Abstract
Purpose
Prediction models for exacerbations in patients with chronic obstructive pulmonary disease (COPD) are scarce. Our aim was to develop and validate a new model to predict exacerbations in patients with COPD.
Patients and methods
The derivation cohort consisted of patients aged 65 years or over, with a COPD diagnosis, who were followed up over 24 months. The external validation cohort consisted of another cohort of COPD patients, aged 50 years or over. Exacerbations of COPD were defined as symptomatic deterioration requiring pulsed oral steroid use or hospitalization. Logistic regression analysis including backward selection and shrinkage were used to develop the final model and to adjust for overfitting. The adjusted regression coefficients were applied in the validation cohort to assess calibration of the predictions and calculate changes in discrimination applying C-statistics.
Results
The derivation and validation cohort consisted of 240 and 793 patients with COPD, of whom 29% and 28%, respectively, experienced an exacerbation during follow-up. The final model included four easily assessable variables: exacerbations in the previous year, pack years of smoking, level of obstruction, and history of vascular disease, with a C-statistic of 0.75 (95% confidence interval [CI]: 0.69–0.82). Predictions were well calibrated in the validation cohort, with a small loss in discrimination potential (C-statistic 0.66 [95% CI 0.61–0.71]).
Conclusion
Our newly developed prediction model can help clinicians to predict the risk of future exacerbations in individual patients with COPD, including those with mild disease.
Introduction
Exacerbations of chronic obstructive pulmonary disease (COPD) have a large impact on morbidity and quality of life and place a huge financial burden on the health care system.Citation1–Citation3 Patients with moderate-to-severe COPD seek medical attention on average two to three times a year for exacerbations, of which one requires hospitalization.Citation4 This fuels the interest in strategies to reduce the number of exacerbations. Adequate prediction models could guide clinicians in subsequent intensified monitoring and treatment of those at increased risk and, thus, reduce the number of exacerbations. The majority of published prediction models developed for patients with COPD, however, aim to predict mortality,Citation5–Citation7 and models specifically developed to predict exacerbations are scarce. Moreover, most of these models are based on severe exacerbations, those requiring hospitalization.Citation8–Citation10 Also, studies that developed prediction models for future exacerbations typically included severely diseased COPD patients recruited from tertiary care centers, and are therefore not representative of the COPD patient population at large.
Severity indices like the BODE (body mass index, obstruction, dyspnea and exercise) IndexCitation5 and the DOSE (dyspnea, obstruction, smoking and exacerbations) IndexCitation11 have also been tested on their ability to predict future exacerbations and showed acceptable performance measures (ie, C-statistics in the range of 0.6–0.7 in their original cohorts). As these scores were not initially developed to predict exacerbations, some potentially important predictors for exacerbations were not considered. Thus, the need remains for an easily applicable prediction model that can be applied to all patients with COPD, including those with mild disease and predicting all exacerbations, including those not requiring hospitalization. Also, the model should be practicable in everyday care, including the primary care setting, in which many patients with (mild) COPD are managed. We set out to develop and externally validate such a model.
Materials and methods
Study design and participants
For the development of the prediction model, we used a cohort of COPD patients, originally set up to screen for heart failure in patients with COPD.Citation12 In brief, patients from primary care enlisted with a general practitioner’s (GP’s) diagnosis of COPD and aged 65 years or over were selected from 51 general practices in the Netherlands between April 2001 and June 2003. At the baseline assessment, the GP’s diagnosis of COPD was reevaluated by newly performed standardized pulmonary function tests (spirometry, body box measurements, and diffusion testing), and presence or absence of COPD was determined by an expert panel including a pulmonologist, applying the Global initiative for chronic Obstructive Lung Disease (GOLD) criteria.Citation13 COPD was established in 243 patients. Follow-up data for all 243 patients was collected over a period of 24 months from study entry measurements by scrutinizing the treating GP’s electronic medical files, including letters from medical specialists.Citation14 The institutional review board of the University Medical Center Utrecht, Utrecht, the Netherlands, approved the study protocol, and all participants gave written informed consent.
The validation cohort originated from the Utrecht General Practitioners Network database, in which collaborating GPs enter all data related to daily patient contacts electronically into a GP information system. For this study we included 793 patients aged 50 years or over with a diagnosis of COPD based on available spirometric data (post-bronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity <70%). This database can be considered as a dynamic cohort. We artificially set the study entry at January 2010 for our study, and baseline characteristics and follow-up data of the eligible patients were determined for 24 months from that period of time onwards.
Outcome
For both cohorts we used the same “operational” definition for exacerbation of COPD, that is, symptomatic deterioration requiring pulsed oral steroid use or hospitalization.Citation15–Citation17
Candidate predictors
The list of candidate predictors to be studied was based on the literature and was limited to those predictors that are readily available in clinical practice. We added predictors related to cardiovascular comorbidity. The list of selected potential predictors included age,Citation10 FEV1,Citation9,Citation10,Citation18,Citation19 previous exacerbations,Citation9,Citation10,Citation19,Citation20 body mass index,Citation18–Citation20 pack years smoked, history of vascular disease,Citation21–Citation23 and history of ischemic heart disease (IHD). All candidate predictors were assessed at study entry for each patient.
Previous exacerbations were considered present if at least one exacerbation occurred in the year prior to the baseline assessment, also defined as symptomatic deterioration requiring pulsed oral steroid use or a hospitalization. For FEV1, the post-bronchodilator value was used, and the percentage of predicted (%predicted) FEV1 according to age, height, and sex was calculated. A history of IHD was defined as a history of myocardial infarction, angina pectoris, percutaneous coronary intervention, or coronary artery bypass grafting. A history of vascular disease was defined as stroke, transient ischemic attack, or peripheral arterial disease.
Missing data
In the validation cohort, follow-up information was missing for three participants (1.3%), and these patients were excluded from the analyses. In six participants (2.5%), data on previous exacerbations were missing. In the validation cohort, post-bronchodilator FEV1 values were missing in eleven patients (1.4%), smoking status in 161 patients (20.3%), and number of pack years in 398 patients (50.2%). All missing values were imputed using multiple regression techniques.Citation24 Rubin’s rulesCitation25 were applied to the 25 multiple imputation sets to calculate the pooled C-statistics. As the exclusion of patients with a missing value (so-called complete-case analysis) in general may lead to biased results, we performed a separate reanalysis that was restricted to “complete cases” of the univariable associations between the number of pack years smoked and exacerbations. The univariable odds ratios were similar to those derived from the imputed dataset, although with broader confidence intervals (CIs).
Data analyses
Model development
Descriptive analyses were used to assess differences in baseline characteristics of patients who experienced an exacerbation in the 2 years after the baseline assessment and those who did not. For model development, we used multivariable logistic regression analysis to examine the independent association between candidate predictors and the occurrence of a COPD exacerbation within 24 months. The strength of the relationship was expressed in odds ratios with 95% CIs. Age, FEV1, and pack years were assessed on their continuous scale. To improve linearity, we log-transformed the number of pack years smoked.
We started with all six preselected candidate predictors in the model and then applied backward-selection procedures, using a P-value<0.20 from the log likelihood ratio test to select predictors for the final model. The model was internally validated using bootstrapping techniques. To correct for overfitting, the beta coefficients of the predictors in the final model were multiplied by the shrinkage factor derived from the 500 bootstrap samples.Citation26 The performance of the final model (after shrinkage) was assessed with the C-statistic and its 95% CI, and calibration was assessed with a calibration plot.
External validation
For validation of the model, the predicted probabilities were calculated for each patient in the validation set using the regression coefficients, after shrinkage, from the original model obtained from the derivation cohort. In a calibration plot, the actual and predicted probabilities were compared across the range of predicted risks. Discrimination in the validation cohort was assessed with the C-statistic.
Data were analyzed using SPSS 20.0 for Windows (IBM Corporation, Armonk, NY, USA) and R (v 2.9.2; The R Foundation for Statistical Computing, Vienna, Austria).
Results
During the 24-month follow-up period, 70 (29.2%) patients experienced at least one exacerbation of COPD in the derivation cohort, and 222 (28.0%) in the validation cohort. For both cohorts, the number of exacerbations increased with increasing severity of COPD and ranged from 22% of patients with mild COPD (GOLD I) to 45% in patients with severe COPD (GOLD III/IV). Disease classification according to the GOLD criteriaCitation13 and other baseline characteristics of the 240 patients from the derivation cohort and 793 patients from the validation cohort, stratified by occurrence of an exacerbation, are summarized in and .
Table 1 Baseline demographic and clinical characteristics of the derivation and validation cohort, with and without exacerbations within 24 months from study entry
Table 2 Disease classification according to the Global initiative for chronic Obstructive Lung Disease (GOLD) criteriaCitation13 for both cohorts, with and without exacerbations within 24 months from baseline assessment
Model development
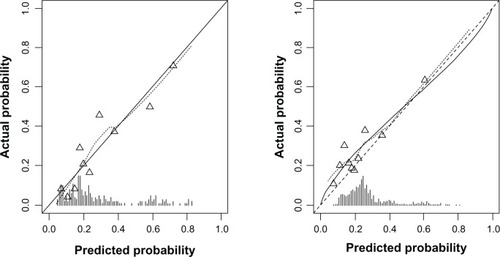
In the multivariable analysis, decreased values of FEV1, previous exacerbations, pack years of smoking, and vascular disease were independent predictors of an exacerbation within 24 months. The final reduced prediction model after backward elimination is shown in . The model discriminated well with a C-statistic after shrinkage of 0.75 (95% CI: 0.69–0.82) and with a shrinkage factor of 0.90. Calibration of the model was good: the predicted and actual risks of exacerbation within 24 months were in close agreement ().
Table 3 Final prediction model to predict exacerbations in COPD patients
Figure 1 Calibration plots of the final prediction model for exacerbations of COPD within the proceeding 24 months in both the derivation and validation cohort.
Abbreviation: COPD, chronic obstructive pulmonary disease.
Model validation
The model showed a moderate discrimination in the validation cohort, with a C-statistic of 0.66 (95% CI: 0.62–0.71). The calibration plot indicated close agreement between the predicted and observed risks ().
Discussion
Our study shows that easy-to-assess variables are independently associated with the occurrence of future COPD exacerbations. A model including these variables from history-taking and spirometry (FEV1 %predicted) could be used by both pulmonologists and GPs in everyday care. After external validation, our model showed moderate discrimination (C-statistic 0.66) and a good calibration.
Our findings that lower levels of FEV1 as %predicted and that a history of previous exacerbations are related to an increased risk of future exacerbations are in line with previous studies.Citation9,Citation10,Citation18–Citation20 Hurst et alCitation20 identified previous exacerbations as the single best predictor of future exacerbations. To examine this claim in our cohort, we tested previous exacerbations as a single value, which resulted in C-statistics of 0.66 and 0.59 in the derivation and validation cohort, respectively. This suggests that other predictors are needed to more adequately predict future exacerbations in a broad sample of patients with COPD. We also evaluated the DOSE index in our derivation cohort.Citation11 This index yielded a C-statistic of 0.65, which is considerably lower than our own model. Unfortunately, we could not evaluate the widely accepted BODE index,Citation5 because we did not perform a 6-minute walking test in any of our cohorts. Importantly, however, when we applied the BODE index without information on exercise capacity, it resulted in a poor C-statistic of 0.61 in the derivation cohort. Moreover, the performance of the BODE index on prediction of future exacerbations is rather poor in other than the original cohorts, with C-statistics of 0.67 and 0.62.Citation6
We also considered cardiovascular diseases because they are a major cause of morbidity and mortality in patients with COPD.Citation27–Citation30 Low-grade systemic inflammation and smoking are key components in the pathophysiology of vascular plaque formation, which may explain the increased rates of cardiovascular disease in COPD.Citation31 Importantly, however, COPD patients with concomitant IHD did not experience a higher incidence of COPD exacerbations in our population, a result similar to previous studies.Citation30,Citation32 On the other hand, a history of vascular disease (including stroke) was positively (and independently) associated with COPD exacerbations in our study. Although the relationship between deep venous thrombosis and exacerbations is well studied,Citation21–Citation23 vascular disease at large, including peripheral arterial disease and stroke, has not been identified before as a predictor of future COPD exacerbations. Peripheral arterial disease is possibly associated with more severe COPD,Citation33 and this might translate into the observed association between vascular disease and the risk of future exacerbations. Increased platelet activation could be a mechanism that plays a role in this association.Citation34
We were not able to include all potential predictors available from the literature, such as exercise capacity, the St George’s Respiratory Questionnaire, oxygen therapy, and gastroesophageal reflux, because these variables were not available in the derivation cohort. Importantly, however, most of these variables are not available in clinical practice, and, thereby, these predictors would not be useful for prediction within individual patients in everyday practice.
It has become increasingly clear that the severity and frequency of exacerbations is only marginally related to disease severity. Because most patients with COPD can be classified as GOLD I and II (ie, mild COPD), the absolute number of exacerbations and overall burden is largest in those with mild COPD.Citation20 This illustrates the importance of including patients with GOLD I and II, as we did in our study.
Patients in the validation cohort were younger; more frequently current smokers; had, in general, milder disease; were more likely to have vascular disease; and experienced fewer exacerbations in the previous year than the derivation cohort. Despite these differences in baseline characteristics (“case mix”), the model yielded acceptable discrimination (C-statistic 0.66) and good calibration, indicating that our findings are generally applicable, including in younger COPD patients. The relatively small sample size of the derivation cohort could be considered a limitation of the study. We chose this data set because the data were prospectively collected, with, as a result, a higher quality of data compared to the validation cohort.
The definition of COPD exacerbations is always a challenge in follow-up studies such as ours. We used symptomatic deterioration requiring pulsed oral steroid use or hospitalization as a definition for exacerbation. This is somewhat different from previous studies that also included treatment with antibiotics.Citation35–Citation37 In Dutch primary care guidelines on COPD, short-course oral corticosteroids are recommended for COPD exacerbations, and addition of antibiotics should only be considered when bacterial infection is suspected.Citation38 Thus, exacerbations of COPD are rarely, if ever, treated solely with antibiotics only in the Netherlands. As a result, with our definition, all exacerbations are captured in the derivation and validation cohort and, as such, our results are generalizable to other populations of COPD, including in other parts of the world.
We used logistic regression modeling to estimate the risk of occurrence of COPD exacerbations within the proceeding 24 months. Cox proportional hazards modeling would also have been a valid approach for risk estimation, but we chose logistic regression analysis because we considered each exacerbation within our predefined time frame of 2 years to be of equal importance, regardless of whether this exacerbation occurred early or late in the follow-up period. Furthermore, calculation of absolute probabilities of exacerbation in future patients is more straightforward using the coefficients of a logistic model than it would be for a Cox survival model due to the unspecified baseline function in the latter.Citation39
To the best of our knowledge, this is the first study providing predictors of future exacerbations in a broad spectrum of patients with COPD, including patients with mild disease. Because we developed and tested the model in a sample of the COPD patient population at large, the applicability of the model is broad and seems applicable to both primary and secondary care. The performance of our prediction model is very promising, and the applicability of the model is facilitated by the limited number of predictors in the model and their ready availability in daily practice. The model helps clinicians to identify COPD patients at high risk of exacerbations and to guide intensified management and monitoring of these patients. To determine the impact of our prediction model on daily practice in terms of patient outcome and the use of health care resources, an implementation study should be performed.
Conclusion
A limited number of easily assessable variables adequately predict future exacerbations of COPD. Cardiovascular disease should be considered in the management of these patients, and adequate treatment of vascular disease could possibly reduce the risk of future exacerbations.
Acknowledgments
We thank the participating patients and the GPs of the Utrecht General Practice Network (HNU) and other GPs for participation in this study. The original study was financially supported by a grant (number 904-61-144) from the Netherlands Organisation for Scientific Research (NWO). The funding source of this study played no role in the design or conduct of the study, data management or analysis, or manuscript preparation, review, and authorization for submission.
Disclosure
The authors report no conflicts of interest in this work.
References
- NiewoehnerDERelation of chronic obstructive pulmonary disease exacerbations to FEV(1) – an intricate tangoRespiration200977222923518840996
- CelliBCrossSGrossmanRImproving the care of COPD patients – suggested action points by the COPD exacerbations taskforce for reducing the burden of exacerbations of COPDPrim Care Respir J200615313914216757393
- WoutersEFEconomic analysis of the Confronting COPD survey: an overview of resultsRespir Med200397Suppl CS3S1412647938
- NiewoehnerDERiceKCoteCPrevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trialAnn Intern Med2005143531732616144890
- CelliBRCoteCGMarinJMThe body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med2004350101005101214999112
- PuhanMAGarcia-AymerichJFreyMExpansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO indexLancet2009374969170471119716962
- ZhangJRuttenFHCramerMJLammersJWZuithoffNPHoesAWThe importance of cardiovascular disease for mortality in patients with COPD: a prognostic cohort studyFam Pract201128547448121602286
- BahadoriKFitzGeraldJMLevyRDFeraTSwistonJRisk factors and outcomes associated with chronic obstructive pulmonary disease exacerbations requiring hospitalizationCan Respir J2009164e43e4919707601
- Garcia-AymerichJMonsóEMarradesRMRisk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM studyAm J Respir Crit Care Med200116461002100711587986
- NiewoehnerDELokhnyginaYRiceKRisk indexes for exacerbations and hospitalizations due to COPDChest20071311202817218552
- JonesRCDonaldsonGCChavannesNHDerivation and validation of a composite index of severity in chronic obstructive pulmonary disease: the DOSE IndexAm J Respir Crit Care Med2009180121189119519797160
- RuttenFHMoonsKGCramerMJRecognising heart failure in elderly patients with stable chronic obstructive pulmonary disease in primary care: cross sectional diagnostic studyBMJ20053317529137916321994
- RabeKFHurdSAnzuetoAGlobal Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- van der LeiJDuisterhoutJSWesterhofHPThe introduction of computer-based patient records in The NetherlandsAnn Intern Med199311910103610418214981
- RuttenFHZuithoffNPHakEGrobbeeDEHoesAWBeta-blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary diseaseArch Intern Med20101701088088720498416
- ShortPMLipworthSIElderDHSchembriSLipworthBJEffect of beta blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort studyBMJ2011342d254921558357
- WanESDemeoDLHershCPClinical predictors of frequent exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD)Respir Med2011105458859421145719
- MarinJMCarrizoSJCasanovaCPrediction of risk of COPD exacerbations by the BODE indexRespir Med2009103337337819013781
- ForemanMGDemeoDLHershCReillyJJSilvermanEKClinical determinants of exacerbations in severe, early-onset COPDEur Respir J20073061124113017715170
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- ChatilaWMThomashowBMMinaiOACrinerGJMakeBJComorbidities in chronic obstructive pulmonary diseaseProc Am Thorac Soc20085454955518453370
- RutschmannOTCornuzJPolettiPAShould pulmonary embolism be suspected in exacerbation of chronic obstructive pulmonary disease?Thorax200762212112517101737
- SchonhoferBKohlerDPrevalence of deep-vein thrombosis of the leg in patients with acute exacerbation of chronic obstructive pulmonary diseaseRespiration19986531731779670296
- DondersARvan der HeijdenGJStijnenTMoonsKGReview: a gentle introduction to imputation of missing valuesJ Clin Epidemiol200659101087109116980149
- RubinDBSchenkerNMultiple imputation in health-care databases: an overview and some applicationsStat Med19911045855982057657
- SteyerbergEWOverfitting and optimism in prediction modelsClinical Prediction Models: A Practical Approach to Development, Validation, and UpdatingNew YorkSpringer Science + Business Media, LLC20098399
- SinDDAnthonisenNRSorianoJBAgustiAGMortality in COPD: role of comorbiditiesEur Respir J20062861245125717138679
- FordESWheatonAGManninoDMPresley-CantrellLLiCCroftJBElevated cardiovascular risk among adults with obstructive and restrictive airway functioning in the United States: a cross-sectional study of the National Health and Nutrition Examination Survey from 2007–2010Respir Res20121311523237325
- HoleDJWattGCDavey-SmithGHartCLGillisCRHawthorneVMImpaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population studyBMJ199631370597117158819439
- PatelARDonaldsonGCMackayAJWedzichaJAHurstJRThe impact of ischemic heart disease on symptoms, health status, and exacerbations in patients with COPDChest2012141485185721940771
- DonaldsonGCHurstJRSmithCJHubbardRBWedzichaJAIncreased risk of myocardial infarction and stroke following exacerbation of COPDChest201013751091109720022970
- ManJPSinDDIgnaszewskiAManSFThe complex relationship between ischemic heart disease and COPD exacerbationsChest2012141483783822474142
- PecciRDe La Fuente AguadoJSanjurjo RivoABSanchez CondePCorbacho AbelairaMPeripheral arterial disease in patients with chronic obstructive pulmonary diseaseInt Angiol201231544445322990507
- MaclayJDMcAllisterDAJohnstonSIncreased platelet activation in patients with stable and acute exacerbation of COPDThorax201166976977421507906
- BurgeSWedzichaJACOPD exacerbations: definitions and classificationsEur Respir J Suppl20034146s53s12795331
- CelliBVestboJJenkinsCRInvestigators of the TORCH StudySex differences in mortality and clinical expressions of patients with chronic obstructive pulmonary disease. The TORCH experienceAm J Respir Crit Care Med2011183331732220813884
- JenkinsCRCelliBAndersonJASeasonality and determinants of moderate and severe COPD exacerbations in the TORCH studyEur Respir J2012391384521737561
- SmeeleIJMvan WeelCvan SchaykCPNHG-Standaard COPD (tweede herziening) [NHG standard COPD (second revision)]Huisarts Wet2007508362379 Dutch
- RoystonPAltmanDGExternal validation of a Cox prognostic model: principles and methodsBMC Med Res Methodol2013133323496923