Abstract
Spirometry is important in the diagnosis and management of chronic obstructive pulmonary disease (COPD), yet it is a common clinical observation that it is underused though the extent is unclear. This survey aims to examine the use of spirometry in the diagnosis and management of COPD patients in a district in Hong Kong. It is a cross-sectional survey involving four clinic settings: hospital-based respiratory specialist clinic, hospital-based mixed medical specialist clinic, general outpatient clinic (primary care), and tuberculosis and chest clinic. Thirty physician-diagnosed COPD patients were randomly selected from each of the four clinic groups. All of them had a forced expiratory volume in 1 second (FEV1) to forced vital capacity ratio less than 0.70 and had been followed up at the participating clinic for at least 6 months for COPD treatment. Of 126 patients who underwent spirometry, six (4.8%) did not have COPD. Of the 120 COPD patients, there were 111 males and mean post-bronchodilator FEV1 was 46.2% predicted. Only 22 patients (18.3%) had spirometry done during diagnostic workup, and 64 patients (53.3%) had spirometry done ever. The only independent factor predicting spirometry done ever was absence of old pulmonary tuberculosis and follow-up at respiratory specialist clinic. Age, sex, smoking status, comorbidities, duration of COPD, percentage predicted FEV1, body mass index, 6-minute walking distance, and Medical Research Council dyspnea score were not predictive. We conclude that spirometry is underused in general but especially by nonrespiratory physicians and family physicians in the management of COPD patients. More effort at educating the medical community is urgently needed.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by advancing airflow obstruction and impairment of gaseous exchange resulting in progressive worsening of shortness of breath. The disease affects 65 million people worldwide and more than 12 million people in the US alone, and is it likely that these figures are grossly under-estimated.Citation1–Citation3 More than 3 million people died from COPD in 2005, and it is predicted that mortality from this disease will continue to increase.Citation2 In Hong Kong, the burden of COPD is also high, with high utilization of health care resources.Citation4–Citation6
Diagnosis of COPD rests on history, physical examination, chest radiograph, and the demonstration of airflow obstruction by spirometry. Although being criticized as overly simplistic,Citation7,Citation8 the spirometric finding of a post-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio of less than 0.70 is still universally accepted as being diagnostic of significant airflow obstruction.Citation1,Citation9–Citation11 Having made the diagnosis, one would like to assess the severity of the disease. Percentage predicted post-bronchodilator FEV1 is objective and reproducible, correlates well with disease severity, and is a good prognostic indicator.Citation12,Citation13 Furthermore, in the subsequent management of COPD, serial FEV1 can serve to follow the progress of the disease and provide guidance on treatment options in different stages of disease evolution.
It is therefore hardly surprising that all the major international COPD management guidelines mandate the use of spirometry in the initial diagnostic evaluation of patients with symptoms suggestive of COPD.Citation1,Citation10,Citation11 However, it is a common observation in daily clinical practice that spirometry is very much underused. In fact, it is not uncommon for patients with severe COPD to have the disease diagnosed and treated for many years, yet have no spirometry done. To examine the extent of the problem, we set out to conduct a survey to observe the use of spirometry in COPD management.
Methods
This is a cross-sectional survey carried out in Kwai Tsing area, Hong Kong, with a population of approximately 570,000. The objective of the survey was to observe what investigations and treatments COPD patients actually receive. Results on treatment aspects have been reported in another paper,Citation14 and this one focuses on use of spirometry.
There were a total of eleven clinics in the public sector which care for COPD patients in the area under study. Very few COPD patients had long-term follow-up in the private sector. The public sector clinics were grouped according to their specialty, and 30 COPD subjects were selected from each group: group 1, one respiratory specialist clinic; group 2, four general medical specialist clinics; group 3, five family medicine clinics or general outpatient clinics (primary care clinics); group 4, one tuberculosis and chest clinic.
For groups 1–3, subject lists were generated from the Hospital Authority Clinical Data Analysis and Reporting System in June 2008. Subjects were randomly selected from the list and were invited to participate in the study by phone call. An appointment was given to verbally consenting subjects to attend a study visit. Recruitment for each group stopped when 30 consenting and evaluable subjects for that group has been accrued. For group 4, since no patient list could be generated, COPD subjects were invited to participate in the study as they attended follow-up at the clinic; workflow was similar to the other groups.
At the study visit, subjects signed an informed consent form and were then checked for study entry criteria. Inclusion criteria were: (1) physician-diagnosed COPD, (2) post-bronchodilator FEV1 to FVC ratio less than 0.70, (3) regular follow-up at the participating clinic for treatment of stable COPD for at least 6 months, and (4) willing and able to comply with study requirements such as performing spirometry and 6-minute walk test. Exclusion criteria were: (1) non-COPD diagnosis as judged by the investigator; (2) subjects attending regular follow-up at another clinic and attending the participating clinic irregularly for acute exacerbation of COPD or other problems; (3) history of significant coexisting chronic lung disease such as asthma, pulmonary fibrosis, bronchiectasis, and restrictive lung disease; and (4) history of lung resection.
When the subjects satisfied all inclusion and none of the exclusion criteria, collection of demographic data, medical data, and smoking history was done. The use of spirometry in the diagnosis and subsequent management of COPD were recorded from the medical records and word of mouth was not accepted. Use of spirometry for diagnostic workup is defined as spirometry done within 6 months before or after making the COPD diagnosis. If the subject had spirometry done in the study center within the previous year, the result was used for study analysis, otherwise spirometry was done for all subjects during the study visit. This was done according to American Thoracic Society/European Respiratory Society 2005 recommendations,Citation15 and the subject must not have had COPD exacerbation in the preceding 4 weeks. Local reference values were used for FEV1 and other spirometric parameters.Citation16 Measurement of body mass index, 6-minute walking distance,Citation17 and dyspnea level using the Medical Research Council dyspnea scaleCitation18 were also done.
After the study visit, subjects continued to attend regular follow-up at their original clinic. Summaries of a subject’s clinical findings and/or treatment recommendations were supplied to the care giver on request.
Data were expressed as percentages, means, and medians, as appropriate. During univariate analysis to compare variables between the groups with and without spirometry ever performed, independent-samples t-test, Mann–Whitney U-test, and chi-square test were used as appropriate. If there was at least one group with expected count less than 5 when comparing distributions, Fisher’s exact test was used. McNemar’s test was used to compare the proportion of patients with spirometry and/or chest radiograph done at diagnosis/ever because the samples were deemed related. With the same standpoint, mean time before study visit of spirometry and chest X-ray were compared by t-test for two related samples. Those variables with P-value less than 0.2 in univariate analysis were subject to logistic regression by backward elimination method, with “significant level of stay” set to 0.10. A P-value of less than 0.05 was considered statistically significant.
The study was approved by the Research Ethics Committee of Hospital Authority Kowloon West Cluster (reference: KW/EX/07-092) and the Ethics Committee of the Department of Health (reference: L/M 243/2008).
Results
Subject recruitment started in June 2008 and was completed in June 2009. A total of 144 subjects were invited to participate in the study. Fourteen subjects refused to participate; two were excluded because of concomitant lung disease, and one died before attending a study visit. The remaining 127 subjects attended study visits. Six were excluded because the diagnosis was judged to be non-COPD, and one was excluded because of failed spirometry. Finally, the data of 120 subjects with 30 from each clinic group were analyzed ().
Figure 1 Subject screening and recruitment summary.
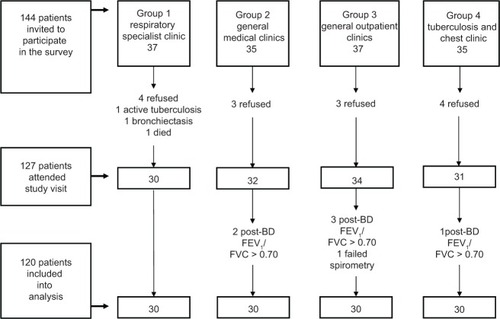
For the six subjects who were excluded during study visits for non-COPD diagnosis, all had a FEV1 to FVC ratio greater than 0.70, and five had a post-bronchodilator FEV1 percentage predicted higher than 80%. Three had chronic bronchitic symptoms and were given the diagnosis of “bronchitis not otherwise specified,” one had mild bronchiectasis, which could explain the symptom of chronic productive cough, while one had no bronchitic symptoms and was considered free from lung disease. The remaining female subject had a very low post-bronchodilator FEV1 of only 40% predicted, and she likely suffered from interstitial lung disease.
For the final 120 subjects, males predominated (111, 92.5%), and mean age was 71.8 years. All were ethnic Chinese, and all but six were either current or ex-smokers. Mean post-bronchodilator FEV1 was 46.2% predicted, and stratification into Global Initiative for Chronic Obstructive Lung Disease (GOLD) stagesCitation1 was: stage I, 10 (8.3%); stage II, 38 (31.7%); stage III, 46 (38.3%); and stage I V, 26 (21.7%). Other characteristics are shown in .
Table 1 Subject characteristics according to whether spirometry was ever performed
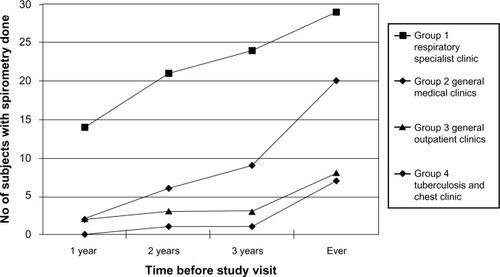
Sixty-four subjects (53.3%) had spirometry ever done prior to study visit and 56 subjects did not. presents data comparing demographic and medical data of the two groups. By univariate analysis, factors significantly associated with spirometry ever done were absence of old pulmonary tuberculosis, more severe disease (lower post-bronchodilator FEV1 percentage predicted and more severe GOLD stage), post-bronchodilator FVC percentage predicted, and clinic group 1 (versus groups 2, 3, and 4 combined). All other factors did not show statistically significant differences between the two groups. These include age, sex, smoking status, number of pack-years, former worker occupation, presence of significant comorbidities, duration of COPD, body mass index, exercise capacity (6-minute walking distance), and severity of dyspnea (Medical Research Council dyspnea score). In the subsequent multivariate analysis (), absence of old pulmonary tuberculosis and clinic group 1 significantly favored spirometry ever done, whereas GOLD stage, post-bronchodilator FEV1 percentage predicted, post-bronchodilator FVC percentage predicted, peak expiratory ratio (FEV1/FVC), and FEV1 bronchodilator reversibility change in volume and percentage were not.
Table 2 Factors associated with spirometry ever done – multivariate analysis (logistic regression by backward elimination)
shows the use of spirometry compared with chest radiograph, which is another important investigation in the management of COPD. Overall, spirometry was performed in only 22 subjects (18.3%) during diagnostic workup, and 64 subjects (53.3%) had it ever done. For those who had spirometry ever done, the mean time interval before study visit was 39.1 months, with a range of 1–132 months. By contrast, chest radiograph was done at diagnostic workup in 96 subjects (80%) and was ever done in 117 subjects (97.5%). Mean time of last order of chest radiograph prior to study visit was much shorter at 12.1 months, with a range of 0.5–84.0 months. All the differences were highly statistically significant.
Table 3 Comparison of use of spirometry and chest radiograph
The timeframe of performing spirometry prior to study visit is depicted in . The figure shows that at all time points, group 1 had a higher proportion of patients having spirometry performed compared with the other groups. Of note is that within 2 years preceding the study visit, 21 subjects (70%) in group 1 had spirometry done, whereas values for groups 2, 3, and 4 were 6 (20%), 3 (10%), and 1 (3.3%) respectively.
Discussion
To our knowledge this is the first cross-sectional survey to examine the use of spirometry in the management of diagnosed COPD patients in Hong Kong. Our data shows that only 18.3% of COPD patients in Kwai Tsing district had spirometry done at diagnosis and 53.3% had it ever done, indicating inconsistent use. Interestingly, this problem appears to be commonplace across the world. A Swedish survey found that of 533 newly diagnosed COPD patients, 59% had spirometry performed and 45% had post- bronchodilator spirometry values. An FEV1 to FVC ratio of less that 0.70 was found in only 30% of patients.Citation19 The Canadian CAGE study involving 1,090 COPD patients from Quebec and Ontario found that 56% had spirometry ever done.Citation20 In the People’s Republic of China, a large survey involving 20,245 COPD subjects from seven provinces/cities showed that only 6.5% were tested with spirometry.Citation21 A recent audit of the US Veterans Health Administration involving 93,724 newly diagnosed COPD patients found that only 36.7% had spirometry performed 2 years before or 6 months after the diagnosis was made.Citation22 This was despite the (then) recent inclusion of this investigation as a performance measure by the United States National Committee for Quality Assurance.Citation22
When we tried to look for factors that favor performance of spirometry, we found as expected that clinic location is the most important factor. This finding suggests that patient factors were not responsible for alerting a doctor to order spirometry in COPD management. Rather, the medical specialty appear to be the important factor, with respiratory physicians most inclined to order spirometry followed by general physicians followed by primary care physicians and tuberculosis and chest physicians. Similar findings were reported by Lee et al,Citation23 in which use of spirometry for newly diagnosed COPD patients was 3.3 times higher for those visiting pulmonologists compared with those visiting primary care alone. One possible explanation for these findings is that patients followed up at respiratory specialist clinics have more severe disease (data not shown), but statistical analysis of our data has indicated that disease severity is not an important factor in this regard. A more plausible explanation might be that specialization towards respiratory medicine increases awareness of the need for spirometry and the proficiency in interpreting the spirometry results. Overseas surveys have observed that use of office spirometry is associated with many practical problems, including availability of spirometer and space, need for calibration and standardization of the spirometer,Citation24 and availability of adequately trained staff.Citation25 Confidence in interpretive skills appears to be an important factor in primary careCitation26 and was not improved by computerized expert report systems.Citation27 Chest radiograph on the other hand is readily available and routinely reported by radiologists, and its higher utilization compared with spirometry may lend support to the above speculation.
A surprise finding is the significant association of the presence of old pulmonary tuberculosis with lack of spirometric assessment. There have not been similar reports elsewhere, and the cause for this finding is not immediately obvious. One would have thought that old pulmonary tuberculosis should be an additional prompt for doctors to order spirometry since there is another lung pathology on top of COPD. However, if doctors can ignore factors like smoking status, significant dyspnea, and poor exercise tolerance, old pulmonary tuberculosis as a prompt to order spirometry may not be a realistic expectation.
Damarla et alCitation28 reported in a retrospective study that of patients admitted to hospital over an 8-year period, only 31% of COPD patients (36% with concomitant respiratory failure) had spirometry done, whereas 78% had two-dimensional echocardiography done for patients with congestive heart failure. For the 219 patients with both conditions, 48% had two-dimensional echocardiography as the only confirmatory test, 34% had both tests performed, and only 2% had spirometry alone. The result is disturbing, since the two tests are very comparable in availability, complexity, ease of interpretation, and utility for treatment guidance. These findings once again suggest that physicians are insufficiently informed on the importance of spirometry in COPD management.
It would appear then that educational workshops with information on the indications, interpretation, and implications of spirometry results, and hands-on workshops on lung function testing may contribute towards solving the current problem. Some published reports focusing on primary care show good short-term results,Citation29,Citation30 but longer-term improvements remain to be seen. Published reports on spirometry workshops with a wider medical audience are lacking. On a different front, spirometry campaigns such as the 2010 World COPD and Spirometry Day may also be useful in increasing public awareness, putting pressure on the medical community to use the test appropriately and consistently. Finally, the setting up of incentive systems like the Quality and Outcomes Framework for general practitioners in the United Kingdom is likely to be helpful.
Our study has the strength that subjects are enrolled from different clinic types, which allows comparisons between them. Also, unlike surveys based on diagnostic and procedural coding, all of our subjects attended a study visit and had clinical and spirometry assessment done to confirm the diagnosis of COPD and whether spirometry was done previously. The study is however limited by its relatively small sample size and its limited location in Hong Kong. Larger, territory-wide studies would be able to give more precise information on the overall situation. Another limitation is the small proportion of female subjects, which probably reflects the low prevalence of smoking amongst Hong Kong women and which severely limits the applicability of our results to this gender.
In conclusion, spirometry is inconsistently used in the management of COPD in Kwai Tsing region, Hong Kong, with most of the problem being seen in nonrespiratory and primary care clinics. A combination of monitoring systems on the use of spirometry in COPD, more education on the importance of spirometry in COPD management, and assistance in interpretation of spirometry results may bring about improvements.
Acknowledgments
We would like to thank Ms Polly Pang and Ms Eva Tam for coordinating the study, Ms Carmen Cheung for performing the lung function tests, Ms Candy Leung for performing the 6-minute walk tests, and Mr WL Wong for statistical support.
Disclosure
This study was supported by a research grant from GlaxoSmithKline (HK) Ltd. The authors have no other conflicts of interest.
References
- Global Initiative for Chronic Obstructive Lung Disease, IncGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease – 2013 update Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdfAccessed April 8, 2013
- World Health Organization [homepage on the Internet]Burden of COPDGenevaWorld Health Organization Available from: http://www.who.int/respiratory/copd/burden/enAccessed June 27, 2013
- ManninoDMBuistASGlobal burden of COPD: risk factors, prevalence, and future trendsLancet200737076577317765526
- Chan-YeungMLaiCKChanKSThe burden of lung disease in Hong Kong: a report from the Hong Kong Thoracic SocietyRespirology200813Suppl 4S133S16518945323
- KoFWWooJTamWPrevalence and risk factors of airflow obstruction in an elderly Chinese populationEur Respir J20083261472147818684847
- LauACIpMSLaiCKVariability of the prevalence of undiagnosed airflow obstruction in smokers using different diagnostic criteriaChest20081331424817989159
- HardieJABuistASVollmerWMRisk of overdiagnosis of COPD in asymptomatic elderly nonsmokersEur Respir J20022051117112212449163
- CerveriICoriscicoAGAccoridiniSUnderestimation of airflow obstruction among young adults using FEV1/FVC < 70% as a fixed cutoff: a longitudinal evaluation of clinical and functional outcomesThorax200863121040104518492741
- JohannessenALehmannSOmenaasERPostbronchodilator spirometry reference values in adults and implications for disease managementAm J Respir Crit Care Med2006173121316132516556696
- QaseemAWiltTJWeinbergerSEDiagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory SocietyAnn Intern Med2011155317919121810710
- National Institute for Health and Care ExcellenceChronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care – 2010 update Available from: http://guidance.nice.org.uk/CG101Accessed April 8, 2013
- AnthonisenNRWrightECHodgkinJEPrognosis in chronic obstructive pulmonary diseaseAm Rev Respir Dis1986133114203510578
- BurrowsBThe course and prognosis of different types of chronic airflow limitation in a general population sample from Arizona: comparison with the Chicago “COPD” seriesAm Rev Respir Dis19891403 Pt 2S92S942782767
- YuWCTaiLBFuSNTreatment of patients with chronic obstructive pulmonary disease as practiced in a defined Hong Kong community: a cross-sectional pilot surveyHong Kong Med J201117430631421813900
- MillerMRHankinsonJBrusascoVATS/ERS Task ForceStandardisation of spirometryEur Respir J200526231933816055882
- IpMSKoFWLauACUpdated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilizationChest2006129238439216478856
- GuyattGHPugsleySOSullivanMJThompsonPJBermanLJonesNJEffect of encouragement on walking test performanceThorax1984398188226505988
- BestellJCPaulEAGarrodRUsefulness of the Medical Research Council (MRC) dyspnea scale as a measure of disability in patients with chronic obstructive pulmonary diseaseThorax199954758158610377201
- ArneMLisspersKStallbergBHow often is diagnosis of COPD confirmed with spirometry?Respir Med2010104455055619931443
- BourbeauJSebaldtRJDayAPractice patterns in the management of chronic obstructive pulmonary disease in primary care practice: the CAGE studyCan Respir J2008151131918292848
- ZhongNWangCYaoWPrevalence of chronic obstructive pulmonary disease in China: a large population-based surveyAm J Respir Crit Care Med2007176875376017575095
- JooMJLeeTAWeissKBGeographic variation of spirometry use in newly diagnosed COPDChest2008134384518347201
- LeeTABartleBWeissKBSpirometry use in clinical practice following diagnosis of COPDChest20061291509151516778268
- EatonTWithySGarrettJESpirometry in primary care practice: the importance of quality assurance and the impact of spirometry workshopsChest199911641642310453871
- PoelsPJSchermerTRJacobsAVariation in spirometry utilization between trained general practitioners in practices equipped with a spirometerScand J Prim Health Care200624818716690555
- BoltonCEIonescuAAEdwardsPHAttaining a correct diagnosis of COPD in general practiceRespir Med20059949350015763457
- PoelsPJSchermerTRSchellekensDPImpact of a spirometry expert system on general practitioners’ decision makingEur Respir J200831849217596275
- DamarlaMCelliBRMullerovaHXPinto-PlataVMDiscrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failureRespir Care200651101120112417005056
- KaminskyDAMarchTWBachandMIrvinCGKnowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physiciansRespir Care200550121639164816318645
- YawnBPEnrightPLLemanskeRFJrSpirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPDChest2007132101162116817550939