Abstract
Background
The use of domiciliary noninvasive positive pressure ventilation (NPPV) in stable chronic obstructive pulmonary disease (COPD) with chronic hypercapnic respiratory failure has yielded variable effects on survival, quality of life, and dyspnea. We hypothesized that use of NPPV in stable COPD and partial pressure of carbon dioxide (PaCO2) <52 mmHg might result in improvement in quality of life and dyspnea.
Methods
Thirty patients with stable COPD (forced expiratory volume in the first second <50% predicted and PaCO2 <52 mmHg) were prospectively randomized to receive domiciliary NPPV (bilevel positive airway pressure, 15/5 cm H2O) or usual therapy for 6 months. Measurements were made at baseline, 6 weeks, 3 months, and 6 months. Primary outcomes were quality of life as assessed by the Chronic Respiratory Disease Questionnaire (CRQ), and dyspnea as measured by the Transitional Dyspnea Index (TDI).
Results
Fifteen subjects in the NPPV arm and 12 controls completed all the study visits. At 6 weeks and 3 months, the NPPV arm showed significant improvement in TDI total score. However, this effect persisted only in the TDI-Task at 6 months (P=0.03). NPPV use was associated with a small improvement in the CRQ-Mastery domain (0.6 versus −0.1, P=0.04). The arterial partial pressure of oxygen (PaO2) in the control arm worsened over the period of the study, whereas it remained stable in the NPPV arm (change −7.2 mmHg versus +2.1 mmHg, respectively, P=0.02).
Conclusion
NPPV resulted in a small improvement in quality of life indices in stable COPD patients with PaCO2 <52 mmHg. Future larger studies will clarify the role of NPPV in this stable subgroup of patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with a progressive decline in lung function and resultant chronic respiratory failure. This leads to significant rates of hospitalization, disability, and death.Citation1 COPD is also associated with sleep-disordered breathing and nocturnal hypoventilation, resulting in nocturnal and daytime hypoxemia and hypercapnia.Citation2,Citation3 These, in turn, can contribute to fatigue, dyspnea, and impaired quality of life. Nocturnal noninvasive positive pressure ventilation (NPPV) can theoretically rest overloaded respiratory muscles, prevent nocturnal hypoventilation, and reset central respiratory drive in patients with hypercapnia. The use of NPPV in late-stage COPD appears logical to change this inexorable course, to alleviate symptoms, and to improve quality of life. While NPPV has a definite role in the management of acute hypercapnic respiratory failure,Citation4 its role in the management of late-stage stable COPD is controversial. Multiple studies have shown no survival benefit for nocturnal NPPV in chronic stable hypercapnic COPD patients.Citation5–Citation10 The effects on quality of life and dyspnea scores in such patients have been inconsistent.Citation8–Citation11 The variable efficacy might have been due to late administration of the intervention in the course of disease, in the presence of advanced hypercapnic respiratory failure. We sought to assess the effect of applying NPPV on indices of health-related quality of life in patients with relative normocapnia. We hypothesized that patients with stable severe COPD and partial pressure of carbon dioxide (PaCO2) <52 mmHg have improved quality of life and reduced dyspnea with the use of NPPV.
Materials and methods
We conducted a prospective, randomized controlled study.Citation12 Subjects were screened from the pulmonary clinic at the University of Iowa Hospital. The protocol was approved by the institutional review board (20010044) and informed consent was obtained from each subject.
Patients
Subjects with stable COPD, defined as a forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) ratio of <0.70, with at least a 10 pack-year smoking history and PaCO2 <52 mmHg were eligible.Citation13 Stable COPD was defined as no exacerbations in the 4 weeks prior to initiation of the study. Only patients with a low clinical probability of having obstructive sleep apnea as assessed using the Berlin Questionnaire were included. This was done to remove the possible confounding effect of sleep apnea.Citation14 Those with diagnoses of congestive heart failure, obstructive sleep apnea, chronic respiratory conditions other than COPD, age <35 years, any disease limiting life expectancy to less than 2 years, active malignancy other than nonmelanotic skin cancer in the previous 2 years, and with any anatomic variation or disease process that precluded wearing a nasal mask were excluded. Subjects could be active or ex smokers, but had to have a stable smoking status for at least 6 months prior to study initiation. Patients could also have undergone pulmonary rehabilitation or be in a maintenance program, but they could not initiate a pulmonary rehabilitation program during the 6-month observation period.
Subjects were randomized to receive or not receive domiciliary NPPV at 15/5 cm H2O. Subjects were randomized in a 1:1 ratio to receive NPPV versus control treatment using a random number generator. Randomization was done using opaque sealed envelopes which were opened during screening visits. For this study, we defined hypercapnia as ≥52 mmHg based on the criteria used for reimbursement for noninvasive ventilation by the Centers for Medicare and Medicaid Services.Citation15 Once subjects were randomized to the NPPV arm, they were initiated on NPPV at home by a respiratory therapist. Subjects were fitted with a nasal-oral contour comfort gel full face mask (Respironics Inc, Murrysville, PA, USA) or nasal pillows (Nellcor Puritan-Bennett Inc, Pleasanton, CA, USA) as needed for optimal comfort and minimal leak. NPPV was initiated at home using a BiPAP® Synchrony ventilator (Respironics Inc) and titrated to final pressures of 15/5 cm H2O within a week. Subjects were instructed to use the ventilator all night or for at least 6 hours every night for the next 6 months. Respiratory therapists called the subjects every day during the first week to ensure optimal usage and to troubleshoot any complications. The therapists also made a home visit to check the use of domiciliary NPPV periodically during the first week and as needed thereafter. All subjects were provided with heated humidification.
A complete history and physical examination was undertaken, with a posterolateral chest radiograph, 12-lead electrocardiogram, lung physiology (spirometry, arterial blood gases on room air), and respiratory muscle strength (negative inspiratory force [NIF]) at baseline. We measured exercise capacity by the six-minute walk test.Citation16 Dyspnea was assessed using the Baseline Dyspnea Index and the Transitional Dyspnea Index (TDI).Citation17 We included a global assessment of health-related quality of life developed for patients with chronic lung disease, ie, the Chronic Respiratory Disease Questionnaire (CRQ).Citation18 Sleep quality was assessed using the Pittsburgh Sleep Quality Index.Citation19 Subjects were reassessed at 6 weeks, 3 months, and 6 months after randomization. The primary outcome was change in the CRQ domains and change in TDI. The minimal clinically important difference in CRQ was defined as a change in the score by 0.5 units,Citation18 and that in TDI by one unit.Citation17 Secondary outcomes were change in NIF, arterial partial pressure of oxygen (PaO2), FEV1, and six-minute walk test. Ventilator use data were downloaded using Encore software (Respironics Inc) at each follow-up visit.
Side effects and compliance
At each visit, subjects on NPPV were asked about side effects, such as dryness of eyes, sinus congestion, skin abrasions, and epistaxis. Compliance was assessed by both patient-reported hours of use at each visit and machine-downloaded data for hours of use. Data obtained from the machine were used for analyses.
Statistical analysis
Results are expressed as the mean (standard deviation) for normally distributed data and median with interquartile range for nonuniformly distributed data. The baseline characteristics of subjects in the NPPV and control arms were compared using the Student’s t-test for continuous variables and the Mann–Whitney U test as appropriate, and the chi-square test for categorical variables. Differences within groups at the end of 6 months were compared with baseline using the paired t-test and between groups using the independent t-test. Repeated-measures analysis of variance was used to assess significant changes over time, and the independent t-test was used to assess where any change occurred between groups.
Distribution of data was assessed using Mauchly’s test of sphericity, and Greenhouse-Geisser correction was used where sphericity could not be assumed. Relationships between outcomes and change from baseline to 6 months in variables that could affect outcomes were assessed using Spearman’s correlation. A P-value<0.05 was considered to be statistically significant. Multivariate analyses for outcomes after adjusting for baseline PaCO2 and NIF were done and showed no change in the results, and hence are not reported. We did not make any sample size justification because this was a pilot study in relatively normocapnic subjects. All analyses were performed using Statistical Package for the Social Sciences version 11.5 software (SPSS Inc., Chicago, IL, USA).
Results
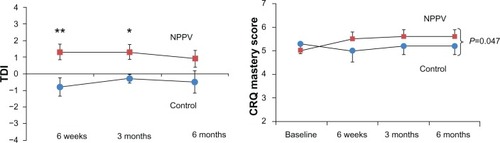
Thirty subjects with COPD were recruited. Twenty-seven completed all assessments, ie, 15 in the NPPV group and 12 in the control group (). The baseline characteristics of subjects who were able to complete the study are shown in . There were a higher proportion of males in the control group. The two groups were well matched for age, body mass index, disease severity, and baseline indices of dyspnea, functional capacity, quality of life, and sleep indices. At 6 weeks and 3 months, NPPV resulted in a significant improvement in TDI in the NPPV arm (data in , ). However, this effect persisted only in the TDI-Task at 6 months (−0.8 versus −1.8, P=0.03). shows the effects of NPPV at the end of 6 months. NPPV did not result in improvement in global quality of life as assessed by the total CRQ score. Subjects using NPPV did experience a small improvement in the mastery domain of the CRQ, a difference in the realm of being clinically significant (0.6 versus −0.1, P=0.04, ). When assessed by repeated measures over time, only the improvement in the mastery domain of CRQ was sustained (data in ).
Table 1 Comparison of baseline characteristics in cases and controls
Table 2 Comparison of outcomes between intervention and control arms at 6 months
Figure 1 Randomization and follow-up of patients in intervention and control arms.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; PaCO2, partial pressure of carbon dioxide in arterial blood; NPPV, noninvasive positive pressure ventilation.
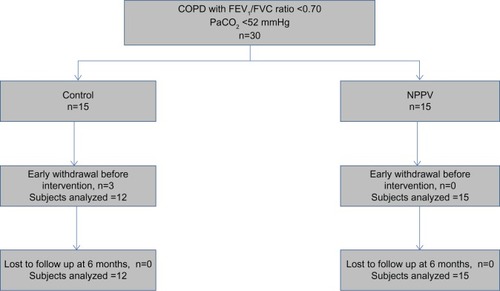
Figure 2 Change in dyspnea measured by TDI and quality of life (CRQ-Mastery domain) over time in intervention and control arms. Dots represent control arm; squares represent intervention arm.
Abbreviations: TDI, Transitional Dyspnea Index; CRQ, Chronic Respiratory Disease Questionnaire; NPPV, noninvasive positive pressure ventilation.
The oxygenation status of controls worsened over the period of the study (−7.2 mmHg), whereas NPPV resulted in conservation of oxygen levels (+2.1 mmHg, P=0.02). There was a trend towards improvement in NIF (delta 7 with NPPV versus delta 0.3 in controls), but this did not reach statistical significance (P=0.14). There was no change in PaCO2. There was also no improvement in six-minute walk distance. NPPV did not result in a change in FEV1. There was no difference in the number of exacerbations in either group during the period of the study, with one exacerbation in each group.
Compliance and side effects
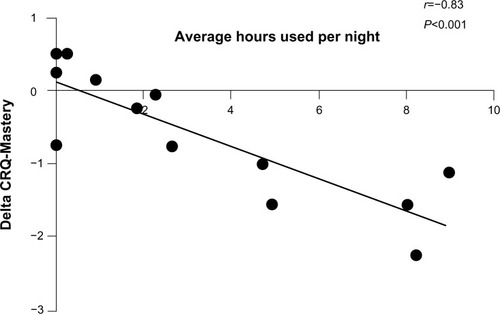
The average duration of NPPV use per night was about 3.1 hours (). More than 40% used it for more than 4 hours per night. More than half (8/15) used it for more than 4 hours in the first week (initial adopters). Of those using NPPV for more than 4 hours per night in the first week, 75% used NPPV for a mean of more than 4 hours per night for the entirety of the study. Eighty-eight percent of initial adopters expressed interest in continuing use of NPPV beyond the duration of the study. Even though there were some adverse effects from using NPPV, these were minimal (). There was no change in the quality of sleep (). Overall, 53% of subjects indicated a willingness to continue using NPPV beyond the period of the study. Interestingly, the improvement in CRQ-Mastery correlated significantly with the average number of hours of NPPV use (Spearman’s r=−0.83, P<0.001, ). More side effects were reported by subjects in the NPPV arm, although this did not preclude them from continued use of the intervention ().
Table 3 Compliance with noninvasive positive pressure ventilation
Table 4 Side effects
Discussion
We showed that there was a small improvement in some quality of life indices in patients with stable COPD administered NPPV for 6 months. There was a significant improvement in CRQ-Mastery and TDI-Task over time with application of NPPV. There was also a beneficial effect on PaO2.
The role of domiciliary nocturnal NPPV in the management of chronic stable COPD is controversial. The current literature is conflicting, but seems to favor its use in patients with hypercapnia, and with high intensity, targeting lowering PaCO2 levels. While multiple retrospective observational studies have shown a survival benefit for nocturnal NPPV in chronic stable hypercapnic COPD patients,Citation5–Citation7,Citation20 these results have not been supported in randomized controlled trials.Citation8–Citation10 These, and other smaller studies, also found inconsistent effects on sleep quality, quality of life, and dyspnea scores in hypercapnic COPD patients using NPPV.Citation8–Citation11,Citation21–Citation23 Most of these trials attempted to use varying intensity of NPPV to reduce daytime hypercapnia and improve gas exchange, and seemed to indicate that adequate lowering of PaCO2 and nocturnal hypoventilation was associated with the most benefits.Citation24 It is possible that the inconsistent effects of NPPV were due to usage of low levels of pressure. Dreher et al showed in a short-duration study that high-intensity NPPV with controlled pressure can significantly reduce nocturnal PaCO2, improve dyspnea scores, lung function tests, and quality of life.Citation25 Based on these, Medicare and Medicaid Services recommended a cutoff of ≥52 mmHg, in addition to other criteria, for initiating domiciliary nocturnal NPPV in stable hypercapnic patients.Citation15 In contrast with these recommendations, we hypothesized that the inconsistent effects seen in previous studies might have been due to the intervention being late in the course of disease.
In patients without hypercapnia, NPPV cannot be targeted toward PaCO2, but might still benefit patients by lowering the work of breathing. Although ours is the first study to target this group specifically, other studies did include subjects who had similar PaCO2 levels (). To our knowledge, no study has compared the effect of NPPV in normocapnic COPD with controls not undergoing another intervention such as rehabilitation and domiciliary oxygen that could confound the results.
Table 5 Comparison of randomized studies of domiciliary NPPV in chronic stable COPD without hypercapnea
NPPV use was associated with some minor benefits in our study in the domain of mastery, and in preservation of PaO2. This supports the results seen in two other short-duration studies aiming to recruit hypercapnic patients, but with PaCO2 levels comparable with our inclusion criteria.Citation21,Citation23 The difference in oxygenation levels was a surprising finding, and is most likely due to NPPV leading to redistribution of airflow toward areas of better perfusion, resulting in improvement in ventilation/perfusion matching.Citation26 Results from short-duration trials cannot be generalized because there can be a significant placebo effect from the mask,Citation27 and there can also be significant problems with long-term compliance.Citation28 A longer-duration randomized study by Casanova et al did not have PaCO2 level as an inclusion criterion but had a mean PaCO2 of 50.7 mmHg. In this study, there was a marginal improvement in Borg dyspnea score and in psychomotor coordination.Citation8 Only the study by Garrod et al had PaCO2 levels comparable with those seen in our study. While they did show an improvement in exercise capacity, oxygenation, and CRQ, the study compared the effect of adding NPPV to an exercise program with exercise alone.Citation11 We showed a change in some quality of life indices, but these were limited by the small number of subjects. In addition to baseline PaCO2 and improvement in hypoventilation, the other major factor that can affect study results is the intensity of NPPV used. Both these comparable randomized controlled studies used inspiratory and expiratory pressures similar to those used in the current study.Citation8,Citation11
Our study has a number of strengths. We studied a group of patients who were not significantly hypercapnic, which is the usual target group for this intervention. We excluded patients with sleep apnea because improvement in sleep can potentially result in improvement in quality of life scores, independent of COPD-related improvement. We meticulously assessed side effects resulting from use of the NPPV mask. There were also some limitations to our study. We did not have a sham arm, and some of the results could be due to a placebo effect. The control subjects were all male, and while we are not aware of any gender differences in response to NPPV, this may have played a role. This study was underpowered to assess end points such as mortality or exacerbation frequency. However, those were not the main aims of our study. Compliance was low, probably due to lack of titration of pressures to each subject, lack of a run-in period, and the nature of the intervention. However, this is not unique to our study.Citation11
In conclusion, use of NPPV is associated with minor improvements in PaO2 and the subdomains of quality of life and dyspnea indices. Whether attainment of the minimal clinically important difference of 0.5 for the mastery domain is worth the cost and inconvenience of NPPV can only be determined by each patient based on cost and tolerance. Nevertheless, this study found a significant difference in this domain. Based on this small study, NPPV has some benefit in COPD patients with PaCO2 <52 mmHg. Better compliance may be associated with improved outcome benefits in this group. Future large studies or meta-analyses in this group will clarify the utility of this intervention.
Acknowledgments
We extend our gratitude to the study subjects, to Charles Dayton for help with the institutional review board approval process, and to Bridget Zimmerman for statistical assistance.
Supplementary material
Table S1 Comparison between groups at each time point and over all time points (repeated-measures analysis of variance)
Disclosure
The study was sponsored by Respironics Inc, which had no input in the design, data, or writing of the paper. Otherwise none of the authors have any conflicts of interest to declare.
References
- [No authors listed]Deaths from chronic obstructive pulmonary disease – United States, 2000–2005MMWR Morb Mortal Wkly Rep200857451229123219008792
- CatterallJRDouglasNJCalverleyPMTransient hypoxemia during sleep in chronic obstructive pulmonary disease is not a sleep apnea syndromeAm Rev Respir Dis1983128124296870065
- O’DonoghueFJCatchesidePGEllisEESleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: prevalence and associated factorsEur Respir J200321697798412797491
- KeenanSPMehtaSNoninvasive ventilation for patients presenting with acute respiratory failure: the randomized controlled trialsRespir Care200954111612619111111
- JonesSEPackhamSHebdenMSmithAPDomiciliary nocturnal intermittent positive pressure ventilation in patients with respiratory failure due to severe COPD: long-term follow up and effect on survivalThorax19985364954989713450
- BudweiserSHitzlAPJorresRAImpact of noninvasive home ventilation on long-term survival in chronic hypercapnic COPD: a prospective observational studyInt J Clin Pract20076191516152217686094
- WindischWKosticSDreherMVirchowJCJrSorichterSOutcome of patients with stable COPD receiving controlled noninvasive positive pressure ventilation aimed at a maximal reduction of Pa(CO2)Chest2005128265766216100151
- CasanovaCCelliBRTostLLong-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPDChest200011861582159011115443
- CliniESturaniCRossiAThe Italian multicentre study on non-invasive ventilation in chronic obstructive pulmonary disease patientsEur Respir J200220352953812358325
- McEvoyRDPierceRJHillmanDNocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trialThorax200964756156619213769
- GarrodRMikelsonsCPaulEAWedzichaJARandomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20001624 Pt 11335134111029341
- University of IowaTrial of Non-invasive Ventilation for Stable COPD Available from: http://clinicaltrials.gov/show/NCT1722773. NLM identifier: NCT1722773Accessed October 24, 2013
- American Thoracic Society/European Respiratory Society Task ForceStandards for the Diagnosis and Management of Patients with COPD. Version 1.2New York, NYAmerican Thoracic Society2004 Available from: http://www.thoracic.org/clinical/copd-guidelines/Accessed October 18, 2013
- NetzerNCStoohsRANetzerCMClarkKStrohlKPUsing the Berlin Questionnaire to identify patients at risk for the sleep apnea syndromeAnn Intern Med1999131748549110507956
- US Department of Health and Human Services, Center for Medicare and Medicaid ServicesDurable medical equipment reference listMedicare Coverage Issues Manual §609Baltimore, MDCMS2002
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function LaboratoriesATS statement: guidelines for the six-minute walk testAm J Respir Crit Care Med2002166111111712091180
- WitekTJJrMahlerDAMinimal important difference of the transition dyspnoea index in a multinational clinical trialEur Respir J200321226727212608440
- GuyattGHBermanLBTownsendMPugsleySOChambersLWA measure of quality of life for clinical trials in chronic lung diseaseThorax198742107737783321537
- BuysseDJReynoldsCF3rdMonkTHBermanSRKupferDJThe Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and researchPsychiatry Res19892821932132748771
- WindischWHaenelMStorreJHDreherMHigh-intensity non-invasive positive pressure ventilation for stable hypercapnic COPDInt J Med Sci200962727619277252
- StrumpfDAMillmanRPCarlisleCCNocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary diseaseAm Rev Respir Dis19911446123412391741532
- Meecham JonesDJPaulEAJonesPWWedzichaJANasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPDAm J Respir Crit Care Med199515225385447633704
- LinCCComparison between nocturnal nasal positive pressure ventilation combined with oxygen therapy and oxygen monotherapy in patients with severe COPDAm J Respir Crit Care Med19961542 Pt 13533588756806
- WijkstraPJNon-invasive positive pressure ventilation (NIPPV) in stable patients with chronic obstructive pulmonary disease (COPD)Respir Med200397101086109314561015
- DreherMStorreJHSchmoorCWindischWHigh-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trialThorax201065430330820388753
- De BackerLVosWDieriksBThe effects of long-term noninvasive ventilation in hypercapnic COPD patients: a randomized controlled pilot studyInt J Chron Obstruct Pulmon Dis2011661562422135493
- JenkinsonCDaviesRJMullinsRStradlingJRComparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trialLancet199935391702100210510382693
- CrinerGJBrennanKTravalineJMKreimerDEfficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failureChest1999116366767510492269