Abstract
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease characterized by airflow limitation that is not fully reversible. Bronchodilator therapy is the cornerstone in COPD treatment. Bronchodilation in COPD is mainly achieved via administration of long- and ultralong-acting β2-agonists and with long-acting muscarinic antagonists. New combinations of bronchodilators with dual-acting muscarinic antagonist and β2-agonist properties have been licensed, and others are currently being developed with the aim of achieving once-daily dosing, and therefore may improve the likelihood of treatment compliance. These combination bronchodilators include glycopyrronium bromide/indacaterol maleate, umeclidinium (UMEC) bromide/vilanterol trifenatate (VI), aclidinium bromide/formoterol and tiotropium bromide/olodaterol (Boehringer Ingelheim, Germany). This review will focus mainly on studies and clinical trials involving the novel fixed-dose combination of UMEC/VI at doses of 125/25 μg and 62.5/25 μg in patients with COPD. Data from large clinical trials involving more than 4,500 COPD patients indicate that UMEC/VI is an effective once-daily treatment in COPD with improved pulmonary function. Future studies assessing the impact of this combination on exacerbations, delay in disease progression, and health status in patients with COPD are warranted.
Introduction
The American Thoracic and the European Respiratory Societies define chronic obstructive pulmonary disease (COPD) as a preventable and treatable disease, characterized by airflow limitation that is not fully reversible, in contrast to asthma, where airflow obstruction is usually reversible.Citation1 It includes chronic obstructive bronchiolitis involving the small airways, and emphysema that results in destruction of lung parenchyma, loss of lung elasticity, and closure of small airways. These new statements of COPD being preventable and treatable underline the importance of carefully optimizing therapy in COPD patients. With the recent increase in the number of available pharmacological agents, there is more choice, improving the decision-making process in managing COPD patients.
In moderate-to-severe disease and in respiratory impairment, the coadministration of different bronchodilator classes has been demonstrated to be more effective in subjective and objective COPD parameters in comparison with the use of a single drug class.Citation2 Bronchodilator treatment in COPD is mainly via administration of long- and ultralong-acting β2-agonists (LABAs) and with long-acting muscarinic antagonists (LAMAs). β2-agonists are sympathomimetic agents that stimulate β2 receptors in airway cells to produce a variety of effects, mainly smooth muscle relaxation and bronchodilation.Citation3 β2-agonists are classified as short-acting, with a 3–6-hour duration of action, and LABAs have a duration of action ≥12 hours. The difference in onset of action is related to the lipophilicity of each of these agents and their ability to activate the receptor.Citation4
The parasympathetic activity in the airways of medium–large caliber is mediated through the muscarinic receptors (M1 and M3) and acts on smooth muscle contraction and mucus secretion of airways while the effects on M2 receptors inhibit acetylcholine release from nerve terminals. Increased cholinergic tone is involved in the pathogenesis of COPD, contributing to both the increase in bronchial smooth muscle tone and mucus hypersecretion.Citation5 In fact, anticholinergics reduce airway tone and improve expiratory flow limitation, hyperinflation, and exercise capacity in patients with COPD. With the pivotal role of bronchodilators in the treatment of obstructive airway diseases, there is a relevant interest in finding novel classes of bronchodilator drugs. Currently, new classes of bronchodilators have been discovered, providing impetus to try new associations of LABA and LAMA in addition to inhaled corticosteroid therapy for the long-term treatment of COPD.
New combinations of bronchodilators with dual-acting muscarinic antagonist and β2-agonist activity are licensed, and others are currently being developed with the aim of achieving once-daily dosing, thereby improving the likelihood of treatment compliance. These include the recently licensed glycopyrronium bromide/indacaterol maleate (QVA149 [Ulitbro®]; Novartis International AG, Basel, Switzerland) (licensed in Japan and Europe) and umeclidinium bromide/vilanterol trifenatate (Anoro®; GlaxoSmithKline plc, London, UK) (licensed in USA). Other fixed-dose combination inhalers, aclidinium bromide/formoterol (Almirall, Barcelona, Spain) and tiotropium bromide/olodaterol (Boehringer Ingelheim, Ingelheim, Germany), are still being assessed. All four of these combination bronchodilator compounds combine two separate molecules in a single novel dry powder delivery device including the Breezehaler®, Ellipta®, Genuair®, and Respimat®, respectively. Another technological advance in the development of combination bronchodilators is the invention of muscarinic receptor antagonist and β2-adrenoceptor agonist molecules. These compounds have the dual pharmacological activities of LAMA and LABA but are bifunctional molecules with muscarinic antagonistic activity at one end, separated by an inert linker portion from the β2-agonist at the other end. These molecules are currently in Phase I and II of development and hold great promise for the future.
In this review, we mainly focus on studies and clinical trials involving the novel, fixed-dose combination of vilanterol trifenatate (VI) and umeclidinium (UMEC), UMEC/VI, at doses of 125/25 μg and 62.5/25 μg in patients with COPD. The association of UMEC/VI (62.5/25 μg) has been newly licensed for once-daily, routine treatment in COPD patients.Citation6 Prior to delving into the combination UMEC/VI bronchodilator use in COPD, we briefly summarize the two components.
UMEC characteristics
UMEC is a novel anticholinergic agent, similar to tiotropium bromide, with a strong affinity to M3 receptors. It is rapidly absorbed (time to reach maximal concentration [Tmax], 5–15 minutes) and presents slow functional reversibility at the M3 receptor, hence manifesting faster onset and prolonged duration of action.Citation7 Moreover, it is safe, as 1%–2% of the total dose is excreted unchanged in the urine (Tmax, 5–15 minutes) after single and repeat doses, and 1.5–1.9-fold accumulation is observed after repeat dosing.Citation8 Single and repeat doses of UMEC were well tolerated and produced clinically relevant 24 hour lung function improvements in patients with COPD.Citation8 The dose-response efficacy of once-daily UMEC in moderate-to-severe COPD patients using 125, 250, and 500 μg UMEC showed significantly improved forced expiratory volume in 1 second (FEV1) versus placebo and was well tolerated.Citation9 In another 12-week study in COPD subjects, besides lung function improvements, patients administered UMEC 62.5 μg and 125 μg had improved breathlessness symptoms and enhanced health status.Citation10
VI characteristics
VI is a potent, selective β2-adrenoreceptor agonist with a long duration of action. It has greater potency than indacaterol and salbutamol and greater intrinsic efficacy than salmeterol.Citation11 VI has significantly greater selectivity for β2-adrenoreceptor than formoterol, indacaterol, and albuterol.Citation12,Citation13 Moreover, it is a metabolically labile LABA that is converted into metabolites with significantly lower β2 activity. Following an inhaled dose, VI shows low systemic absorption,Citation14 is rapidly absorbed (median Tmax of 10 minutes), and shows no safety issues, especially cardiovascular.Citation15,Citation16 A single dose of VI (25–100 μg) was not only well tolerated in COPD patients, but it was also reported to have a rapid onset (5 minutes) and prolonged bronchodilation (25 hours), hence it was suitable for once-daily administration.Citation17 These efficacy and safety endpoints were also confirmed in moderate-to-severe COPD subjects in a 28-day Phase IIb study.Citation18
Adverse effects
UMEC per se, being an anticholinergic, has a good safety and tolerability profile. More recently approved inhaled anticholinergic agents are well tolerated because they are very poorly absorbed following inhalation. Anticholinergic compounds are more commonly associated with the manifestation of dry mouth, constipation, dyspepsia, gastroesophageal reflux, urinary difficulties, papillary dilatation, blurred vision with the possibility of worsening glaucoma symptoms, and paradoxical bronchoconstriction.Citation19–Citation24 The latter has been postulated to arise from blockade of prejunctional M2 receptor on airway cholinergic nerves, which normally inhibit acetylcholine release. Of note, alarm has been raised about possible associations of anticholinergics with cardiovascular morbidity and mortality.Citation25 However, the UPLIFT trial failed to find an increased risk in mortality or cardiovascular morbidity during treatment in patients with COPD.Citation26 Controversially, a systematic review and meta-analysis observed that tiotropium administered via the Respimat® preparation was associated with a statistically significant increased risk of mortality, probably related to a greater absorption of tiotropium into the systemic circulation due to the new formulation.Citation27 However, this has been disproved in a study of over 17,000 patients conducted more recently.Citation28,Citation29
Similarly, VI seems safe and well tolerated; however, being a β2-agonist, it has potential side effects related to the stimulation of the β-adrenergic receptor. This is mainly due to the widespread distribution of β2-adrenoreceptors, which when β2 agonists are absorbed into the systemic circulation, result in undesired responses. These effects comprise palpitations with heart rate elevation, arrhythmias (particularly supraventricular tachycardias), nervousness, tremor, anxiety, hypokalemia, glycogenolysis and hyperglycemia, and transient airway obstruction.Citation30–Citation34
UMEC and VI
In December 2013, the combination bronchodilator UMEC/VI (62.5/25 μg) in a single inhaler was approved in the USA.Citation6 The development program for UMEC/VI leading to US Food and Drug Administration (FDA) approval implicated several Phase I studies and four large 24-week pivotal Phase II/III trials. We have summarized the studies discussed in with the main endpoints assessed.
Table 1 Characteristics of the clinical studies on UMEC/VI
Phase I UMEC/VI studies
UMEC (500 μg) and VI (50 μg) administered separately and in combination using a novel dry powder inhaler, Ellipta®, in 16 healthy Japanese males showed that in all treatment arms, the administered medication was well tolerated.Citation35 Pharmacokinetic (PK) evaluation demonstrated rapid absorption (Tmax 5 minutes for both) followed by rapid elimination with median Tlast (time to last measurable concentration) of 4–5 hours for UMEC and median Tlast of 1.5–2.0 hours for VI.
In another study by the same group, the effects of 10 days of inhaled UMEC/VI 125/25 μg and 500/100 μg (supratherapeutic dose) and UMEC alone (500 μg) on the QT interval (Fridericia’s correction [QTcF]) in 103 healthy subjects was compared with placebo and moxifloxacin treatment (positive control).Citation36 Although there was no observed difference in QTcF between UMEC/VI or UMEC monotherapy and placebo (while moxifloxacin showed a significant increase in QTcF), treatment with UMEC/VI and UMEC alone were associated with an increase in heart rate compared with placebo. The maximum increase of heart rate was observed 10 minutes postdose, with a rapid normalization. Moreover, 20% of the participants treated with UMEC/VI (500/100 μg) reported palpitation without any electrocardiograph abnormalities.
To evaluate the effects of oral administration of verapamil (240 mg), an antiarrhythmic agent frequently used by patients with COPD and cardiovascular related comorbidities, the PK, pharmacodynamics, safety, and tolerability of inhaled UMEC 500 μg monotherapy and UMEC/VI 500/25 μg combination therapy has also been conducted.Citation37 Administration of UMEC alone and in combination with VI was well tolerated and did not show clinically relevant increases in systemic exposure for either drug.
Two additional studies have excluded significant adverse effects with the use of UMEC/VI in patients with moderate hepatic and severe renal impairment.Citation38,Citation39
Phase II UMEC/VI studies
Feldman et alCitation40 conducted a double-blind, placebo-controlled (DBPC) Phase II study that assessed the safety, tolerability, PK, and pharmacodynamics of inhaled UMEC/VI (500/25 μg) (n=42) administered once daily via the Ellipta® inhaler over 28 days compared to placebo (n=9) in subjects with COPD. UMEC/VI was noninferior to placebo for the primary end point: the mean change from baseline in 0–6 hours postdose weighted mean pulse rate after 28 days of treatment. Also, no significant differences were observed in blood pressure, minimum and maximum heart rate, and QTcF between the two treatment groups. Eleven (26%) patients reported adverse events in the UMEC/VI group compared to one patient in the placebo group; however, no serious adverse events were reported in either treatment groups. PK results confirmed rapid absorption (median Tmax ~6 minutes for both drugs), followed by a rapid decline in plasma concentrations, indicating rapid distribution and elimination of both drugs, with no evidence of accumulation.
Donohue et alCitation41 conducted a DBPC randomized, parallel-group study for the evaluation of the efficacy and safety of 24-week treatment with once-daily UMEC/VI (62.5/25 μg) compared with UMEC 62.5 μg and VI 25 μg monotherapies in 1,532 patients with moderate-to-severe COPD. All treatment groups resulted in significant improvements in trough FEV1 versus placebo (72–167 mL; all P<0.001), but increases with UMEC/VI combination was greater than that observed with monotherapies (52–95 mL; all P≤0.004). Compared to placebo, UMEC/VI was associated with improved rescue medication use, transition dyspnea index (TDI), health-related quality of life questionnaires compared to placebo. No significant changes in vital signs, electrocardiography, or laboratory parameters were noted.
Phase III UMEC/VI studies
In a Phase III DBPC parallel-group 24-week study, the efficacy and safety of once-daily UMEC/VI (125/25 μg) was compared to its component monotherapies and placebo in 1,493 patients with COPD.Citation42 All the active treatments resulted in significantly improved trough FEV1 versus placebo (124–238 mL; all P<0.001); in fact, the combination therapy resulted in significantly greater functional benefit compared to the monotherapy-treated arms of the study (79–114 mL; all P<0.001). Additionally, UMEC/VI combination treatment showed improvements in the TDI, rescue medication use, and health-related quality of life. The study did not report any safety concerns, and the numbers of adverse events were similar across all study arms.
The UMEC/VI 125/25 μg and 62.5/25 μg combinations have been compared with tiotropium (18 μg) and VI alone (25 μg) in a randomized, double-blind, active-controlled 24-week study involving 843 COPD patients.Citation43 Significant improvements in least squares mean change from baseline trough FEV1 and 0–6 hours weighted mean FEV1 was observed with both combination treatments compared with the monotherapies (P<0.005).
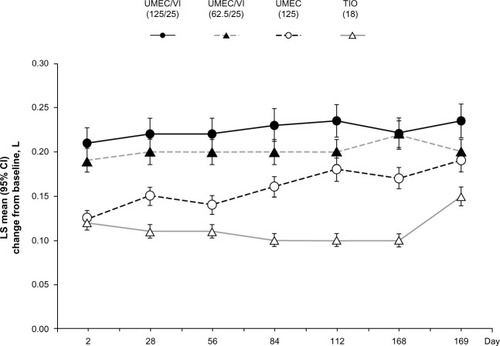
In another randomized, double-blind, active-controlled 24-week study in 869 COPD patients, the combination of UMEC/VI (125/25 μg and 62.5/25 μg) was compared with tiotropium (18 μg) and UMEC alone (125 μg).Citation44 Treatment with UMEC/VI 125/25 μg resulted in a statistically significant improvement in trough FEV1 at day 169 compared with tiotropium (P=0.003) but not UMEC 125 μg (P=0.142). An improvement was also observed with UMEC/VI 62.5/25 μg versus tiotropium (P=0.018) but not versus UMEC 125 μg (P=0.377).
Efficacy data
In all the Phase III placebo-controlled studies following 24-weeks treatment, trough FEV1 was significantly higher with both doses of UMEC/VI (62.5/25 μg and 125/25 μg) when compared with UMEC alone, VI alone, and placebo ().Citation41,Citation42 When both doses of UMEC/VI were compared with tiotropium and VI, a significant improvement in trough FEV1 was observed at day 169 and later ().Citation43 Interestingly, in a yet to be published trial, the preliminary data indicate that the comparison of UMEC/VI 125/25 μg and UMEC 125 μg with respect to trough FEV1 was not statistically significant ().Citation44 Statistically significant improvements in 0–6 hours postdose weighted mean FEV1 were also observed for UMEC/VI (62.5/25 μg and 125/25 μg doses) over VI and tiotropium.Citation43
Figure 1 Least squares mean (95% CI) change from baseline for trough FEV1.
Notes: Reprinted from Respir Med, 107, Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A, Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD, pages 1538–1546.Citation41 Copyright © 2013, with permission from Elsevier. Placebo not drawn.
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; LS, least squares; UMEC, umeclidinium; VI, vilanterol.
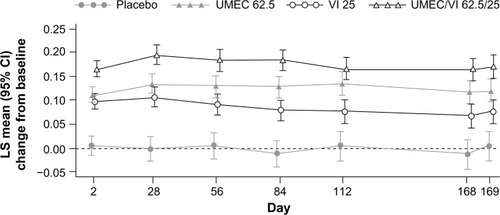
Figure 2 Least squares mean (95% CI) change from baseline for trough FEV1 in the treatment arms.
Note: Reprinted from Lancet Respir Med, 2, Decramer M, Anzueto A, Kerwin E, et al, Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials, pages 472–486.Citation43 Copyright © 2014, with permission from Elsevier.
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second.
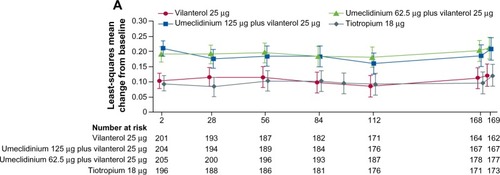
Figure 3 Least squares mean (95% CI) change from baseline for trough FEV1 in the treatment arms.
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; LS, least squares; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol; FDA, US Food and Drug Administration.
Additional findings in several Phase III studies showed that treatment with UMEC/VI was associated with significant improvements in some patient-centered secondary outcomes, like TDI, St George’s Respiratory Questionnaire (SGRQ) score, rescue albuterol use, and time to first COPD exacerbation.Citation41,Citation42 Moreover, both 125/25 μg and 62.5/25 μg UMEC/VI doses had significantly more patients achieving the minimal clinical important difference in TDI (P<0.001).
The incidence of COPD exacerbations observed during the studies was lower in UMEC/VI-treated patients compared with placebo (8% versus 14%).Citation41,Citation42 Moreover, patients on UMEC/VI had a lower risk of exacerbation versus placebo in the analysis of time to first exacerbation. However, it must be considered that the cited studies were not properly designed to measure exacerbations as a primary outcome, and all studies and data need to be considered for longer periods.
Safety data
Phase I and II studies have not revealed any significant adverse events in patients treated with the combination UMEC/VI at all considered dosages (125/25 μg and 62.5/25 μg). One study in particular analyzed the effects of a supratherapeutic dose (500/125 μg), observing only the occurrence of transient palpitations.Citation35 No clinically significant treatment-related modification in vital signs, electrocardiogram, or clinical laboratory parameters were observed for UMEC/VI compared with placebo. A 52-week safety Phase III study was also very recently completed but not yet published or presented.Citation45 In this study, the most common adverse events reported were headache and nasopharyngitis. However, the FDA approbation includes a warning on the use of UMEC/VI in patients with cardiovascular disease and has recommended postmarketing studies to better assess safety of the drug combination.Citation6 The FDA has included paradoxical bronchospasm, cardiovascular effects, acute narrow-angle glaucoma, and worsening of urinary retention as serious adverse effects.Citation6 More importantly, the drug carries a boxed warning stating that LABAs raise the risk for asthma-related death.Citation6 In fact, the safety and efficacy of the UMEC/VI combination have not been studied in patients with asthma, and hence is not approved for asthma treatment. It is also not proposed to be used as a rescue therapy for sudden bronchospasm.
Clinical perspectives
Currently, UMEC/VI (62.5/25 μg) combination bronchodilator is the only licensed therapy for use in COPD in the USA and is awaiting approval in other countries.
GOLD 2014 practice guidelines recommend the use of a LAMA or a LABA for symptom relief in patients with stable, relatively milder disease (GOLD stage A or B).Citation2 To maximize the effects of bronchodilation in patients with COPD not well controlled with long-acting bronchodilator therapy, adding a molecule with an alternative mechanism of action to bronchodilator activity is the preferred recommendation by various guidelines. In particular, guidelines suggest the use of dual therapy LAMA/LABA treatment alternative for patients in GOLD stage B–D. The combination of two classes of bronchodilators may not only improve lung function, but also lower the risk of adverse effects compared to increasing the dose of a single bronchodilator.
However, the measurement of FEV1 alone may not adequately reflect the overall health of a patient. Evidence suggests that the association of LAMA/LABA show greater improvements in patient-centered outcomes, such as dyspnea symptoms, use of rescue medications, and quality of life, compared to the drugs used individually. Moreover, the combination of LAMA/LABA was seen to better prevent exacerbations of COPD compared to monotherapy.
As discussed, the development of a new combination product relies on the development of the individual ingredient components. The selection of an appropriate dose and dosing frequency for each component is impacted by safety concerns specific to each drug class. LAMA and LABA bronchodilators cause smooth muscle relaxation in the airways, leading to airway expansion and improved lung function. UMEC and VI are intended as maintenance treatment to relieve the symptoms associated with COPD. In the clinical trials mentioned above, UMEC and VI were administered once daily in a single-dose dry powder, inhaled via a novel delivery device at 62.5 μg or 125 μg and 25 μg, respectively.
There was strong statistical evidence of beneficial effects of both UMEC/VI 62.5/25 μg and 125/25 μg as compared to placebo, with respect to the primary and secondary endpoints, in addition to supportive trends across a range of other spirometric and nonspirometric endpoints of interest. However, it is important to note that there was evidence of superiority against placebo for the proposed 62.5/25 μg dose of the combination product from only one published primary efficacy study.Citation41 Overall, the combination of UMEC/VI has been reported to be safe and well tolerated, besides being effective in improving lung function and symptoms, in clinical studies of over 4,500 patients with COPD.
COPD guidelines underline the concept of adherence to treatment as a cornerstone in the treatment of COPD, especially because the lack of adherence to therapy is common in these patients given the high frequency of comorbidities, often the advanced age, and the presence of complex multiple treatments. It is known that patients’ adherence was robustly related with dosing frequency.Citation31 Therefore once-daily dosing should be a strategic weapon to improve adherence. In this perspective, the development of combination treatment such as UMEC/VI could be of great interest to the health community. With this concept in mind, a number of novel monotherapy LAMAs and LABAs, as well as combination bronchodilators, are in development, some in innovative delivery devices (Morjaria, unpublished data, 2014).Citation47 These would not only add to the armamentarium of therapies for this debilitating condition, but also provide patients much-needed therapeutic alternatives, which have been previously lacking.
We are aware of the important effects of this combination on respiratory functional parameters; however, we are still lacking important data concerning the frequency of exacerbations, hospitalizations, and mortality of COPD patients. Moreover, new guidelines underline that symptoms and not just airflow limitation now have to be taken into account to guide the management of COPD.Citation48,Citation49 Although the combination of UMEC/VI has demonstrated good efficacy and safety in trials conducted to date, studies assessing the impact of this combination on exacerbations, delay in disease progression, and health status in patients with COPD are still needed. Also, evaluations of comparisons between UMEC/VI and new LAMA and LABA compounds will be mandatory before deciding the indication for these new drugs in the treatment for COPD.
Disclosure
MM has received honoraria for speaking, and financial support to attend meetings/advisory board meetings from Chiesi, GlaxoSmithKline/Allen and Hanburys, and Mundipharma. JBM has received honoraria for speaking, and financial support to attend meetings/advisory board meetings from Wyeth, Chiesi, Pfizer, Merck Sharp and Dohme, Boehringer-Ingelheim, Teva, GlaxoSmithKline/Allen and Hanburys, Napp, Almirall, and Novartis. AR reports no conflicts of interest in this work.
References
- QaseemAWiltTJWeinbergerSEAmerican College of PhysiciansAmerican College of Chest PhysiciansAmerican Thoracic SocietyEuropean Respiratory SocietyDiagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory SocietyAnn Intern Med2011155317919121810710
- Global Inititaive for Obstructive Lung Disease [homepage on the Internet]Global strategy for the diagnosis, management and prevention of COPD2011 [updated Jan 2014]. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.htmlAccessed May 18, 2014
- DonohueJFOharJABronchodilator therapy of airway diseaseChungKFBarnesPJPharmacology and Therapeutics of Airway DiseaseNew YorkInforma Healthcare USA2009198225
- OharJADonohueJFMono- and combination therapy of long-acting bronchodilators and inhaled corticosteroids in advanced COPDSemin Respir Crit Care Med201031332133320496301
- GrossNJCoESkorodinMSCholinergic bronchomotor tone in COPD. Estimates of its amount in comparison with that in normal subjectsChest19899659849872805869
- US Food and Drug AdministrationFNA News Release12182013 Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm379057.htmAccessed May 18, 2014
- SalmonMLuttmannMAFoleyJJPharmacological characterization of GSK573719 (umeclidinium): a novel, long-acting, inhaled antagonist of the muscarinic cholinergic receptors for treatment of pulmonary diseasesJ Pharmacol Exp Ther2013345226027023435542
- Tal-SingerRCahnAMehtaRInitial assessment of single and repeat doses of inhaled umeclidinium in patients with chronic obstructive pulmonary disease: two randomised studiesEur J Pharmacol20137011–3404823276660
- DecramerMMaltaisFFeldmanGBronchodilation of umeclidinium, a new long-acting muscarinic antagonist, in COPD patientsRespir Physiol Neurobiol2013185239339923026438
- TrivediRRichardNMehtaRChurchAUmeclidinium in patients with COPD: a randomised, placebo-controlled studyEur Respir J2014431728123949963
- MalerbaMRadaeliAMorjariaJBTherapeutic potential for novel ultra long-acting β2-agonists in the management of COPD: biological and pharmacological aspectsDrug Discov Today2012179–1049650422119310
- BarrettVJEmmonsAFordAJKnowlesRIn vitro pharmacological characterisation of GW642444, a novel long acting β2-agonist (LABA) using human recombinant β1/2/3 adrenoceptor cAMP assays [Abstract]Am J Respir Crit Care Med2010181A4451
- ProcopiouPABarrettVJBevanNJSynthesis and structure-activity relationships of long-acting beta2 adrenergic receptor agonists incorporating metabolic inactivation: an antedrug approachJ Med Chem201053114522453020462258
- FordAJHughesSSmithCSomersGRanshawLThe therapeutic index of vilanterol trifenatate [Abstract]Eur Respir J201024208s
- KempsfordRNorrisVSiedererSGW642444, a novel inhaled long-acting β2 adrenoceptor agonist (LABA), at single doses of 25, 50 and 100 mcg, is well tolerated and demonstrates prolonged bronchodilation in asthmatic patients [Abstract]Am J Respir Crit Care Med2010181A5413
- KempsfordRNorrisVSiedererSGW642444, a novel inhaled long-acting β2 adrenoceptor agonist (LABA), at single doses of 25, 50 and 100 mcg, is well tolerated and demonstrates prolonged bronchodilation in COPD patients [Abstract]Am J Respir Crit Care Med2010181A4447
- KempsfordRNorrisVSiedererSVilanterol trifenatate, a novel inhaled long-acting beta2 adrenoceptor agonist, is well tolerated in healthy subjects and demonstrates prolonged bronchodilation in subjects with asthma and COPDPulm Pharmacol Ther201326225626423232038
- HananiaNAFeldmanGZachgoWThe efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trialChest2012142111912722241764
- KestenSCelliBDecramerMLeimerITashkinDTiotropium HandiHaler in the treatment of COPD: a safety reviewInt J Chron Obstruct Pulmon Dis2009439740920037679
- KizerKMBessDTBedfordNKBlurred vision from ipratropium bromide inhalationAm J Health Syst Pharm199956991410344619
- MulpeterKMWalshJBO’ConnorMO’ConnellFBurkeCOcular hazards of nebulized bronchodilatorsPostgrad Med J1992687961321331533281
- PrasEStienlaufSPinkhasJSidiYUrinary retention associated with ipratropium bromideDICP19912599399401835224
- StephensonASeitzDBellCMInhaled anticholinergic drug therapy and the risk of acute urinary retention in chronic obstructive pulmonary disease: a population-based studyArch Intern Med20111711091492021606096
- MannJSHowarthPHHolgateSTBronchoconstriction induced by ipratropium bromide in asthma: relation to hypotonicityBr Med J (Clin Res Ed)19842896443469
- LeeTAPickardASAuDHBartleBWeissKBRisk for death associated with medications for recently diagnosed chronic obstructive pulmonary diseaseAnn Intern Med2008149638039018794557
- TashkinDPCelliBSennSUPLIFT Study InvestigatorsA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- SinghSLokeYKEnrightPLFurbergCDMortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trialsBMJ2011342d321521672999
- WiseRAAnzuetoACalverleyPThe Tiotropium Safety and Performance in Respimat Trial (TIOSPIR), a large scale, randomized, controlled, parallel-group trial-design and rationaleRespir Res2013144023547660
- WiseRAAnzuetoACottonDTIOSPIR InvestigatorsTiotropium Respimat inhaler and the risk of death in COPDN Engl J Med2013369161491150123992515
- TashkinDPFabbriLMLong-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agentsRespir Res20101114921034447
- KnudsonRJConstantineHPAn effect of isoproterenol on ventilation-perfusion in asthmatic versus normal subjectsJ Appl Physiol19672234024066020222
- WagnerPDDantzkerDRIacovoniVETomlinWCWestJBVentilation-perfusion inequality in asymptomatic asthmaAm Rev Respir Dis19781183511524707879
- PhilipsonLHBeta-agonists and metabolismJ Allergy Clin Immunol2002110Suppl 6S313S31712464941
- TesfamariamBWaldronTSeymourAAQuantitation of tremor in response to beta-adrenergic receptor stimulation in primates: relationship with hypokalemiaJ Pharmacol Toxicol Methods199840420120510465154
- KelleherDLMehtaRSJean-FrancoisBMSafety, tolerability, pharmacodynamics and pharmacokinetics of umeclidinium and vilanterol alone and in combination: a randomized crossover trialPLoS One2012712e5071623284643
- KelleherDTombsLCraterGPreeceABrealeyNMehtaRA placebo- and moxifloxacin-controlled thorough QT study of umeclidinium monotherapy and umeclidinium/vilanterol combination in healthy subjects [Abstract]Am J Respir Crit Care Med2013187A1487
- MehtaRKelleherDPreeceAHughesSCraterGEffect of verapamil on systemic exposure and safety of umeclidinium and vilanterol: a randomized and open-label studyInt J Chron Obstruct Pulmon Dis2013815916723569370
- MehtaRHardesKKelleherDPreeceATombsLBrealeyNEffect of moderate hepatic impairment (MHI) on umeclidinium (UMEC) and vilanterol (VI) pharmacokinetics (PK) [Abstract]Eur Respir J201342Suppl 57A3641
- KelleherDHardesKBrealeyNTombsLPreeceAMehtaREffect of severe renal impairment (SRI) on umeclidinium (UMEC) and vilanterol (VI) pharmacokinetics (PK) [Abstract]Eur Respir J201342Suppl 57P4148
- FeldmanGWalkerRRBrooksJMehtaRCraterG28-Day safety and tolerability of umeclidinium in combination with vilanterol in COPD: a randomized placebo-controlled trialPulm Pharmacol Ther201225646547122955035
- DonohueJFMaleki-YazdiMRKilbrideSMehtaRKalbergCChurchAEfficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPDRespir Med2013107101538154623830094
- CelliBRCraterGKilbrideSA 24-week randomized, double-blind, placebo-controlled study of the efficacy and safety of once-daily umeclidinium/vilanterol 125/25 mcg in COPD [Abstract]Am J Respir Crit Care Med2013187A2435
- DecramerMAnzuetoAKerwinEEfficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trialsLancet Respir Med20142647248624835833
- clinicaltrials.gov [homepage on the Internet]DB2113374: a multicenter trial comparing the efficacy and safety of GSK573719/GW642444 with GSK573719 and with tiotropium over 24 weeks in subjects with chronic obstructive pulmonary disease (COPD). ClinicalTrials.gov identifier NCT13169132011 [updated January 9, 2014]. Available from: http://clinicaltrials.gov/show/NCT1316913Accessed May 18, 2014
- clinicaltrials.gov [homepage on the Internet]A 52 week, multi-centre, open-label study to evaluate the safety and tolerability of GSK573719/GW642444 125 mcg once-daily in combination with GW642444 25 mcg once-daily via novel dry powder inhaler (nDPI) in Japanese subjects with chronic obstructive pulmonary disease. ClinicalTrials.gov identifier NCT13763882011 [updated February 13, 2014]. Available from: http://clinicaltrials.gov/show/NCT1376388Accessed May 18, 2014
- FDA Advisory Committee Briefing DocumentANORO™ ELLIPTA™ (Umeclidinium Bromide/Vilanterol Inhalation Powder) For Treatment of Chronic Obstructive Pulmonary Disease Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM367414.pdfAccessed May 28, 2014
- PrakashABabuKSMorjariaJBNovel anti-cholinergics in COPDDrug Discov Today20131821–221117112623872011
- CazzolaMSegretiAMateraMGNew developments in the combination treatment of COPD: focus on umeclidinium/vilanterolDrug Des Devel Ther2013712011208
- FeldmanGJEdinAThe combination of umeclidinium bromide and vilanterol in the management of chronic obstructive pulmonary disease: current evidence and future prospectsTher Adv Respir Dis20137631131924004659
