January 2022 until December 2023. All procedures used in this study adhered to the institutional and national research committee’s ethical requirements and the Declaration of Helsinki. All patients who participated in this study were adults and had given written informed consent. In this study, we calculated the minimum number of subjects using the multiple linear regression equation, resulting in a requirement of 90 patients. For path analysis, following the rule of thumb suggests a minimum of 5 observations per estimate parameter, and considering a total of 15 estimated parameters, a minimum sample of 75 patients was necessary. Eventually, we used the larger minimum sample size, which is 90 patients.
Out of all patients referred or diagnosed with COPD (n = 620) in four different hospitals from January 2022 to December 2023, we included only 114 patients aged 40–80 years, with a FEV1/FVC value of <70%, no exacerbations in the previous month, and had basic literacy skills (elementary school level). Exclusion criteria included subjects with a history of severe anxiety disorders and depression (evaluated using General Anxiety Disorder-7 [GAD-7] and Patient Health Questionnaire-9 [PHQ-9]), communication difficulties, history of head injury, stroke, brain tumor, epilepsy, brain injury, active pulmonary tuberculosis, post-TB obstruction syndrome, cystic fibrosis, interstitial lung disease, lung cancer, and history of COVID-19 in the last one month, history of lung or brain surgery, lung or brain radiotherapy, and individuals with alcoholism or a history of drug abuse.
Spirometry
A spirometry test (EasyOnePro®, ndd Medical Technologies) was done to obtain lung function data of vital capacity (VC), forced vital capacity (FVC), and forced expiratory volume at 1s (FEV1) pre- and post-bronchodilator. Variables used in this study are FEV1/FVC (%) for diagnosing COPD and FEV1 pp (%) for classifying COPD severity based on GOLD criteria. All spirometry assessment performed is in accordance with the American Thoracic Society (ATS).Citation15
Quantitative CT
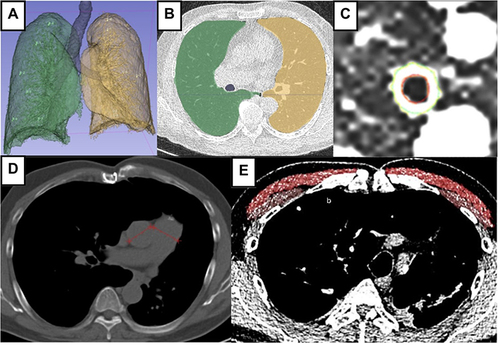
All thoracic CT acquisitions use 128-slice HRCT (Siemens Perspective) with full inspiration without contrast with the following parameters: 120−140 kVP, 30−60 mAs, and matrix size 512 × 512 pixels. The CT image was then reconstructed using a standard algorithm with a cut thickness of 0.625 mm and a cut interval of 5 mm, a window width of 1500 HU, and a window level of −600 HU. All the images were reconstructed and transferred to workstations for QCT assessment (%LAA−950, %WA, PA:A, PMA, and PMD) using 3D Slicer software version 5.30. The %LAA−950 parameter was performed throughout the lungs with a density limit setting of <- 950 HU. We restricted the measurement of %WA in the 3rd generation bronchi in the right upper lobe of the lung due to its location being perpendicular to the imaging plane, making it appear circular in the standard axial imaging plane and less susceptible to motion artifact.Citation16–19 Moreover, Nakano et alCitation20 showed that the assessment of large airway dimensions can reflect pathological abnormalities in the smaller airways and evaluation of the airway walls in smaller airways increased the risk of overestimation in CT scans. The ratio of PA:A was taken from the largest diameter value of the pulmonary artery and aorta in one level in the axial cut. The ratio of PA:A >1 is used due to numerous studies that have demonstrated its correlation with more severe COPD manifestations and its significant link to the presence of pulmonary hypertension.Citation21–23 PMA and PMD measurements were performed at the axial cut just above the aortic arch, and segmentation was carried out to describe the region of interest (ROI) (). An agreement regarding the location determination of %WA, pulmonary artery and aorta diameter assessment, as well as pectoralis delineation was reached by three radiologists.
Figure 1 QCT evaluation using 3D slicer software. (A and B) Density evaluation based on %LAA−950 after lung segmentation to define emphysema. (C) Airway evaluation in the third generation of the right lung resulting in %WA. (D) An HRCT axial view, mediastinum window to measure PA:A ratio and (E) Measurement PMA and PMD on the level right above the aortic arch showing pectoralis muscle segmentation (bright red = major pectoralis muscle; brownish red = minor pectoralis muscle).
HIF-1α Analysis
From peripheral venous, 3 mL of blood was collected. The blood sample was centrifuged at 3000 rpm for 10 minutes at 25°C to separate serum sample from blood products. Blood serum was then stored at −80°C before the examination was carried out simultaneously. HIF-1α examination was performed using Human HIF-1α ELISA Kit ab171577 (SimpleStep ELISA® microplate) reagent. The detection technique used in this assessment is colorimetric, set at a wavelength of 450 nm, using a microplate spectrophotometer [Multiskan GO, Thermo scientific] through SkanIt 3.2 software.
Cognitive Assessment Tool
All included subjects’ cognitive functions were assessed by two neurologists using the Indonesian version of the Montreal Cognitive Assessment (MoCA-Ina). This test primarily examined visuospatial/executive skills (trail-making test, copying a 3D cube, and drawing a clock following verbal commands), naming ability (identifying three animal figures), memory performances (memorizing and recalling a five-word list after a delay), attention capabilities (direct and inverse span, interference inhibition test, and a serial subtraction of 100–7), language proficiency (repetition of two complex sentences and phonological fluency), abstraction skills (quick analogy test). This test only took about 10 minutes with a total score of 30 and the common score used for diagnosing MCI is 26.Citation24
Statistical Analysis
Statistical analysis of this study used Statistical Program for Social Science (SPSS) version 26. The comparison of subjects’ characteristics was analyzed with univariate analysis of variance (ANOVA), while variables were categorical using the chi-square test. All data are presented as absolute numbers, mean ± standard deviation (SD), and percentages. Correlation analyses using the Pearson test assessed the clinical and radiological parameters’ correlation with cognitive scores. Variables with a correlation score <0.25 were then included in multiple linear regression analysis to evaluate the association between variables. Path analysis using Jeffrey's Amazing Statistics Program (JASP) was conducted to find the magnitude of influence and interaction between clinical and radiological parameter variables with cognitive scores.
Results
There were 114 subjects in GOLD I–IV included in this study, and subjects’ characteristics were stratified by GOLD (). Significant differences between GOLD I–IV were seen among clinical parameters, except education duration, Brinkman index, MoCA-Ina score, and HIF-1α. However, among QCT parameters, only %LAA−950 (p < 0.001), indicating emphysema, showed significant differences between GOLD, and others do not show substantial differences.
Table 1 Participants’ Characteristics
showed that only education duration positively correlates with MoCA-Ina score (r = 0.40; p < 0.001). In the categorical comparison (), besides education duration (p = 0.008), BMI was significantly associated with MoCA-Ina score (p = 0.003). No other QCT or clinical parameters were correlated with the MoCA-Ina score.
Table 2 Correlation of QCT Parameters, Lung Function, HIF-1α, and Clinical Factors with MoCA-Ina Score
Table 3 Comparison of QCT Parameters, Lung Function, HIF-1α, and Clinical Factors with MoCA-Ina Score
Variables analyzed in the multiple linear regression are BMI, education duration, HIF-1α, %LAA, %WA, and FEV1 pp. The data distribution was normal, and there was no heteroscedasticity. All variables had tolerance values greater than 0.1, and the VIF values were less than 10, indicating no multicollinearity. The Durbin-Watson value of 2.223 was greater than the dU of the Durbin-Watson table value of 2.2131, showing no autocorrelation. According to the linear regression results, education duration (p = 0.01) and %LAA−950 (p = 0.02) can be independent predictors of CI ().
Table 4 Predictor Value Toward CI
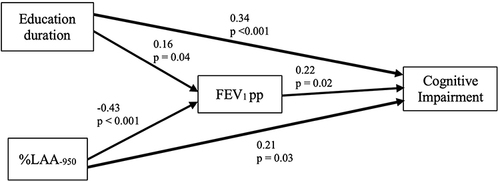
To understand the role of the MoCA-Ina score, we used path analysis. As shown in , and , the final model highlights our result that education duration and %LAA−950 significantly impact cognitive impairment (CI), both directly and indirectly, through FEV1 pp. The model fits the data well, indicated by a CFI of 0.99, RMSEA of 0.04, and a chi-square value of 4.73 at 12 degrees of freedom (p = 0.32).
Table 5 Qualification Measurement of Path Analysis with Cognitive Impairment
Table 6 The Regression Coefficient of Path Analysis with Cognitive Impairment
Figure 2 Path analysis model with cognitive impairment. Both %LAA−950 and education duration directly and indirectly through FEV1 pp contribute to CI development.
Discussion
All subjects in this study have a mean MoCA-Ina score ranging from 22 to 23, indicating the presence of CI across various stages of COPD. Despite the absence of statistical differences, the MoCA-Ina score decreased with increasing GOLD severity. However, there is still a significant positive correlation with education duration, as anticipated. According to the theory of cognitive reserve, which states that education can preserve cognitive abilities, even in the face of physiological and anatomical changes associated with aging and illness.Citation25 This preservation potentially inhibits the manifestation of clinical symptoms and signs of disease and thus, explains our findings. The consistent educational duration data observed across all GOLD groups likely contribute to the limited variations in MoCA-Ina scores among different COPD severity groups.Citation26,Citation27
BMI has significant differences among GOLD severity, indicating that lower weight is associated with worsening lung function. This inverse relationship where increased COPD severity leads to weight loss and cachexia may be driven by higher energy consumption, reduced peripheral oxygen availability, muscle disuse, and systemic inflammation.Citation28 Despite its significant differences, there was no correlation between BMI and CI. Diverse theories exist about the BMI-CI connection. Mun, et alCitation29 stated that higher BMI has been linked to better neurocognitive scores, suggesting minimal CI risk. While Qu, et alCitation30 reported midlife obesity is associated with higher dementia risk. The obesity paradox theory suggests that in older adults, obesity may protect cognitive function due to increased availability of vitamins and microelements, infrequent occurrence of adipose tissue dysfunction, elevated estrogen levels, and metabolic medications, including metformin, statin, and acetylsalicylic acid, which may improve clinical condition and also delaying the aging process.Citation31
Emphysema, as indicated by %LAA−950, significantly differs across the GOLD group. The increase in %LAA−950 confirms emphysema progression in advanced COPD stages.Citation32 This aligns with recent findings that local changes in alveolar micromechanics within damaged lung may drive lung injury progression that showed an increase in %LAA−950 along GOLD stages.Citation33–36 This study noted a decrease in %WA with increasing GOLD severity, consistent with previous research.Citation32 This occurrence may be attributed to the impact of the airway size and wall thickness on the %WA result. When both factors increase in size or other factors, such as lung distortion, alter the airway’s standard size and shape, the %WA value may not accurately reflect obstruction. In emphysematous conditions, lung parenchyma destruction destabilizes the airway, leading to collapse.Citation37 Another explanation could be that higher GOLD grades are more associated with irreversible airway damage and degradation, while lower GOLD grades are associated with a higher of %WA due to the presence of smooth muscle hypertrophy and hyper-responsiveness of the airway.Citation32
Although the education duration is the only variable that correlates with the MoCA-Ina score, our multivariate regression analysis has revealed that both %LAA and education duration can independently predict CI. This indicates that emphysema quantification and CI are better understood when considering multiple predictors simultaneously. Path analysis reveals that CI, measured by MoCA-Ina score, is directly influenced by %LAA−950 and education duration and indirectly through FEV1 pp. Emphysema’s direct correlation with CI may involve reduced acetylcholine (ACh) production, a crucial neurotransmitter that influences neuronal excitability and is linked to an oxygen-dependent enzyme. It is proposed that even brief episodes of hypoxia, lasting as little as 15 minutes, could lead to a decline in ACh synthesis.Citation7 In non-hypoxic, CI may occur due to the presence of arterial stiffness. Emphysema is thought to correlate with arterial stiffness due to shared susceptibility to elastin degradation both in the lungs and major vessels.Citation38 Maclay et alCitation38 observed systemic inflammation and reactive oxygen species, which are abundant among COPD patients, also contribute to arterial stiffness through extrapulmonary elastin degradation. However, the specific biomolecular mechanism linking COPD and CI remains unclear, as the HIF-1ɑ result did not correlate with GOLD severity or MoCA-Ina score.
Our study found that HIF-1α has neither direct nor indirect effect on CI. HIF, a transcription factor activated by hypoxia, includes HIF-1 and HIF-2 responding to acute hypoxia due to a reduction of prolyl hydroxylase (PHD) activity.Citation39 HIF-1 expression peaks after 4 hours of hypoxia, decreasing significantly by 8 hours, while HIF-2 expression peaks at 8 hours and remains elevated for 24 hours.Citation40 During prolonged hypoxia, there is a sequential overlap of HIF-1 and HIF-2 responses, referred to as the HIF switch. Cellular oxygen redistribution leads to PHD reactivation, restarting HIF-1α degradation. Reduced histone deacetylase 2 and 7 (HDAC-2 and HDAC-7) in severe COPD may inhibit HIF-1α activation, leading to inadequate hypoxic response.Citation41,Citation42 The absence of HIF-1α expression in our study may be due to the predominance of severe COPD subjects and stable condition participants, none experiencing acute hypoxia during recruitment. We suggested that low-grade inflammation, a hallmark of COPD, is linked to various extrapulmonary manifestations and could contribute to cognitive decline. COPD patients have elevated levels of inflammatory markers like TNF-α, IL-6, IL-8, CRP, and nitrites/nitrates (NOx).Citation43 These markers can cross the blood–brain barrier, potentially triggering neuroinflammation and affecting cognitive function, similar to the mechanism involved in AD.Citation44,Citation45
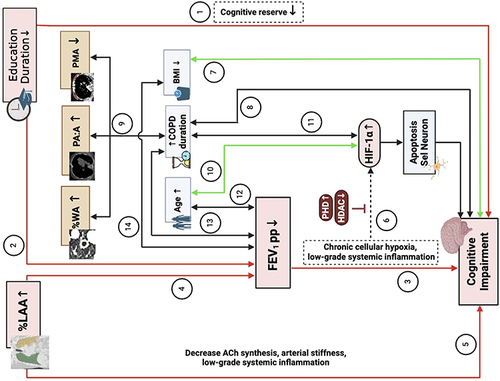
Considering the result of our study and other literature, we proposed mechanisms of CI development in individuals with COPD (). Firstly, shorter education duration affects CI both directly and indirectly through a decreased cognitive reserve and FEV1 pp reduction. Destruction of the lung parenchyma, represented by %LAA, will also, directly and indirectly, result in CI due to a larger emphysematous area, which will have a lower FEV1 pp value, indicating hypoxia. Other QCT parameters such as %WA, PA:A, and PMA are supposedly affected by the COPD duration to influence CI significantly. HIF-1α serves as a mediator that triggers neuronal cell apoptosis. However, low-grade systemic inflammation may also play a role in CI development in chronic conditions.
Figure 3 Proposed Mechanism of CI Development in COPD. ① Duration of education affects directly in CI, ② Duration of education affects indirectly in CI through FEV1 pp, ③ FEV1 pp affects directly in CI, ④ %LAA indirectly affects in CI through FEV1 pp, ⑤ %LAA directly affects in CI, ⑥ Chronic cellular hypoxia and low-grade inflammation induce HIF-1α expression that cause neuron cell apoptosis and CI, ⑦ BMI correlates with CI, ⑧ Duration of COPD correlates with CI, ⑨ Duration of COPD that correlates with %WA, PA:A, and PM, ⑩ Age correlates with HIF-1α, ⑪ Duration of COPD correlates with CI, ⑫ Age correlates with FEV1, ⑬ Duration of COPD correlates with FEV1, ⑭ BMI correlates with FEV1.
The study’s strengths include its multicentre design at a referral hospital, the high response rate, and the use of identical CT scan models. This study offers new insights into COPD, highlighting how QCT parameters, particularly %LAA, can represent lung function and predict CI. Limitations of this study are not considering comorbidities like diabetes or SLE that cause low-grade inflammation, not incorporating other lung parameters and lung vessel disease that could contribute to a broader comprehension of the correlation between those variables and not confirming brain pathology through brain imaging. Thus, we suggest future research to consider low-grade inflammation causes, quantitative assessment of small pulmonary vessels, and brain imaging to strengthen findings and explore other mechanisms of CI in COPD.
Conclusion
In conclusion, cognitive impairment in COPD patients is directly and indirectly influenced by %LAA and education duration through FEV1 pp. No link was found between HIF-1α and CI in COPD patients, suggesting low-grade systemic inflammation might pose a better mechanism. Our study highlights the importance of using QCT parameters, lung function, and clinical variables in evaluating and managing COPD.
Ethical Approval and Consent to Participate
This study received two ethical approvals, that is, Ethical Committee of Persahabatan Hospital (no: 02/KEPK-RSUPP/01/2022), which allow us to recruit participants from Persahabatan Hospital from January 2022. Another ethical approval released by Faculty of Medicine, University of Indonesia (no: KET-133/UN2.F1/ETIK/PPM.00.02/2022), which allow us to recruit participants from three other hospitals (Cipto Mangunkusumo Hospital, Gatot Soebroto Army Hospital, and Jakarta Islamic Hospital Cempaka Putih) from February 2022. All procedures used in this study adhered to the institutional and national research committee’s ethical requirements and the Declaration of Helsinki. All participants in this study are adults and have given a written informed consent before enrolled in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We are grateful to all participants of the project and the research members for their valuable contributions and assistance with the data collection process so that we can complete this scientific paper.
Additional information
Funding
References
- GOLD Report - Global Initiative for Chronic Obstructive Lung Disease – GOLD. 2024; Available from:. https://goldcopd.org/2024-gold-report/. Accessed February 15, 2024
- Siraj RA. Comorbid Cognitive Impairment in Chronic Obstructive Pulmonary Disease (COPD): current Understanding, Risk Factors, Implications for Clinical Practice, and Suggested Interventions. Medicina. 2023;59(4). doi:10.3390/medicina59040732
- Thaipisuttikul P, Jaikla K, Satthong S, et al. Rate of conversion from mild cognitive impairment to dementia in a Thai hospital-based population: a retrospective cohort. Alzheimer's Dementia. 2022;8(1)
- Rong B, Liu Y, Li M, Fu T, Gao W, Liu H. Correlation of serum levels of HIF-1α and IL-19 with the disease progression of COPD: a retrospective study. Int J Chron Obstruct Pulmon Dis. 2018;13:3791–3803. doi:10.2147/COPD.S177034
- Dodd JW. Lung disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther. 2015;7(1). doi:10.1186/s13195-015-0116-3
- Li J, Huang Y, Fei GH. The evaluation of cognitive impairment and relevant factors in patients with chronic obstructive pulmonary disease. Respiration. 2013;85(2):98–105. doi:10.1159/000342970
- Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35(4):913–922. doi:10.1183/09031936.00125109
- Kurashima K, Fukuda C, Nakamoto K, et al. CT-diagnosed emphysema and prognosis of chronic airflow obstruction: a retrospective study. BMJ Open. 2013;3(11):3541. doi:10.1136/bmjopen-2013-003541
- Han MLK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi:10.1148/radiol.11110173
- Fernandes L, Fernandes Y, Mesquita A. Quantitative computed tomography imaging in chronic obstructive pulmonary disease. Lung India. 2016;33(6):646. doi:10.4103/0970-2113.192880
- Schroeder JD, McKenzie AS, Zach JA, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2013;201(3):W460–W470. doi:10.2214/AJR.12.10102
- Bak SH, Kwon SO, Han SS, et al. Computed tomography-derived area and density of pectoralis muscle associated disease severity and longitudinal changes in chronic obstructive pulmonary disease: a case control study. Respir Res. 2019;20(1). doi:10.1186/s12931-019-1191-y
- Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi:10.1056/NEJMoa1203830
- McDonald MLN, Diaz AA, Ross JC, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014;11(3):326–334. doi:10.1513/AnnalsATS.201307-229OC
- Graham BL, Steenbruggen I, Barjaktarevic IZ, et al. Standardization of Spirometry 2019 UpdateAn Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):E70–E88.
- Yamashiro T, Matsuoka S, San José Estépar R, et al. Quantitative assessment of bronchial wall attenuation with thin-section CT: an indicator of airflow limitation in chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2010;195(2):363–369. doi:10.2214/AJR.09.3653
- Takayanagi T, Suzuki S, Katada Y, et al. Comparison of Motion Artifacts on CT Images Obtained in the Ultrafast Scan Mode and Conventional Scan Mode for Unconscious Patients in the Emergency Department. AJR Am J Roentgenol. 2019;213(4):153–161. doi:10.2214/AJR.19.21456
- Dudurych I, Muiser S, McVeigh N, et al. Bronchial wall parameters on CT in healthy never-smoking, smoking, COPD, and asthma populations: a systematic review and meta-analysis. Eur Radiol. 2022;32(8):5308. doi:10.1007/s00330-022-08600-1
- Koyama H, Ohno Y, Nishio M, et al. Three-dimensional airway lumen volumetry: comparison with bronchial wall area and parenchymal densitometry in assessment of airway obstruction in pulmonary emphysema. Br J Radiol. 2012;85(1020):1525. doi:10.1259/bjr/22602417
- Nakano Y, Wong JC, De Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142–146. doi:10.1164/rccm.200407-874OC
- Schneider M, Ran H, Pistritto AM, et al. Pulmonary artery to ascending aorta ratio by echocardiography: a strong predictor for presence and severity of pulmonary hypertension. PLoS One. 2020;15(7):e0235716. doi:10.1371/journal.pone.0235716
- Malenfant S, Brassard P, Paquette M, et al. Compromised Cerebrovascular Regulation and Cerebral Oxygenation in Pulmonary Arterial Hypertension. J Am Heart Assoc. 2017;6(10). doi:10.1161/JAHA.117.006126
- Wu XG, Shi YJ, Wang XH, et al. Diagnostic value of computed tomography‐based pulmonary artery to aorta ratio measurement in chronic obstructive pulmonary disease with pulmonary hypertension: a systematic review and meta‐analysis. Clin Respir J. 2022;16(4):276–283. doi:10.1111/crj.13485
- Sun R, Ge B, Wu S, et al. Optimal cut-off MoCA score for screening for mild cognitive impairment in elderly individuals in China: a systematic review and meta-analysis. Asian J Psychiatr. 2023;87:103691. doi:10.1016/j.ajp.2023.103691
- Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: a non-parametric systematic review. Psychol Med. 2006;36(8):1065–1073. doi:10.1017/S0033291706007744
- Meng X, D’Arcy C, Laks J. Education and Dementia in the Context of the Cognitive Reserve Hypothesis: a Systematic Review with Meta-Analyses and Qualitative Analyses. PLoS One. 2012;7(6). doi:10.1371/journal.pone.0038268
- Clouston SAP, Smith DM, Mukherjee S, et al. Education and Cognitive Decline: an Integrative Analysis of Global Longitudinal Studies of Cognitive Aging. J Gerontol B Psychol Sci Soc Sci. 2020;75(7):E151–E160. doi:10.1093/geronb/gbz053
- Sun Y, Milne S, Jaw JE, et al. BMI is associated with FEV1 decline in chronic obstructive pulmonary disease: a meta-analysis of clinical trials. Respir Res. 2019;20(1):1–10. doi:10.1186/s12931-019-1209-5
- Mun YS, Park HK, Kim J, et al. Association Between Body Mass Index and Cognitive Function in Mild Cognitive Impairment Regardless of APOE ε4 Status. Dement Neurocogn Disord. 2022;21(1):30. doi:10.12779/dnd.2022.21.1.30
- Qu Y, Hu HY, Ou YN, et al. Association of body mass index with risk of cognitive impairment and dementia: a systematic review and meta-analysis of prospective studies. Neurosci Biobehav Rev. 2020;115:189–198. doi:10.1016/j.neubiorev.2020.05.012
- Puzianowska-Kuznicka M, Kuryłowicz A, Walkiewicz D, et al. Obesity Paradox in Caucasian Seniors: results of the PolSenior Study. J Nutr Health Aging. 2019;23(9):796–804. doi:10.1007/s12603-019-1257-z
- Konietzke P, Wielpütz MO, Wagner WL, et al. Quantitative CT detects progression in COPD patients with severe emphysema in a 3-month interval. Eur Radiol. 2020;30(5):2502–2512. doi:10.1007/s00330-019-06577-y
- Martini K, Frauenfelder T. Emphysema and lung volume reduction: the role of radiology. J Thorac Dis. 2018;10(Suppl 23):S2719–S2731. doi:10.21037/jtd.2018.05.117
- Harmouche R, Ross JC, Diaz AA, et al. A Robust Emphysema Severity Measure Based on Disease Subtypes. Acad Radiol. 2016;23(4):421–428. doi:10.1016/j.acra.2015.12.021
- Mizusawa H, Shiraishi O, Shiraishi M, et al. Quantitative emphysema on computed tomography imaging of chest is a risk factor for prognosis of esophagectomy: a retrospective cohort study. Medicine. 2023;102(41):E35547.
- Knudsen L, Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem Cell Biol. 2018;150(6):661–676. doi:10.1007/s00418-018-1747-9
- Martinez CH, Richardson CR, Han MLK, et al. Chronic obstructive pulmonary disease, cognitive impairment, and development of disability: the health and retirement study. Ann Am Thorac Soc. 2014;11(9):1362–1370. doi:10.1513/AnnalsATS.201405-187OC
- Maclay JD, McAllister DA, Rabinovich R, et al. Systemic elastin degradation in chronic obstructive pulmonary disease. Thorax. 2012;67(7):606–612. doi:10.1136/thoraxjnl-2011-200949
- Ginouvès A, Ilc K, Macías N, et al. PHDs overactivation during chronic hypoxia “desensitizes” HIFα and protects cells from necrosis. Proc Natl Acad Sci U S A. 2008;105(12):4745–4750. doi:10.1073/pnas.0705680105
- Jaśkiewicz M, Moszyńska A, Króliczewski J, et al. The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell Mol Biol Lett. 2022;27(1):1–19. doi:10.1186/s11658-022-00408-7
- Yasuo M, Mizuno S, Kraskauskas D, et al. Hypoxia inducible factor-1α in human emphysema lung tissue. Eur Respir J. 2011;37(4):775–783. doi:10.1183/09031936.00022910
- To M, Yamamura S, Akashi K, et al. Defect of adaptation to hypoxia in patients with COPD due to reduction of histone deacetylase 7. Chest. 2012;141(5):1233–1242. doi:10.1378/chest.11-1536
- Fard MT, Cribb L, Nolidin K, Savage K, Wesnes K, Stough C. Is there a relationship between low-grade systemic inflammation and cognition in healthy people aged 60-75 years? Behav Brain Res. 2020;383.
- Dyer AH, McNulty H, Caffrey A, et al. Low-Grade systemic inflammation is associated with domain-specific cognitive performance and cognitive decline in older adults: data from the TUDA study. Neurobiol Aging. 2024;134:94–105. doi:10.1016/j.neurobiolaging.2023.11.008
- Kipinoinen T, Toppala S, Rinne JO, et al. Association of Midlife Inflammatory Markers with Cognitive Performance at 10-Year Follow-up. Neurology. 2022;99(20):E2294–E2302. doi:10.1212/WNL.0000000000201116
