Abstract
Purpose
Breathlessness is a predominant symptom of chronic obstructive pulmonary disease (COPD), making it a valuable outcome in addition to lung function to assess treatment benefit. The phosphodiesterase-4 inhibitor roflumilast has been shown to provide small but significant improvements in dyspnea, as measured by the transition dyspnea index (TDI), in two 1-year studies in patients with severe to very severe COPD.
Patients and methods
To provide a more comprehensive assessment of the impact of roflumilast on dyspnea, post hoc analyses of four 1-year roflumilast studies (M2-111, M2-112, M2-124, and M2-125) in patients with moderate to very severe COPD were conducted.
Results
In this pooled analysis (N=5,595), roflumilast significantly improved TDI focal scores versus placebo at week 52 (treatment difference, 0.327; P<0.0001). Roflumilast was associated with significantly greater TDI responders and significantly fewer TDI deteriorators (≥1-unit increase or decrease from baseline, respectively) versus placebo at week 52 (P<0.01, both); these significant differences were apparent by week 8 and maintained until study end (P<0.05, all). At study end, the postbronchodilator forced expiratory volume in 1 second improvement in TDI responders was significantly greater with roflumilast versus placebo (P<0.05). Similar to the overall population, improvements in TDI focal scores at week 52 were small but consistently significant over placebo in patients with chronic bronchitis, regardless of exacerbation history, concomitant treatment with short-acting muscarinic antagonists or long-acting β2-agonists, or pretreatment with inhaled corticosteroids.
Conclusion
This analysis shows that patients treated with roflumilast to reduce exacerbation risk may also experience small but significant improvements in dyspnea, with accompanying improvements in lung function.
Introduction
Current recommended treatment goals for chronic obstructive pulmonary disease (COPD) include reduction of breathlessness, improvement of exercise capacity and health status, and prevention of exacerbations.Citation1 As dyspnea is a predominant symptom of COPD, an appreciation of impaired lung function and breathlessness severity may better reflect the impact of treatment.Citation1 While treatment guidelines recommend a relatively simple approach to evaluating dyspnea (ie, modified Medical Research Council ScaleCitation1), more sophisticated tools exist to assess breathlessness in more detail.Citation2,Citation3 One example is the transition dyspnea index (TDI),Citation4 a validated tool that assesses the impact of a patient’s daily activities on breathlessness.Citation5 It has been shown to reliably denote distinct changes in breathing difficulty due to therapeutic intervention.Citation6–Citation10
The phosphosdiesterase-4 inhibitor roflumilast, approved to reduce the risk of exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations, has been shown to reduce markers of inflammation in COPD patient airways.Citation11 As breathlessness is thought to arise primarily from mechanical limitation of inspiratory effort,Citation12 we wondered whether an agent with potential anti-inflammatory activities like roflumilast would impact dyspnea. Small but significant improvements in dyspnea, measured by the TDI, have been previously reported with 1 year of roflumilast treatment compared with placebo in two studies in COPD patients;Citation13 however, these data have not been examined in detail, nor was the sample size in previously reported studies large enough to explore relevant clinical associations. In addition, significant lung function improvements with roflumilast treatment have also been reported in placebo-controlled studies in patients with COPD,Citation13–Citation15 but have not yet been evaluated with regard to dyspnea-related outcomes. Thus, to more comprehensively evaluate the impact of roflumilast on breathlessness, including any potential relationship with lung function improvement, individual and pooled analyses of four 1-year clinical studies with roflumilast are presented here. Results from this analysis may have potential implications in the pharmacological management of COPD with regard to the current recommended treatment goals of reduction of COPD symptoms and prevention of exacerbations.Citation1
Material and methods
Study design and patients
Data were pooled from four 1-year placebo-controlled, double-blind, multicenter, Phase 3 clinical trials (M2-111, M2-112, M2-124, and M2-125) with once-daily roflumilast 500 μg, which were designed to enroll patients with forced expiratory volume in 1 second (FEV1), 50% predicted. Patients in M2-124 and M2-125, but not M2-111 and M2-112, were required to have chronic bronchitis and a history of exacerbations. Full details of methodology and patient selection have been reported elsewhere.Citation13–Citation15 All four studies were approved by local ethical review committees and performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Assessments
Dyspnea was measured at baseline using the baseline dyspnea index (BDI) total score and during treatment (weeks 4, 8, 12, 20, 28, 36, 44, and 52) using the TDI focal score, which evaluates changes from the baseline measurement. BDI total score ranges from 0 (no dyspnea) to 12 (severe dyspnea); TDI focal score ranges from −9 (major deterioration) to 9 (major improvement).
TDI responders were defined as patients who experienced a clinically meaningful improvement (change of ≥1 unit from baseline) in TDI focal score;Citation5 TDI deteriorators were patients who experienced a ≥1 unit deterioration from baseline. TDI nonresponders and non-deteriorators were those patients not meeting these thresholds. Outcomes assessed in TDI responders/nonresponders were TDI focal score at week 52, change from baseline to week 52 in postbronchodilator FEV1, and percentage of patients achieving a clinically meaningful improvement in FEV1 (≥100 mL)Citation16 from baseline to week 52.
Statistical analysis
Data were pooled for all randomized patients who took ≥1 dose of study medication in each study. Data analysis was performed post hoc for the overall patient population from the four studies, as well as for the following six subpopulations of patients with: 1) concomitant inhaled corticosteroid (ICS) treatment (M2-111 and M2-112); 2) chronic bronchitis (M2-111, M2-112, M2-124, and M2-125); 3) chronic bronchitis and concomitant short-acting muscarinic antagonist (SAMA) treatment (M2-111, M2-112, M2-124, and M2-125); 4) chronic bronchitis and a history of exacerbations (M2-111, M2-124, and M2-125); 5) chronic bronchitis, a history of exacerbations, and concomitant long-acting β2-agonists (LABA) treatment (M2-124 and M2-125); and 6) chronic bronchitis, a history of exacerbations, and ICS pretreatment (M2-124 and M2-125). Patients included in the subpopulation analyses were required to have non-missing baseline BDI and post-baseline TDI assessments.
Treatment comparisons on continuous variables were performed at week 52 (last observation carried forward [LOCF]) using analysis of covariance with terms for treatment group, BDI, age, smoking status, sex, ICS pretreatment, country/region, and study. Treatment comparisons on dichotomous/categorical variables were performed using a Cochran–Mantel–Haenszel test (pooled data were stratified by study) at week 52 (LOCF) and weeks 4, 8, 12, 20, 28, 36, 44, and 52 (observed cases). Statistical significance was assessed using an alpha level of 0.05.
Results
In M2-124 and M2-125, the proportion of patients pretreated with ICS was equal in both the roflumilast and placebo groups (42%); the proportions of patients who received concomitant ICS or LABA were also similar in both roflumilast (21% and 49%, respectively) and placebo (23% and 51%, respectively) groups. Similarly, in M2-111 and M2-112, the proportions of patients who received concomitant ICS or LABA were comparable in both roflumilast (61% and 8%, respectively) and placebo (60% and 9%, respectively) groups. For the overall pooled population, patient demographic and clinical characteristics were similar between treatment arms (). Of randomized patients who took ≥1 dose of study medication, 1,921/2,864 (67.1%) and 2,083/2,913 (71.5%) patients completed the trials in the roflumilast and placebo groups, respectively.
Table 1 Demographics and baseline characteristics
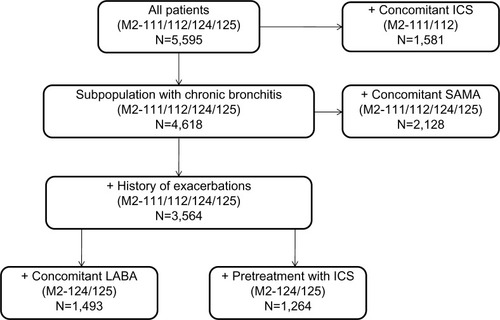
At week 52, roflumilast significantly improved mean TDI focal score versus placebo (roflumilast, 0.368; placebo, 0.041; difference [95% confidence interval {CI}], 0.327 [0.166–0.488]; P<0.0001). Between-group differences in TDI focal score for the pooled population were similar to those in the individual studies () and generally favored roflumilast. Significantly higher percentages of roflumilast-treated patients achieved clinically meaningful improvements in TDI focal score (TDI responders) versus placebo after 52 weeks of treatment (LOCF; 39.0% versus 33.9%, respectively; P=0.0001) (). Furthermore, the significantly higher percentage of TDI responders observed at week 52 was apparent by week 8 and maintained until study end (all, P<0.01) (). At week 52, roflumilast-treated patients were more likely to demonstrate clinically meaningful improvements in TDI than placebo-treated patients (); generally similar results were observed in the individual trials. Values of I2 for both analyses in were 0, indicating lack of observed statistical heterogeneity.Citation17 Overall, 20 patients need to be treated with roflumilast to have one additional patient with clinically meaningful improvements in TDI focal score over 1 year (crude number needed to treat, 19.9; 95% CI, 13.3–40.0). Conversely, roflumilast treatment was associated with significantly fewer TDI deteriorators versus placebo after 52 weeks (LOCF; 20.4% versus 23.7%, respectively; P=0.0022) (). This significant between-group difference was also apparent by week 8 and maintained for the remainder of the study (all, P<0.05).
Figure 1 Improvements in dyspnea in patients treated with roflumilast or placebo at week 52. (A) Differences and 95% CIs between roflumilast- and placebo-treated patients for TDI focal score and (B) odds ratios and 95% CIs for TDI responders in the roflumilast and placebo groups at week 52 (last observation carried forward) for the four pooled studies and for each individual study.
Notes: TDI focal scores for M2-124 and M2-125 reported in (A) are from the prespecified ANCOVA reported in the individual studies.Citation13 All other data in A and B are based on the ANCOVA model used in the current post hoc analysis, as no prespecified ANCOVA analyses were performed for these outcomes in the individual studies.
Abbreviations: ANCOVA, analysis of covariance; CI, confidence interval; TDI, transition dyspnea index.
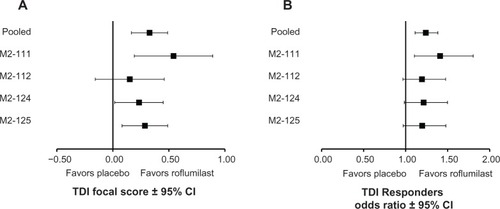
Figure 2 TDI responders (A) and deteriorators (B) for the overall population over time.
Abbreviations: LOCF, last observation carried forward; n, number of responders; N, number of patients analyzed; TDI, transition dyspnea index.
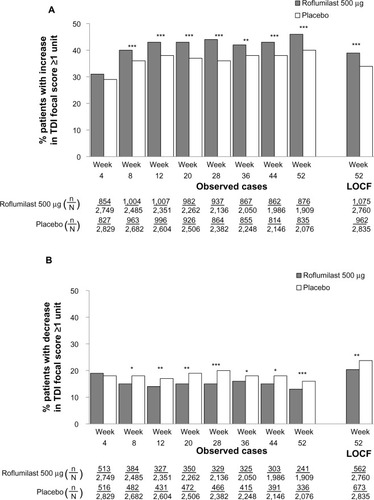
To explore the potential association of breathlessness with improved lung function, clinically meaningful improvements in TDI focal score and FEV1 were evaluated. In the overall population, the proportion of patients who achieved the minimum clinically important difference (MCID) of 100 mL in FEV1 (ie, FEV1 responders) was significantly greater with roflumilast versus placebo by week 4, which was maintained until study end (all, P<0.0001) ().
Figure 3 Percentage of patients who achieved a clinically meaningful (≥100 mL) improvement from baseline in postbronchodilator FEV1 (ie, FEV1 responders) over time for the overall population.
Abbreviations: FEV1, forced expiratory volume in 1 second; LOCF, last observation carried forward; n, number of responders; N, number of patients analyzed.
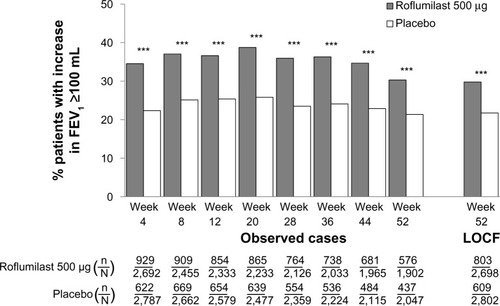
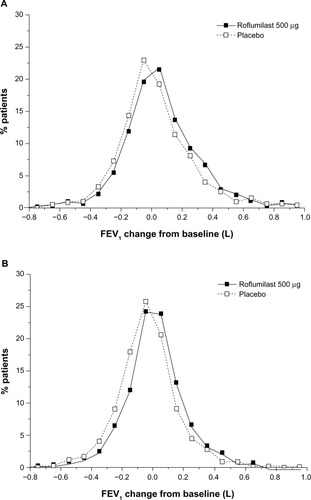
Among TDI responders, roflumilast led to significantly higher percentages of patients who achieved MCID in FEV1 (36%) versus placebo (29%; P<0.01) at study end. Furthermore, the magnitude of FEV1 improvement was significantly greater with roflumilast versus placebo in both TDI responders (P<0.05) and nonresponders (P<0.0001) (). Consistent with this observed treatment effect in the magnitude of FEV1 improvement with roflumilast in both TDI responders and nonresponders, a greater proportion of patients treated with roflumilast versus placebo demonstrated improvements from baseline in FEV1, a treatment effect (ie, shift to the right in favor of roflumilast) also observed in both TDI responders and nonresponders (). In addition, as the roflumilast curve is generally above that of placebo across the positive x-axis in both TDI responders and nonresponders, there were also a greater percentage of roflumilast-treated patients than placebo-treated patients for most levels of positive FEV1 change from baseline.
Figure 4 Mean change from baseline in postbronchodilator FEV1 in TDI responders and nonresponders at week 52.
Note: *P<0.05; ***P<0.001 versus placebo.
Abbreviations: FEV1, forced expiratory volume in 1 second; TDI, transition dyspnea index.
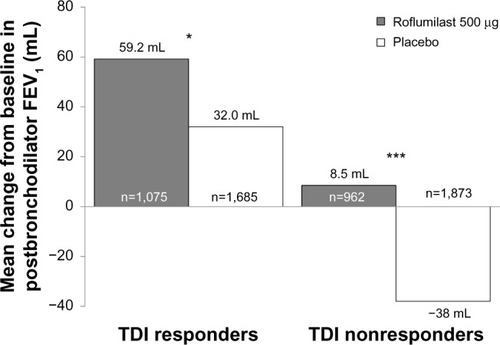
Figure 5 Frequency distribution of FEV1 improvements from baseline in TDI responders (A) and nonresponders (B) at study end.
Abbreviations: FEV1, forced expiratory volume in 1 second; TDI, transition dyspnea index.
Further analysis of the pooled ITT population revealed that particular baseline characteristics were associated with a significantly greater percentage of TDI responders (). These characteristics included being a former smoker (versus current), no concomitant use of LABAs or SAMAs (versus with), and no concomitant or pre-ICS use (versus with). To further analyze whether patient characteristics such as chronic bronchitis, history of exacerbations, or concomitant medications had an effect on TDI response, six subpopulations were examined (). In COPD patients with chronic bronchitis, roflumilast significantly improved TDI focal scores versus placebo (P<0.05) regardless of exacerbation history, concomitant treatment with SAMA or LABA, or pretreatment with ICS (). In the patient subpopulation treated with concomitant ICS, roflumilast was associated with significantly less deterioration in TDI focal score versus placebo (P=0.0114). TDI responders and deteriorators for each subpopulation are presented in –. In all subpopulations, treatment with roflumilast was associated with more TDI responders and fewer TDI deteriorators versus placebo at all treatment weeks; however, differences were not statistically significant at all treatment weeks.
Table 2 Percentage of TDI responders at week 52 (LOCF) according to baseline characteristics in pooled ITT population (N=5,595)
Table 3 TDI focal scores for subpopulations at week 52 (LOCF)
Discussion
As breathlessness in COPD patients worsens gradually over time and is related to lung function, physical activity, and quality of life, dyspnea has been considered a surrogate marker for disease progression.Citation18 Moderate improvements in breathlessness with roflumilast have previously been reported in patients with severe to very severe COPD.Citation13 To further characterize the effect of roflumilast on dyspnea, data from four 1-year roflumilast studies were pooled for those patients with or without clinically meaningful responses in TDI. In addition, evaluation of the corresponding FEV1 improvements among TDI responders explored the relationship between breathlessness and lung function.
The results from the current analysis confirm previous findings that roflumilast significantly improves breathlessness compared with placebo, as measured by TDI, in patients with severe to very severe COPD.Citation13 Furthermore, these results show that a significantly greater percentage of roflumilast-treated patients (40%) experienced clinically meaningful improvements in TDI focal score versus placebo (36%) starting at week 8 (P≤0.001); this 4% between-group difference was maintained to 1 year (LOCF; week 52; roflumilast, 39%; placebo, 34%; P<0.01 for all). Conversely, significantly fewer roflumilast-treated patients experienced clinically meaningful deteriorations in dyspnea compared with placebo-treated patients.
In patients with COPD, improvements in lung function have been reported to occur with decreased breathlessness.Citation19–Citation21 Patients with COPD in several 1-year studies of various inhaled bronchodilators exhibited improvements in trough FEV1 over placebo (range, 108–160 mL) at study endpoint, which were accompanied by improvements in TDI focal scores (range, 0.6–1.1 units).Citation22–Citation24 As roflumilast is an anti-inflammatory agent, and its effects on lung function in the individual studies pooled here (ie, between-treatment change in prebronchodilator FEV1 range, 36–58 mL)Citation13–Citation15 are lower than those seen for bronchodilators, it is not surprising that the effect of roflumilast on dyspnea reported in this pooled analysis (0.327 units) is lower in magnitude versus those of bronchodilators. However, consistent with studies demonstrating correlations between dyspnea and lung function improvements,Citation19,Citation21,Citation25 the present analysis demonstrates that roflumilast-treated patients with clinically meaningful improvements in dyspnea (TDI responders) had a significantly greater magnitude of improvement in FEV1 and were more likely to have a clinically meaningful improvement in lung function compared with placebo-treated patients.
Furthermore, the present analysis is consistent with a previous study that reported significant lung function improvements occurring earlier than significant reductions in breathlessness.Citation26 In the individual 1-year roflumilast studies, small but significant treatment-associated improvements in lung function were demonstrated as early as week 4.Citation14,Citation27 In the current analysis, significantly more patients achieved the FEV1 MCID with roflumilast treatment versus placebo as early as week 4 and at every study visit thereafter. In comparison, more roflumilast-treated patients achieved the TDI focal score MCID at week 4 compared with placebo; however, this difference did not reach significance until week 8, at which point it was maintained until study end. Together, these time courses suggest that significant lung function improvements may be observed earlier than significant dyspnea improvements. The mechanisms by which roflumilast may lead to improved airflow are not fully defined, but an anti-inflammatory effect has been suggested.Citation11,Citation28
Similar to results in the overall population, improvements in TDI focal scores at study end were small but consistently significant over placebo in patients with chronic bronchitis, regardless of exacerbation history, concomitant SAMA or LABA treatment, or ICS pretreatment. Although greater proportions of TDI responders were observed with roflumilast versus placebo in the subgroups of patients receiving concomitant ICS, LABA, or SAMA treatment throughout the treatment period, these differences were not significant at every study visit. In contrast, significantly greater percentages of TDI responders were consistently observed with roflumilast versus placebo from treatment weeks 8 through 52 in patients with chronic bronchitis, a history of exacerbations, and ICS pretreatment.
Consistent with the results presented here, a 6-month placebo-controlled study examining the effects of roflumilast in patients with moderate to severe COPD and chronic bronchitis who were already being treated with tiotropium demonstrated that significantly more roflumilast+tiotropium patients achieved clinically meaningful improvements in TDI focal score compared with placebo+tiotropium patients,Citation29 with a between-group difference (0.4 units) that was generally similar to that reported in the current analysis. In addition, compared with placebo, significantly more patients receiving roflumilast+tiotropium in the 6-month studyCitation29 achieved clinically meaningful differences in an instrument that assessed dyspnea associated with specific activities of daily living, the University of California, San Diego Shortness of Breath Questionnaire (SOBQ),Citation30 further demonstrating the positive impact of roflumilast treatment on breathlessness. Numerical improvements in TDI focal scores were also observed with the addition of roflumilast to salmeterol in a separate 6-month study in patients with moderate to severe COPD; however, these differences were not significantly different from patients treated with salmeterol and placebo (treatment difference of 0.1 unit).Citation31 It is of note that the inclusion criteria for the roflumilast+tiotropium trial (ie, chronic cough and sputum production and frequent use of short-acting β2-agonists during the run-in period while being treated with tiotropium ≥3 months prior to study enrollment) led to the recruitment of more symptomatic patients compared with patients recruited in the roflumilast+salmeterol trial. As such, the variation in the magnitude of treatment effect of roflumilast on dyspnea in tiotropium-treated patients versus salmeterol-treated patients may have been due to these differences in patients’ baseline characteristics between the two studies. Inclusion of both of these 6-month roflumilast trials in a recent meta-analysis by Pan et alCitation32 demonstrated a moderate but significant increase in TDI focal scores with roflumilast (weighted mean difference of 0.30 units; 95% CI, 0.14–0.46), similar to what was reported in the current analysis that focused on 1-year studies alone.
Increased dyspnea is strongly associated with reduced quality of life in COPD patients.Citation33,Citation34 In M2-111, the St George’s Respiratory Questionnaire (SGRQ),Citation35 a disease-specific quality of life instrument that assesses causes and impacts of dyspnea, was used to evaluate health status. Compared with placebo at week 52, roflumilast provided moderate but significant improvements in SGRQ total score, and a significantly greater percentage of patients achieved a clinically meaningful improvement in SGRQ with roflumilast (P<0.05 for both).Citation36 Furthermore, pooled data in patients with chronic bronchitis and/or emphysema (M2-111 and M2-112) demonstrated a significant difference in SGRQ total score improvement versus placebo after 52 weeks of treatment.Citation15 This suggests that roflumilast may similarly provide clinically meaningful improvements in quality of life in some COPD patients, possibly due to a reduction of breathlessness.
Several limitations of the present analysis should be noted. As all analyses were performed post hoc, and the included component studies were not designed to detect clinically meaningful improvements in dyspnea, these comparisons should be interpreted with caution and used primarily to generate hypotheses for future studies. Findings may not be generalizable to all patients with COPD, as inclusion criteria for each individual study contributing to this pooled analysis may have excluded patients typically seen in clinical practice. The use of LOCF in the current analysis may not have taken into account missing data that were nonrandom (ie, treatment-related discontinuations). The average placebo-adjusted improvements in dyspnea observed in the analyses presented here were small in magnitude; however, patients who improved their TDI focal score by ≥1 unit were observed with roflumilast treatment throughout the studies, suggesting that some patients treated with roflumilast may experience clinically meaningful improvements in breathlessness. Furthermore, the studies included in this analysis were only 1 year in duration. Since worsened breathlessness has been associated with an increased risk for COPD exacerbations,Citation37 and the effects of roflumilast on dyspnea may be related to a reduction in exacerbation rates, it is possible that longer studies in which more exacerbations would be prevented with roflumilast may enable observation of greater magnitudes of dyspnea improvement. As two of the four studies included in this pooled analysis had a population enriched with patients with a history of exacerbations, it is unclear whether COPD patients with chronic bronchitis who are frequent exacerbators would experience greater improvements in breathlessness compared with non-exacerbators.
Finally, this analysis was based on monotherapy trials that limited concomitant use of medications commonly used to treat COPD, such as fixed-dose ICS/LABA, long-acting muscarinic antagonist, or theophylline. While data derived from subgroups of patients who were pretreated with ICS or who received concomitant SAMA, LABA, or ICS treatment during the studies can be suggestive of potential treatment combinations, this may not be directly applicable to patients who are simultaneously prescribed multiple medications to manage their COPD.Citation38
Conclusion
In conclusion, these results further explore the effects of roflumilast on breathlessness, demonstrating small but generally consistent improvements in dyspnea with roflumilast treatment over placebo. Furthermore, patients that had reduced breathlessness also experienced accompanying improvements in lung function. Patients being treated with roflumilast to reduce their risk of exacerbations may also experience reduced dyspnea, regardless of patient characteristics such as chronic bronchitis, history of exacerbations, or previous (ICS) or concomitant (SAMA, LABA, or ICS) treatment. The added benefit of reducing breathlessness with roflumilast treatment, although small in magnitude, may provide an added value to consider in the use of this phosphodiesterase-4 inhibitor in the management of COPD.
Acknowledgments
The authors would like to thank Dana Creanga, PhD, a contractor employed by Forest Research Institute, Inc., at the time of these analyses, for assistance with data analysis. Medical writing and editorial support was provided by Prescott Medical Communications Group (Chicago, IL, USA) and was funded by Forest Research Institute, Inc. Studies were sponsored by Takeda Pharmaceuticals International GmbH (Zürich, Switzerland). Analyses were performed by Forest Research Institute (Jersey City, NJ, USA), a wholly owned subsidiary of Forest Laboratories, Inc., (New York, NY, USA). The sponsors were kept informed throughout the development of the manuscript. All scientific decisions were made by the authors independently and without restrictions of any kind. The sponsors had no involvement in the decision to submit the manuscript for publication.
Supplementary materials
Figure S1 TDI responders (A) and deteriorators (B) over time for the subpopulation of patients with chronic bronchitis.
Notes: *P<0.05; **P≤0.01; ***P≤0.001 versus placebo.
Abbreviations: LOCF, last observation carried forward; n, number of responders; N, number of patients analyzed; TDI, transition dyspnea index.
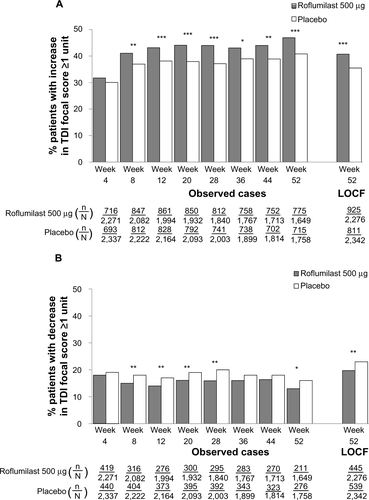
Figure S2 TDI responders (A) and deteriorators (B) over time for the subpopulation of patients with chronic bronchitis and a history of exacerbations.
Notes: *P<0.05; **P≤0.01; ***P≤0.001 versus placebo.
Abbreviations: LOCF, last observation carried forward; n, number of responders; N, number of patients analyzed; TDI, transition dyspnea index.
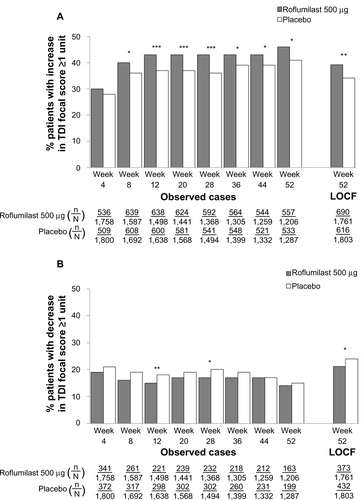
Figure S3 TDI responders (A) and deteriorators (B) over time for the subpopulation of patients with chronic bronchitis, a history of exacerbations, and pretreatment with inhaled corticosteroids.
Notes: *P<0.05; ***P≤0.001 versus placebo.
Abbreviations: LOCF, last observation carried forward; n, number of responders; N, number of patients analyzed; TDI, transition dyspnea index.
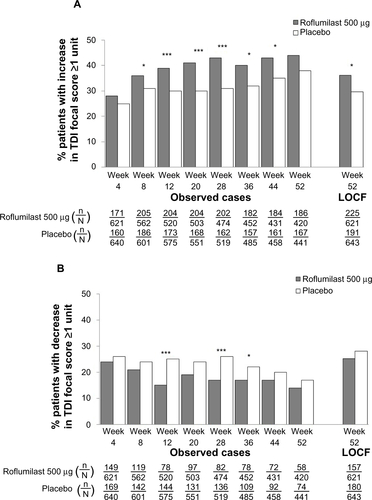
Figure S4 TDI responders (A) and deteriorators (B) over time for the subpopulation of patients with chronic bronchitis, a history of exacerbations, and concomitant long-acting β2-agonist treatment.
Notes: *P<0.05; **P≤0.01; ***P≤0.001 versus placebo.
Abbreviations: LOCF, last observation carried forward; n, number of responders; N, number of patients analyzed; TDI, transition dyspnea index.
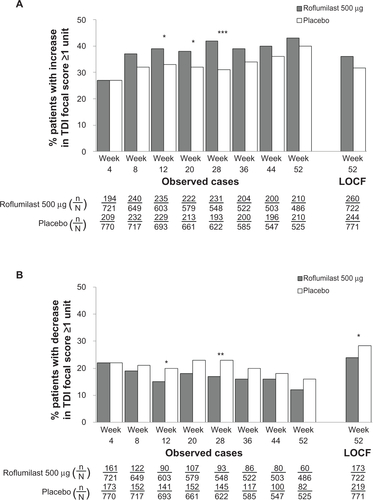
Figure S5 TDI responders (A) and deteriorators (B) over time for the subpopulation of patients with chronic bronchitis and concomitant short-acting muscarinic antagonist treatment.
Notes: *P<0.05; **P≤0.01.
Abbreviations: LOCF, last observation carried forward; n, number of responders; N, number of patients analyzed; TDI, transition dyspnea index.
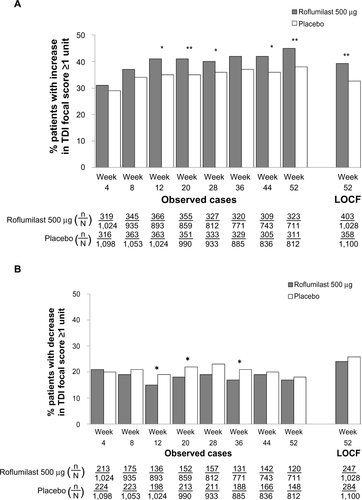
Figure S6 TDI responders (A) and deteriorators (B) over time for the subpopulation of patients with concomitant inhaled corticosteroid treatment.
Notes: *P<0.05; **P≤0.01; ***P≤0.001 versus placebo.
Abbreviations: LOCF, last observation carried forward; n, number of responders; N, number of patients analyzed; TDI, transition dyspnea index.
Disclosure
Stephen I Rennard has served as a consultant or participated in advisory boards for: ABIM, Able Associates, Adelphi Research, Almirall, APT, Aradigm, Argenta, AstraZeneca, BI (ACCP), Biostrategies, BoomCom, Britnall and Nicolini, Capital Research, Chiesi, Clinical Advisors, CommonHealth, Complete Medical Group, Consult Complete, COPDForum, DataMonitor, Decision Resources, Defined Health, Dey, Dunn Group, Easton Associates, Enterprise Analysis, Equinox, Forest, Fulcrum, Gerson Lehman, GSK, Guidepoint, Hoffman LaRoche, IMS, Informed, Inspire, Insyght, KOL Connection, Leerink Swan, M Pankove, MDRx Financial, MedaCorp, Medimmune, Mpex, Novartis, Nycomed, Oriel, Otsuka, Pearl, Pennside Partners, Pfizer, Pharma Ventures, Pharmaxis, Pick Research, Prescott, Price Waterhouse, Propagate, Pulmatrix, Pulmonary Reviews, Quadrant, Reckner Associates, Recruiting Resource, Reviews and Trends in COPD/Convergent Health Solutions, Roche, Sacoor, Schering, Schlesinger Medical, Scimed, Smith Research, Sudler and Hennessey, Talecris, Theravance, UBC, Uptake Medical, and Vantage Point. He has received lecture fees from AAAAI, Am Col Osteopathic Physicians, Asan Medical Center, ATS, AstraZeneca, California Soc Allergy, Convergent Health Solutions for Reviews and Trends in COPD, COPD Foundation, Creative Educational Concepts, Dey, Duke, France Foundation, Information TV, University of California-Los Angeles, Network for Continuing Education, Novartis, Nycomed, Otsuka, Pfizer, Sarasota Mem Hospital, Spanish Thoracic Society, University of Washington, University of Alabama-Birmingham, University of Pittsburgh, University of British Columbia, University of California-Davis, VA Sioux Falls. He has received industry-sponsored grants from AstraZeneca, Biomarck, Centocor, GlaxoSmithKline, Mpex, Nabi, Novartis, Otsuka, and Pfizer. Peter M A Calverley has given presentations at symposia sponsored by Astra Zeneca, Boehringer, GlaxoSmithKline, and Nycomed, and has received fees for advising Astra Zeneca, Boehringer, GlaxoSmithKline, Novartis, and Nycomed. Shawn X Sun, Stavros Tourkodimitris, and Paul Rowe are employees of Forest Research Institute, Inc. Udo M Goehring and Dirk Bredenbröker are employees of Takeda Pharmaceuticals International GmbH.
References
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- American Thoracic SocietyDyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic SocietyAm J Respir Crit Care Med199915913213409872857
- ParshallMBSchwartzsteinRMAdamsLAn official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspneaAm J Respir Crit Care Med2012185443545222336677
- MahlerDAWeinbergDHWellsCKThe measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexesChest19848567517586723384
- MahlerDAWitekTJJrThe MCID of the transition dyspnea index is a total score of one unitCOPD2005219910317136969
- JonesPWMahlerDAGaleRProfiling the effects of indacaterol on dyspnoea and health status in patients with COPDRespir Med2011105689289921397482
- JonesPWSinghDBatemanEDEfficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN studyEur Respir J201240483083622441743
- KaplanAEffect of tiotropium on quality of life in COPD: a systematic reviewPrim Care Respir J201019431532521042697
- MahlerDAWellsCKEvaluation of clinical methods for rating dyspneaChest19889335805863342669
- NanniniLCatesCJLassersonTJCombined corticosteroid and long-acting beta-agonist in one inhaler versus placebo for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20074CD00379417943798
- GrootendorstDCGauwSAVerhooselRMReduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPDThorax200762121081108717573446
- O’DonnellDEHyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary diseaseProc Am Thorac Soc20063218018416565429
- CalverleyPMRabeKFGoehringUMRoflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trialsLancet2009374969168569419716960
- CalverleyPMSanchez-TorilFMcIvorAEffect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2007176215416117463412
- RennardSICalverleyPMGoehringUMReduction of exacerbations by the PDE4 inhibitor roflumilast – the importance of defining different subsets of patients with COPDRespir Res2011121821272339
- CazzolaMMacNeeWMartinezFJOutcomes for COPD pharmacological trials: from lung function to biomarkersEur Respir J200831241646918238951
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- HalpinDMTashkinDPDefining disease modification in chronic obstructive pulmonary diseaseCOPD20096321122519811377
- JonesPWDonohueJFNedelmanJCorrelating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysisRespir Res20111216122206353
- MahlerDAWardJWatermanLAPatient-reported dyspnea in COPD reliability and association with stage of diseaseChest200913661473147919696126
- WestwoodMBourbeauJJonesPWRelationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic reviewRespir Res2011124021477298
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J200219221722411866001
- DahlRChungKFBuhlREfficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPDThorax201065647347920522841
- KerwinEHebertJGallagherNEfficacy and safety of NVA237 versus placebo and tiotropium in patients with moderate-to-severe COPD over 52 weeks: The GLOW2 studyEur Respir J20124051106111423060624
- WatsonLSchoutenJPLofdahlCGPredictors of COPD symptoms: does the sex of the patient matter?Eur Respir J200628231131816707516
- AmbrosinoNFoglioKBalzanoGTiotropium and exercise training in COPD patients: effects on dyspnea and exercise toleranceInt J Chron Obstruct Pulmon Dis20083477178019281092
- Forest Research Institute Core Presentation for the April 7, 2010 meeting of the Pulmonary-Allergy Drugs Advisory Committee Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM208711.pdfAccessed April 1, 2013
- RabeKFBatemanEDO’DonnellDRoflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trialLancet2005366948556357116099292
- RennardSISunSXTourkodimitrisSEffect of roflumilast treatment added to tiotropium on dyspnea in patients with chronic obstructive pulmonary disease [abstract]Am J Respir Crit Care Med2012185A2261
- EakinEGResnikoffPMPrewittLMValidation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San DiegoChest199811336196249515834
- FabbriLMCalverleyPMIzquierdo-AlonsoJLRoflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trialsLancet2009374969169570319716961
- PanLGuoYZZhangBDoes roflumilast improve dyspnea in patients with chronic obstructive pulmonary disease? A meta-analysisJ Thorac Dis20135442242923991297
- CurtisJRDeyoRAHudsonLDPulmonary rehabilitation in chronic respiratory insufficiency. 7. Health-related quality of life among patients with chronic obstructive pulmonary diseaseThorax19944921621708128407
- HajiroTNishimuraKTsukinoMA comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPDChest199911661632163710593787
- JonesPWQuirkFHBaveystockCMThe St George’s Respiratory QuestionnaireRespir Med199185Suppl B25311759018
- RennardSCalverleyPMASunSXEffect of roflumilast treatment on health-related quality of life in patients with chronic obstructive pulmonary disease [abstract]Respir Care2011101641
- JenkinsCRCelliBAndersonJASeasonality and determinants of moderate and severe COPD exacerbations in the TORCH studyEur Respir J2012391384521737561
- FranssenFMSpruitMAWoutersEFDeterminants of polypharmacy and compliance with GOLD guidelines in patients with chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2011649350122069360