Abstract
Background
Congestive heart failure is underdiagnosed in patients with chronic obstructive pulmonary disease (COPD). Pulmonary congestion on chest radiograph at admission for acute exacerbation of COPD (AECOPD) is associated with an increased risk of mortality. A standardized evaluation of chest radiographs may enhance prognostic accuracy.
Purpose
We aimed to evaluate whether a standardized, liberal assessment of pulmonary congestion is superior to the routine assessment in identifying patients at increased risk of long-term mortality, and to investigate the association of heart failure with N-terminal prohormone of brain natriuretic peptide (NT-proBNP) concentrations.
Material and methods
This was a prospective cohort study of 99 patients admitted for AECOPD. Chest radiographs obtained on admission were routinely evaluated and then later evaluated by blinded investigators using a standardized protocol looking for Kerley B lines, enlarged vessels in the lung apex, perihilar cuffing, peribronchial haze, and interstitial or alveolar edema, defining the presence of pulmonary congestion. Adjusted associations with long-term mortality and NT-proBNP concentration were calculated.
Results
The standardized assessment was positive for pulmonary congestion in 32 of the 195 radiographs (16%) ruled negative in the routine assessment. The standardized assessment was superior in predicting death during a median follow up of 1.9 years (P=0.022), and in multivariable analysis, only the standardized assessment showed a significant association with mortality (hazard ratio 2.4, 95% confidence interval [CI] 1.2–4.7) (P=0.016) and NT-proBNP (relative concentration 1.8, CI 1.2–2.6) (P=0.003).
Conclusion
By applying a standardized approach when evaluating pulmonary congestion on chest radiographs during AECOPD, a group of patients with increased risk of dying, possibly due to heart failure, is identified.
Background
During recent years, increasing attention has been paid to the role of comorbidities in patients with chronic obstructive pulmonary disease (COPD). Cardiovascular diseases (CVD) are among the extrapulmonary manifestations of COPD. The incidence of and mortality from CVD, such as cerebral stroke, myocardial infarction (MI), heart failure (HF), and arrhythmias are higher among COPD patients than in the general population, even after adjusting for smoking and other important confounders.Citation1–Citation4 Accordingly, increased awareness of concomitant CVD in COPD is warranted. In review articles on the use of new radiological imaging modalities in COPD, particular attention is paid to the presence of emphysema, bronchiectasis, or vascular remodeling.Citation5,Citation6 There is little emphasis on the signs of heart disease and what can still be learned from the standard chest radio-graphs, though this remains central in the diagnosis of HF.Citation7 Reportedly, only 16% of chest radiographs taken during acute exacerbation of COPD (AECOPD) show abnormal findings,Citation8 but when observed, these are findings that will have therapeutic consequences. Pulmonary congestion, a sign of congestive HF, is among the abnormalities that can be found. HF is associated with increased mortality, although effective treatment exists. However, it has been shown that heart disease often remains undiagnosed in COPD patients,Citation9–Citation11 Therefore, radiographic evidence of such could be important to the clinicians.
In a previous study, we found that signs of pulmonary congestion on chest radiographs taken on admission for AECOPD was strongly and independently associated with long-term mortality.Citation12 These radiographs were investigated by dedicated study physicians, and the prevalence of pulmonary congestion on admission was 16%. Our impression was that in everyday practice, radiological signs of HF remain undetected in a high proportion of COPD patients.
The natriuretic peptides B-type natriuretic peptide (BNP) and the amino terminal fragment of the BNP prohormone (NT-proBNP) are established markers of HF.Citation7 BNP and chest radiographs have been found to be independently associated with HF in patients presenting with acute dyspnea.Citation13 Both BNP and NT-proBNP are independently associated with worse prognosis during and after AECOPD.Citation14–Citation17
Radiological evaluation of HF in patients with COPD may be difficult.Citation18 Therefore, in the present study we wanted to a) investigate whether a standardized investigation of pulmonary congestion on chest radiograph taken on admission for AECOPD could predict mortality better than routine evaluation of the radiographs, and b) validate the standardized assessment with regard to the presence of HF, as evaluated by NT-proBNP levels.
Methods
During 23 months in 2005 and 2006 we prospectively included 99 unselected patients as they were admitted with AECOPD. Among these, 41 patients had data recorded on one or more readmissions during the inclusion period, and in total, we gathered data from 219 admissions. The details regarding patient inclusion and data gathering have been described in previous papers.Citation12,Citation19 On admission, we recorded medical history, clinical data, electrocardiograms (ECG), and chest radiographs. In addition to a qualitative analysis of prior MI (pathological Q, loss of R, and T inversion) and acute ischemia (ST segment depression or elevation), the ECG analysis included a cardiac infarction injury score (CIIS), where a score ≥20 indicates high probability of prior MI.Citation20 In one study of COPD patients without a history of CVD, CIIS ≥20 was associated with increased mortality.Citation9 The ECG analyses were conducted by two independent investigators blinded for all other information, and discrepancies were settled by a third investigator.
Chest radiographs were obtained routinely on admission, the standard being posteroanterior and lateral projections. In clinically deranged patients unable to cooperate or stand up, only the frontal plane image with anteroposterior projection was obtained. For the “standardized assessment,” the radiographs were examined by two of the investigators, a pulmonologist, and a radiology fellow, blinded to all clinical data. In each case, they cooperated to determine the presence or absence of Kerley B lines, enlarged vessels in the lung apex (redistribution), peribronchial cuffing, perihilar haze, and interstitial or alveolar edema. If any of these features were present, it was considered positive for pulmonary congestion. The investigators also recorded the presence of infiltrates and pleural effusions. On admission, the radiographs had routinely been examined by first the radiology fellow on call and then by one of the senior radiologists (a minority of the radiographs were evaluated by a senior radiologist only). The radiologists were unaware that the patient under investigation was to be included in any study. Their descriptions (“routine assessment”) therefore reflect the normal everyday radiologic evaluation and were retrieved from the hospital’s electronic records, and it was noted whether or not pulmonary congestion had been reported.
Blood collected on admission was stored at −80°C for subsequent analysis of NT-proBNP and creatinine concentrations. From the hospital records, we also recorded the discharge diagnoses with regard to concomitant HF or infection (International Classification of Diseases [ICD]-10 codes I50 or J10–22).Citation21 The patients were followed with respect to survival until the end of 2008 or death.
The study was approved by the Data Inspectorate and the Regional Ethics Committees South East. All patients provided written informed consent.
Statistical analyses
Baseline analyses
Using the 99 baseline observations, we first analyzed the crude mortality rates associated with the two radiological assessments and compared them using a logrank test. We also calculated the two assessments’ sensitivity and specificity for identifying patients at risk of death during the observation period and compared the two assessments, using receiver-operating characteristics (ROC).
For both radiological assessments, we then investigated the univariable baseline associations between pulmonary congestion and covariables. We compared clinical and biochemical data in these groups, using the Kruskal–Wallis test for continuous variables (age, body mass index [BMI], forced expiratory volume in 1 second/forced vital capacity [FEV1/FVC] ratio, mean arterial pressure, serum creatinine, leucocyte count, serum C-reactive protein [CRP], hemoglobin, heart rate, arterial oxygen saturation [SaO2], arterial partial pressures of O2 [PaO2] and CO2 [PaCO2], and arterial pH) and chi-square or Fisher’s exact test for categorical data (smoking status [current or recent versus previous or never], history of coronary artery disease, HF, diabetes mellitus, and arterial hypertension, atrial fibrillation, peripheral edema, and chest pain on admission, patient posture, infiltrate on chest radiograph, and prior MI, acute ischemia, and CIIS ≥20 in ECG).
Bivariable analyses
If patients with and without pulmonary congestion had different values of a covariable in any radiological assessment (P<0.20 in the baseline analyses), this covariable was investigated with regard to an association with mortality and NT-proBNP concentration.
Analysis of mortality
To investigate the association between the relevant covariables and mortality, we calculated the mortality rates in each category of the covariables, with continuous variables categorized by quartiles, and compared these with survival, using an age-adjusted logrank test.
Analysis of NT-proBNP
Due to the skewed distribution of NT-proBNP concentrations, the natural logarithm of the NT-proBNP concentration (lnBNP) was used as the dependent variable in these analyses. The associations between lnBNP and the relevant categorized covariables were then compared using Student’s t-test or analysis of variance (ANOVA). In each category of the covariables, we also calculated the geometric mean of NT-proBNP concentration, that is, the antilog of the mean lnBNP value.
Multivariable analyses
In the baseline and bivariate analyses, only the 99 baseline observations were included in the analyses. However, for the multivariable analyses, we also used data from later admissions, in an extended Cox model (survival)Citation22,Citation23 and a linear mixed model (LMM) (associations with NT-proBNP).Citation24,Citation25
Analysis of mortality
All variables that were associated with both pulmonary congestion (P<0.20 with either radiological assessment) and mortality (logrank P<0.20) were included in a Cox regression analysis with time-dependent covariables (ie, allowing update of a patient’s data on repeat admissions). Using a backward elimination procedure, the model was reduced by removing variables with P>0.05 provided that the coefficient of the association between mortality and any radiological assessment changed less than 20%. Age and sex were kept in the model by convention, and both radiological assessments were kept for comparison. The proportional hazards assumption was checked by the Martingale residuals.
Analysis of NT-proBNP
The variables that were associated with pulmonary congestion (in the baseline analyses) and NT-proBNP concentration (P<0.20 in bivariable analysis) were included in a LMM along with age, sex, and both radiological assessments. The advantage of the LMM is that unbalanced data (the number of observations and the time elapsed between them are different between the patients) can be analyzed without introducing bias.Citation25 We could therefore include all observations in this analysis. The model was manually backward reduced by removing variables with P>0.05 unless their removal increased the Akaike information criterion (AIC) statistic (ie, resulting in poorer model fit).
All the analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline analyses
The mean age at inclusion was 71.5 years (standard deviation [SD] 9.0), and the mean FEV1/FVC was 45% (SD 0.14%). The chest radiograph was missing for one admission, thus, 218 admissions, from 99 patients, constituted the study sample. The patient characteristics, grouped by evaluation of their chest radiographs at inclusion, are shown in for those variables that differed (P<0.20) within any radio-graphic assessment. Lung function was included to better characterize the cohort. Among the variables that were not significantly different at baseline were smoking status (48% active or recent smokers), history of coronary artery disease (27%), history of diabetes mellitus (8%), leucocyte count (mean 11.1 × 109/L, SD 5.0), serum creatinine (median 66 μmol/L, interquartile range [IQR] 54–88), and CIIS (38% had a score ≥20).
Table 1 Characteristics of patients with and without pulmonary congestion at baseline, assessed by standardized and routine procedure
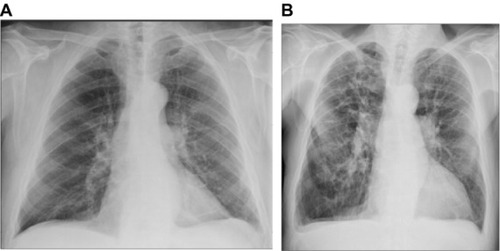
Of all 218 radiographs, 195 (89%) were ruled negative for pulmonary congestion, by routine clinical assessment. In 32 of these (16%), the standardized assessments were positive. The corresponding numbers using the index observations only were 10 of 85 radiographs (12%). The features suggesting pulmonary congestion in the 32 radiographs were interstitial edema in two cases, enlarged vessels in the apex in 22 cases, Kerley B lines in one case, perihilar haze in 13 cases, and peribronchial cuffing in eight cases. shows two radiographs (selected at random), which were considered negative by the routine assessment but positive for apical blood vessels and perihilar haze by the standardized assessment.
Figure 1 Randomly selected chest radiographs assessed negative in the routine assessment and positive in the standardized assessment.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NT-proBNP, N-terminal prohormone of brain natriuretic peptide.
In the ten baseline observations that were negative in the routine assessment but positive in the standardized assessment, the median NT-proBNP concentration was 1,027 pg/mL (IQR 400–3,426). Conversely, among the eight baseline observations that were positive in the routine assessment and negative in the standardized assessment, the median NT-proBNP concentration was 397 pg/mL (IQR 271–1,859).
Survival analyses
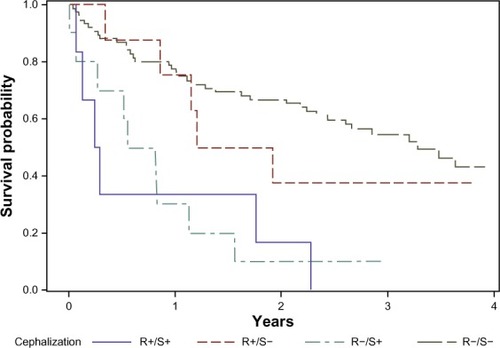
During a median follow-up time of 1.9 years, 57 patients died. Of these, 15 had pulmonary congestion by the standardized assessment (sensitivity 26%, specificity 98%, ROC area 0.62 [95% confidence interval (CI) 0.56–0.68]), and eleven had pulmonary congestion by the routine assessment (sensitivity 19%, specificity 93%, ROC area 0.54 [0.496–0.63]). Comparing the ROC, the standardized assessment proved superior in predicting death (P=0.022, ). shows patient survival, stratified by radiological assessment. The overall mortality rate was 28.7 per 100 patient-years (95% CI: 22.1–37.2). Among the patients positive and negative for pulmonary congestion by the standardized assessment, the mortality rates were 111 (95% CI: 67.1–185) and 22.7 (95% CI: 16.8–37.2), respectively (logrank P<0.0001). The corresponding mortality rates by the routine assessment were 56.0 (95% CI: 31.0–101) and 25.7 (95% CI: 19.2–34.3), with logrank P=0.039.
Figure 2 ROC curves for the routine (red dashed) and standardized (blue) assessments.
Notes: Age-adjusted logrank P<0.0001 between R−/S− and R−/S+; age-adjusted P=0.049 between R+/S− and R−/S+. The results were based on the 99 index admissions.
Abbreviations: COPD, chronic obstructive pulmonary disease; R−, radiological assessment negative; R+, radiological assessment positive; S−, standardized assessment negative; S+, standardized assessment positive; ROC, receiver-operating characteristics.
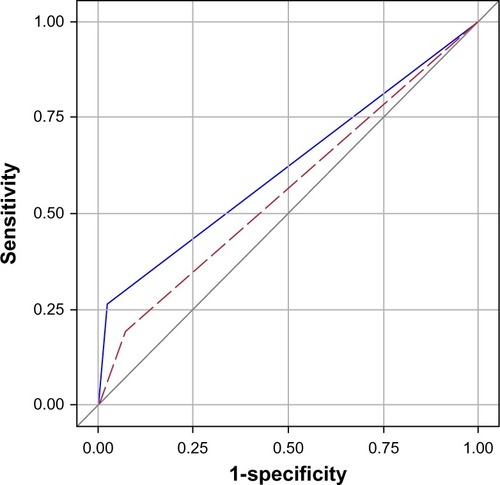
Figure 3 Survival after acute exacerbation of COPD, stratified by radiologic evaluation of pulmonary congestion on admission.
Abbreviations: AUC, area under the curve; ROC, receiver operating characteristics.
Of the variables in , PaCO2, serum CRP, and ECG changes were not associated with mortality (age-adjusted logrank P>0.20). There was a trend towards increasing mortality with lung function below the mean (FEV1 <0.9 L and FEV1/FVC <0.45 [age-adjusted logrank P=0.093 and P=0.103, respectively]), but as this was not associated with any radiological assessment (), it was not included in further analyses. Patient survival, by the remaining covariables, is shown in and . Reducing the model resulted in the final model shown in . Of the two radiological evaluations, only the standardized assessment proved significantly associated with long-term mortality. The other variables in the model were age, sex, peripheral edema, and patient posture. As three observations had missing data on peripheral edema, this analysis was based on 215 observations.
Table 2 Number of mortalities (with mortality rates per 100 patient-years) in patients with AECOPD, by selected dichotomous covariables
Table 3 Number of mortalities (with mortality rates per 100 patient-years) in patients with AECOPD, by quartiles of selected continuous covariables
Table 4 Hazard ratios (with 95% confidence intervals) for dying during a median of 1.9 years follow up after acute exacerbation of COPD
Validation against NT-proBNP
Several of the variables that differed between the radiological assessments also showed an association with NT-proBNP concentration. These variables were age, BMI, history of HF, edema and atrial fibrillation on admission, heart rate, mean arterial pressure, SaO2, PaO2, PaCO2, serum CRP, and patient posture. and show the geometric means of baseline NT-proBNP concentrations and the corresponding P-values for these variables.
Table 5 Geometric mean (with number of observations) of baseline NT-proBNP concentration (pg/mL), by selected dichotomous variables
Table 6 Geometric mean of baseline NT-proBNP concentrations (pg/mL), by quartiles of selected continuous variables
When these variables were included in a LMM, a model with fixed effects only (ie, no random effects) and a spatial linear covariance structure had the lowest AIC (the best fit). Reduction of the full model resulted in the final model shown in , adjusting for sex and statistically significant associations. With the standardized assessment, the NT-proBNP concentrations were 50% higher in patients with pulmonary congestion than in patients without (ratio 1.8 [95% CI: 1.2–2.6] [P=0.003]). Using the routine assessment, there was no significant difference in NT-proBNP concentration (ratio 1.6 [0.96–2.6] [P=0.069]).
Table 7 Relative concentration of NT-proBNP in patients with acute exacerbation of COPD
Discussion
In this study, a standardized, liberal assessment of pulmonary congestion on chest radiographs obtained on admission for AECOPD identified a group of patients at increased risk of dying, which was not reported by routine radiological assessment. The standardized assessment was also more strongly associated with NT-proBNP concentrations, indicating that this assessment more accurately identified concomitant HF with pulmonary congestion. A higher awareness of HF among COPD patients is warranted as it may have therapeutic consequences.
Although we applied accepted criteria for diagnosing pulmonary congestion,Citation8,Citation26 our standardized assessment may be regarded as liberal. Applying a liberal definition inherently carries a risk of misclassifying normal radiographs as pathological. This error is expected to increase the sensitivity, but attenuate the specificity. However, in the present study, compared with the routine assessment, the association with both mortality and NT-proBNP became stronger using the standardized assessment. Both the sensitivity and specificity were higher, meaning that the standardized assessment not only identified more patients at risk of dying, but also, acquitted more patients with a better prognosis. Consequently, it was superior in identifying patients at risk of dying, as reflected by the greater (though admittedly still low) area under the curve (AUC) value in the ROC analysis. Hence, in order to detect patients at risk, clinicians as well as radiologists should pay more attention to the pulmonary vasculature, particularly the upper lung fields, in COPD patients hospitalized with worsening of dyspnea. It is not unlikely that such findings are overlooked as the underlying cause of the exacerbation in these patients is expected to be an acute inflammatory or infectious process in the airways.
We investigated the hypothesis that the standardized assessment identified more patients with increased NT-proBNP concentrations than did the routine assessment. The two assessments concurred with regard to pulmonary congestion in the majority of cases, and the NT-proBNP concentrations could not be expected to be significantly different between them. However, when compared in a multivariable analysis, only the standardized assessment remained associated with NT-proBNP. Among the observations that were negative in the routine assessment but positive in the standardized assessment, the NT-proBNP concentrations were relatively high. In two studies,Citation27,Citation28 NT-proBNP concentrations below 264 and 1,000 pg/mL, respectively, were optimal to rule out HF in AECOPD, while 1,800 pg/mL and 2,500 pg/mL, respectively, were suggested to confirm the presence of concomitant HF. In light of this, many of the patients identified by the standardized, but not by the routine assessment, may have suffered from HF (as well as from COPD exacerbation) on admission. However, the diagnosis of left HF cannot be established by NT-proBNP alone as natriuretic peptides may originate from the right ventricle, particularly in these patients.
In all, 44% of the patients with pulmonary congestion found by the standardized assessment had a history of HF compared with 29% found with the routine assessment. The study investigators were blinded for all clinical data, but the clinicians who performed the initial assessment were not. Thus, despite potential information of known HF, the routine assessment was less likely to diagnose pulmonary congestion. There may have been some systematic clinical bias in that the radiologists were informed that the radiograph under investigation was from a COPD patient with (yet another) acute exacerbation. This may influence the radiologists to describe it as such, without specifically looking for signs of HF. Such an approach should be discouraged on the basis of our findings.
Radiological infiltrates and serum CRP both showed some association with the standardized assessment, mortality, and the NT-proBNP level, and one might speculate whether we have identified patients with lower airway infections rather than HF. The review of the patient records does not support this as the proportion of patients with a discharge diagnosis of pneumonia/airway infection was similar across the groups (data not shown).
The radiology fellow and the pulmonologist performing the standardized assessment had about the same experience as the attending and senior physicians, respectively, performing the routine assessments. The investigators, including a pulmonologist, performing the standardized assessment were of course aware that they did this as part of a study; the radiologists who performed the routine assessments were not. Moreover, one of our hypotheses was that unrecognized HF in COPD patients is common. Thus, the awareness of the investigators performing the standardized assessment was higher, and the threshold to rule in pulmonary congestion may have been lower. As they were blinded to the clinical data and objectively determined the presence or absence of predefined radiological features, we have sought to minimize this potential bias. However, it is noteworthy, if it proves true, that one can identify COPD patients at increased risk of dying simply by increasing the awareness of pulmonary congestion. Indeed, this is a key message from this study: To minimize the potential prejudice and subjectivity in the interpretations, radiographs from patients presenting with AECOPD should be systematically examined for signs of left HF as this may be challenging to diagnose clinically. If such signs are present, clinicians should take this into consideration. When identified, heart disease in COPD patients should be treated according to guidelines, as emphasized in the latest GOLD revision.Citation29 Therefore, in contrast to the recommendation made in a recent review,Citation8 we suggest chest radiography, an inexpensive and readily available procedure, to be performed as a routine examination in AECOPD.
In the multivariable analyses, we used data from 218 observations from 99 patients. By using statistical models described in standard textbooks,Citation22–Citation25 this can be done without introducing bias or inflating the results. Particularly regarding the survival analysis, each patient contributes, with observation time from inclusion to death or censoring. However, the patients are allowed to change on readmissions (for example from congestion to not congestion or from CRP first quartile to CRP third quartile).
Limitations
Our study sample was quite small but was unselected. In spite of only 99 included patients, we showed a statistically significant difference in mortality between the two radiological assessments. Based on our results, a much larger sample would be needed to show significant differences in NT-proBNP.
This was a small single-center study, and we do not know to what extent the present findings can be generalized. The applicability of the assessment should be validated in other cohorts of COPD patients and different populations. Finally, there may be residual confounding in the material, ie, unidentified factors associated with radiologic interpretation and mortality.
Conclusion
By applying a standardized and liberal approach when evaluating pulmonary congestion on chest radiographs during AECOPD, a group of patients at increased risk of dying was identified. The observation that NT-proBNP concentrations were increased in these patients suggests that this may have been due, at least partially, to left-sided congestive heart failure. The systematic assessment of chest radiographs of COPD patients hospitalized for worsening of dyspnea may give important information that may guide their treatment.
Acknowledgments
The study was financed by The Norwegian Association of Heart and Lung Patients, through funds from the Norwegian Extra Foundation for Health and Rehabilitation. They played no role in the study design, collection of data, writing of the manuscript, or the decision to submit for publication.
The authors thank Dr Anke Neukamm, Akershus University Hospital, and Dr Lars Øivind Høiseth, Oslo University Hospital, for their assistance in analyzing the ECGs.
Disclosure
Torbjørn Omland has received speaker’s honoraria from Abbott Diagnostics, Siemens Healthcare Diagnostics, and Roche Diagnostics; and research grant support from Abbott Diagnostics and Roche Diagnostics, through Akershus University Hospital. The authors report no other conflicts of interest in this work.
References
- CurkendallSMDeLuiseCJonesJKCardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patientsAnn Epidemiol2006161637016039877
- FimognariFLScarlataSConteMEIncalziRAMechanisms of atherothrombosis in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis200831899618488431
- HuiartLErnstPSuissaSCardiovascular morbidity and mortality in COPDChest200512842640264616236937
- SidneySSorelMQuesenberryCPDeLuiseCLanesSEisnerMDCOPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care ProgramChest200512842068207516236856
- MullerNCoxsonHChronic obstructive pulmonary disease. 4: Imaging the lungs in patients with chronic obstructive pulmonary diseaseThorax2002571198298512403883
- ShakerSBDirksenABachKSMortensenJImaging in chronic obstructive pulmonary diseaseCOPD20074214316117530508
- McMurrayJJAdamopoulosSAnkerSDESC Committee for Practice GuidelinesESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESCEur Heart J201233141787184722611136
- CardinaleLVolpicelliGLamorteAMartinoJVeltriAndreaRevisiting signs, strengths and weaknesses of Standard Chest Radiography in patients of Acute Dyspnea in the Emergency DepartmentJ Thorac Dis20124439840722934143
- BrekkePHOmlandTSmithPSøysethVUnderdiagnosis of myocardial infarction in COPD – Cardiac Infarction Injury Score (CIIS) in patients hospitalised for COPD exacerbationRespir Med200810291243124718595681
- BuajordetIEbbesenJErikssenJBrørsOHilbergTFatal adverse drug events: the paradox of drug treatmentJ Intern Med2001250432734111576320
- MacchiaARodriguez MoncalvoJJKleinertMUnrecognised ventricular dysfunction in COPDEur Respir J2012391515821700606
- HøisethADNeukammAKarlssonBDOmlandTBrekkePHSøysethVElevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary diseaseThorax201166977578121653926
- KnudsenCWOmlandTCloptonPDiagnostic value of B-Type natriuretic peptide and chest radiographic findings in patients with acute dyspneaAm J Med2004116636336815006584
- ChangCLRobinsonSCMillsGDBiochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPDThorax201166976476821474497
- MedinaAMMartelesMSSáizEBPrognostic utility of NT-proBNP in acute exacerbations of chronic pulmonary diseasesEur J Intern Med201122216717121402247
- StolzDBreidthardtTChrist-CrainMUse of B-type natriuretic peptide in the risk stratification of acute exacerbations of COPDChest200813351088109418339792
- HøisethADOmlandTHagveTABrekkePHSøysethVNT-proBNP independently predicts long term mortality after acute exacerbation of COPD – a prospective cohort studyRespir Res2012139723107284
- HawkinsNMPetrieMCJhundPSChalmersGWDunnFGMcMurrayJJHeart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiologyEur J Heart Fail200911213013919168510
- HøisethADOmlandTHagveTABrekkePHSøysethVDeterminants of high-sensitivity cardiac troponin T during acute exacerbation of chronic obstructive pulmonary disease: a prospective cohort studyBMC Pulm Med2012122222651225
- RautaharjuPMWarrenJWJainUWolfHKNielsenCLCardiac infarction injury score: an electrocardiographic coding scheme for ischemic heart diseaseCirculation19816422492567249294
- World Health OrganizationThe International Classification of Diseases (ICD) Available from: http://www.who.int/classifications/icd/en/Accessed November 1, 2013
- HosmerDWJrLemeshowSMaySApplied Survival Analysis: Regression Modeling of Time-to-Event Data2nd edHoboken, NJWiley-Interscience2008
- KleinbaumDGKleinMSurvival Analysis. A Self-Learning Text2nd edNew York, NYSpringer2005
- DigglePJHeagertyPLiangKYZegerSLAnalysis of Longitudinal Data2OxfordOxford University Press2002
- FitzmauriceGMLairdNMWareJHApplied Longitudinal AnalysisNew York, NYJohn Wiley & Sons2004
- NovellineRASquire’s Fundamentals of Radiology6th edBoston, MAHarvard University Press2004
- AbrougFOuanes-BesbesLNciriNAssociation of left-heart dysfunction with severe exacerbation of chronic obstructive pulmonary disease: diagnostic performance of cardiac biomarkersAm J Respir Crit Care Med2006174999099616840745
- BaggishALSiebertULainchburyJGA validated clinical and biochemical score for the diagnosis of acute heart failure: the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Acute Heart Failure ScoreAm Heart J20061511485416368291
- goldcopd.org [homepage on the Internet]From the global strategy for the diagnosis, management and prevention of COPDGlobal Initiative for Chronic Obstructive Lung Disease (GOLD)2013 Available from: http://www.goldcopd..org/Accessed October 31, 2013