Abstract
Background
Indacaterol is a novel, once-daily, inhaled, long-acting b2-agonist for patients with chronic obstructive pulmonary disease (COPD). The study objective was to evaluate the efficacy of indacaterol on quality of life and pulmonary function in patients with COPD in a real-world setting, and also to evaluate its inhaler device (Breezhaler®), which is important for both adherence and management.
Methods
Twenty-eight outpatients with COPD were treated with indacaterol (150 μg once daily for 8 weeks), and the effects on pulmonary function were evaluated using a questionnaire survey with the modified Medical Research Council (mMRC) dyspnea scale and COPD assessment test (CAT) before and after treatment. Similar investigations were also performed separately among different baseline medications. Moreover, original questionnaire surveys for indacaterol and its device were performed.
Results
Overall, mMRC dyspnea scale and CAT scores significantly improved (1.96±1.04 to 1.57±1.07 and 17.39±8.23 to 12.82±8.42, respectively; P<0.05). Significant improvements in forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were also observed on pulmonary function tests (2.91±0.66 L to 3.07±0.65 L and 1.46±0.60 L to 1.58±0.59 L, respectively; P<0.05). Replacement therapy from salmeterol to indacaterol significantly improved mMRC and FVC values, but did not significantly improve CAT scores or other pulmonary functions. Add-on therapy with indacaterol significantly improved mMRC score, CAT score, FVC, and FEV1, regardless of whether tiotropium was used as a baseline treatment. All subjects in a questionnaire survey found the inhaler device easy to use. There were no serious adverse events leading to treatment discontinuation.
Conclusion
Indacaterol is thought to be effective and well tolerated as a bronchodilator for the management of COPD. Treatment with indacaterol in addition to a long-acting muscarinic antagonist was also useful.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide and results in an economic and social burden that is both substantial and increasing.Citation1,Citation2 It was reported that the overall lifetime risk of physician-diagnosed COPD at 80 years of age was 27.6% in a longitudinal cohort study.Citation3 Improvements in quality of life (QoL), such as exercise tolerance and physical activity, and the improvement of pulmonary function in addition to the prevention of exacerbation are expected in the medication management of patients with COPD. The principal therapies for COPD management are inhaled medications. However, various devices for inhalation exist. Satisfaction with the inhaler device is also positively correlated with improved adherence and clinical outcomes.Citation4 Especially for elderly patients, it is thought that the merits of these devices reflect treatment successes. Indacaterol (Onbrez®; Novartis Pharma, Tokyo, Japan) is a novel, inhaled, long-acting b2-agonist (LABA) providing a fast onset of actionCitation5 and 24 hours of bronchodilationCitation6–Citation8 on once-daily dosing. A dose of 150 μg of powder contained in a capsule is inhaled using its inhaler device (Breezhaler®; Novartis Pharma).
In the present study, an evaluation of the efficacy of indacaterol on QoL (the modified Medical Research Council [mMRC] dyspnea scale and COPD assessment test [CAT] scores) and pulmonary function was performed for patients with COPD in an open study in a real-world setting. Additionally, similar analyses were investigated in a salmeterol-to-indacaterol replacement group and in an indacaterol add-on group based on the presence or absence of the long-acting muscarinic antagonist (LAMA) tiotropium. Finally, a questionnaire survey was conducted for the inhaler device, which is important for both adherence and management.
Materials and methods
Subjects
Twenty-eight outpatients with COPD who had been commuting to the Department of Respiratory Medicine and Allergology at Nara Hospital, Kinki University Faculty of Medicine, Ikoma, Japan, were enrolled. COPD was defined according to the Global initiative for chronic Obstructive Lung Disease (GOLD) 2011 criteria.Citation9 Subjects were over 40 years old, had >10 pack-years of smoking status, and had stable disease with no exacerbations during the 3 months prior to the study. Twenty-six patients (92.9%) were men and the mean age was 75.7±7.0 years. Maximum inspiratory flow using an In-Check Oral Inspiratory Flow Meter® (Matsuyoshi and Co, Ltd, Tokyo, Japan) was 78.2±20.7 L/min (using an adaptor for Diskus; A/A/D). The mean Brinkman Index was 1,175 (58.75 pack-years). Regarding the severity of airflow limitation,Citation9 six patients were classified as mild (GOLD1), twelve were moderate (GOLD2), six were severe (GOLD3), and four were very severe (GOLD4). Two patients were undergoing long-term oxygen therapy and two had bronchial asthma complications. As baseline medications, 19 patients were receiving tiotropium, ten were receiving salmeterol, two were receiving theophylline, seven were receiving an inhaled glucocorticosteroid, and seven were receiving mucolytics (). All patients were ex-smokers and were not on active smoking cessation pharmacotherapy.
Table 1 Clinical characteristics of the subjects (n=28)
Protocol (study design)
This study was performed in a real-world setting and was an open-label clinical trial with no randomization, no placebo group, and no blinding. All subjects visited the hospital in the morning and answered the mMRC and CAT questionnaires. A pulmonary function test using a CHESTAC-33 (CHEST MI, Tokyo, Japan) was performed 2–4 hours after the patients took their baseline medications. After receiving inhaler instructions from a pharmacist at the hospital, subjects started to inhale indacaterol (150 μg) once daily, starting the next morning. Eight weeks (±1 week) later, they visited the hospital at a similar time in the morning. Then, they answered the mMRC, CAT, and original questionnaires for indacaterol (Onbrez) and the Breezhaler device (it was confirmed that no subjects experienced problems with Breezhaler usage during the visit). Spirometry was also performed 2–4 hours after indacaterol inhalation and after the administration of other morning medications. In addition, adverse events were investigated during the 8 week (±1 week) follow-up visit.
All ten patients taking salmeterol as a baseline controlling medication were switched to indacaterol. Other baseline medications, except for salmeterol, were continued.
Second, similar subanalyses were performed on the basis of different baseline medications: the groups treated with and without an LAMA (tiotropium) and the groups treated with and without an LABA (salmeterol).
The study protocol was approved by the Institutional Review Board at Nara Hospital, Kinki University Faculty of Medicine, and informed consent was obtained from all subjects.
Statistical analysis
Data are summarized as the mean ± standard deviation. Statistical differences before and after treatment with indacaterol were assessed using paired t-tests. Statistical analyses were performed using JMP® version 10.0.2 statistical software (SAS Institute Japan, Tokyo, Japan), and a difference of P<0.05 was considered statistically significant.
Results
As one subject among the 28 patients could not perform the pulmonary function test after treatment, resulting from a mistake, their pulmonary function data were excluded from the analyses. In the questionnaire survey, some blank responses were also excluded from the analyses. All patients continued treatment with indacaterol during the study period, had no exacerbations, and did not require the use of a rescue inhaler.
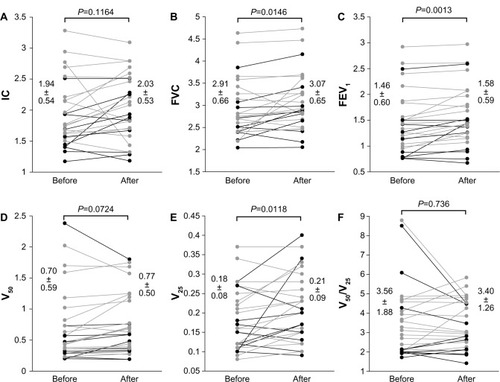
Pre- and post-treatment mMRC dyspnea scale scores were significantly improved by treatment with indacaterol (1.96±1.04 to 1.57±1.07; P=0.0013) (). CAT scores also significantly improved from 17.39±8.23 to 12.82±8.42 (P=0.0003) after treatment with indacaterol (). Scores for each item on the CAT questionnaire are shown in . Scores for questions one to seven of the total of eight questions were significantly improved (P<0.05). In pulmonary function tests, treatment with indacaterol significantly improved forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and flow at 25% forced vital capacity (V25) (P<0.05 for each) ().
Figure 1 Comparison of mMRC dyspnea scale and CAT scores pre- and post-treatment with indacaterol. The mMRC dyspnea scale (A) and the CAT score (B) significantly improved after treatment with indacaterol (P=0.0013 and P=0.0003, respectively).
Notes: Add-on group with indacaterol (n = 18) for gray lines. Replacement group with indacaterol (n = 10) for black lines.
Abbreviations: CAT, chronic obstructive pulmonary disease assessment test; mMRC, modified Medical Research Council.
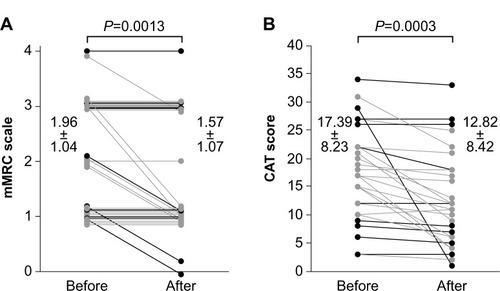
Figure 2 Comparison of scores for each item of the CAT questionnaire pre- and post-treatment with indacaterol. Scores for questions one to seven of the total of eight questions were significantly improved by treatment with indacaterol (*P<0.05 for each).
Abbreviation: CAT, chronic obstructive pulmonary disease assessment test.
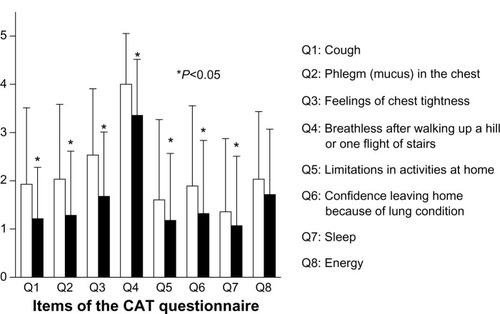
Figure 3 Pulmonary function tests pre- and post-treatment with indacaterol. The IC (A), FVC (B), FEV1 (C), V50 (D), V25 (E), and V50/V25 (F) pulmonary functions were compared pre- and post-treatment with indacaterol. FVC, FEV1, and V25 were significantly improved by treatment with indacaterol (P<0.05 for each).
Abbreviations: IC, inspiratory capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; V25, flow at 25% forced vital capacity; V50, flow at 50% forced vital capacity.
Subanalyses performed based on treatment with or without an LAMA (tiotropium) as a baseline medication are shown in . In patients treated with tiotropium, mMRC scale, CAT score, FVC, and V25 significantly improved (2.33±1.12 to 1.67±1.12, 13.78±4.99 to 10.22±4.87, 1.45±0.65 L to 1.64±0.56 L, and 0.17±0.10 L to 0.26±0.10 L; P<0.05 for each). In patients treated with tiotropium, mMRC scale score, CAT score, FVC, and FEV1 significantly improved (1.79±0.98 to 1.53±1.07, 19.11±8.99 to 14.05±9.53, 2.92±0.68 L to 3.08±0.66 L, and 1.47±0.60 L to 1.56±0.61 L; P<0.05 for each).
Table 2 Subanalyses based on treatment with or without LAMA
Subanalyses based on groups who had not been treated with salmeterol, those who received indacaterol add-on therapy (the add-on group), and those who had been treated with salmeterol and switched to indacaterol (replacement group) are shown in . In the add-on group, mMRC scale score, CAT score, FEV1, and V25 significantly improved (1.94±1.00 to 1.50±0.86, 17.28±6.44 to 12.17±6.60, 1.63±0.59 L to 1.75±0.55 L, and 0.20±0.09 L to 0.22±0.08 L; P<0.05 for each). In the replacement group, mMRC and FVC significantly improved (2.00±1.15 to 1.70±1.42 and 2.66±0.52 L to 2.84±0.61 L; P<0.05 for each).
Table 3 Subanalyses in the indacaterol add-on and salmeterol-to-indacaterol replacement
Original questionnaire surveys for indacaterol and the Breezhaler device were conducted, and responses were obtained from 26 subjects. A total of 61.5% of the total responded “I want to continue Onbrez”, and all subjects responded “Yes” for device ease of handling and ease of use. Furthermore, 24 subjects (92.3%) responded that they felt like they could inhale ().
Table 4 Questionnaire survey for indacaterol (Onbrez®) and the Breezhaler® device
Responses for reasons the patients felt they could inhale were obtained from 21 of those 24 subjects. Reasons included (multiple answers were allowed) 71.4% responding “By the sweet taste when I inhale”, 52.4% responding “I can confirm the inhalation because the inhalation capsule is transparent”, and 47.6% responding “By hearing the sound when I inhale” ().
The most frequent adverse event observed was coughing immediately after inhalation, in six patients. In addition, three reports of hoarseness and two reports of dry mouth were observed in four subjects. The two patients who complained of dry mouth were both treated with tiotropium as a baseline medication. However, these symptoms were mild and did not lead to discontinuation of indacaterol treatment. All patients continued treatment with indacaterol after the present study ().
Table 5 Adverse events
Discussion
Once, it was considered that anticholinergics (muscarinic antagonists) demonstrated a greater bronchodilation effect than b2-agonists in patients with COPD.Citation9,Citation10 In fact, of the long-acting bronchodilators, tiotropium showed a higher efficacy than salmeterol.Citation11 However, indacaterol, a new LABA, was shown to be at least as effective as tiotropium and with a faster onset of action.Citation12 Indacaterol has shown a powerful bronchodilation effect with a mechanism different from that of the LAMAs, and its efficacy has been shown. Pharmacologic therapy in COPD is used to reduce symptoms, reduce the frequency and severity of exacerbations, and improve health status and exercise tolerance.Citation13 There are several validated questionnaires to assess symptoms in patients with COPD. GOLD recommends the use of the mMRC questionnaire or the CAT. The well-known mMRC questionnaire assesses only disability resulting from breathlessness; however, the CAT covers a broader range of the impact of COPD on the patient’s daily life and well-being.Citation13 In the present study, these two questionnaires were used for the assessment of the patients’ QoL, and the efficacy of bronchodilation was assessed by spirometry. Because inhaler adherence is very important in the management of COPD,Citation14 questionnaire surveys for indacaterol (Onbrez) and its inhaler device (Breezhaler) were performed.
It has been reported that patients receiving indacaterol had clinically significant improvements in symptoms of dyspnea compared with placebo.Citation15 Among the 23 eligible patients, 14 were receiving tiotropium 18 μg/day (by a Handihaler®), and nine were receiving no therapy. Treatment with indacaterol improved both pulmonary function and QoL.Citation16 Add-on therapy with indacaterol also improved expiratory reserve volume and exercise capacity, although a significant difference was not noted in the improvement of FEV1.Citation17 Overall, indacaterol therapy improved the QoL of patients with COPD in the present study. In addition, FVC, FEV1, and V25 were improved in pulmonary function tests. Significantly more patients receiving indacaterol reported less need for a rescue inhaler (short-acting b2-agonist) than LABA recipients.Citation18,Citation19 In the present study, no patients used a short-acting b2-agonist in a rescue capacity. Indacaterol might be a useful option, even for the treatment of acute exacerbations of COPD.Citation20
Indacaterol was at least as effective as tiotropium in improving clinical outcomes.Citation21,Citation22 However, a comparison of indacaterol and tiotropium under blinded conditions confirmed a statistically significant improved effect of indacaterol over tiotropium for dyspnea (transition dyspnea index) and health status (the St George’s Respiratory Questionnaire).Citation23 Furthermore, compared with tiotropium monotherapy, indacaterol plus tiotropium provided greater bronchodilation and lung deflation (reflected by an increased resting inspiratory capacity).Citation24 Regardless of the use or nonuse of tiotropium, add-on therapy with indacaterol significantly improved mMRC scale score, CAT score, and FEV1 in the present study. These results support COPD guideline recommendations to combine bronchodilators with different mechanisms of action.
Using trough FEV1 as a measure of therapeutic effect, indacaterol was superior to other b2-agonists, tiotropium, and placebo at weeks 12, 26, and 52. Indacaterol had a greater effect on the transition dyspnea index than salmeterol.Citation25 Although this study did not consider the trough value, the switch from salmeterol did not significantly improve pulmonary function, except for FVC. However, mMRC score was significantly improved, even in the present small sample size. Therefore, it is thought that indacaterol treatment contributed to improvements of QoL more than salmeterol.
Adherence is crucial for optimizing clinical outcomes in COPD, with nonadherence resulting in significant health and economic burdens.Citation26 Adherence in patients with COPD is affected by multiple factors associated with the patient, their clinician, and society.Citation27 Patient-related factors include health beliefs and self-efficacy. Treatment-related factors include each treatment requiring a different technique for administration, in addition to the need for multiple inhalers, and are especially burdensome for older COPD patients. In the present study, indacaterol and its device seemed to be regarded favorably by patients with COPD. These results may contribute to better clinical outcomes through satisfactory adherence.
The most frequently observed adverse event was coughing immediately after inhalation; hoarseness and dry mouth were also observed. However, these adverse events were mild and did not result in the discontinuation of indacaterol treatment. Indacaterol demonstrated excellent tolerability.
A limitation of the present study is the small sample size. There were no analyses of the patients’ adherence as distinguished from inhaler device preference; therefore, future investigations regarding inhaler adherence are needed. However, despite the small sample size and regardless of the quality of adherence, pulmonary functions and QoL were improved. In addition, there are no analyses comparing inhaler devices in the present study with other inhaler devices, and further studies are needed to better clarify patient preferences.
Conclusion
In summary, indacaterol is both safe and effective in the management of COPD. Furthermore, add-on therapy with an LAMA is also effective in improving clinical outcomes. The inhaler device is easy to use and was well regarded by the patients, which can contribute to medication adherence.
Disclosure
The authors report no conflicts of interest in this work.
References
- LopezADShibuyaKRaoCChronic obstructive pulmonary disease: current burden and future projectionsEur Respir J20062739741216452599
- MathersCDLoncarDProjections of global mortality and burden of disease from 2002 to 2030PLoS Med20063e44217132052
- GershonASWarnerLCascagnettePVictorJCToTLifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population studyLancet201137899199621907862
- MäkeläMJBackerVHedegaardMLarssonKAdherence to inhaled therapies, health outcomes and costs in patients with asthma and COPDRespir Med20131071481149023643487
- BalintBWatzHAmosCOwenRHigginsMKramerBINSURE Study InvestigatorsOnset of action of indacaterol in patients with COPD: comparison with salbutamol and salmeterol-fluticasoneInt J Chron Obstruct Pulmon Dis2010531131820856830
- DahlRChungKFBuhlREfficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPDThorax20106547347920522841
- BleeckerERSilerTOwenRKramerBBronchodilator efficacy and safety of indacaterol 150 μg once daily in patients with COPD: an analysis of pooled dataInt J Chron Obstruct Pulmon Dis2011643143822003288
- KornmannODahlRCentanniSOnce-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparisonEur Respir J20113727327920693243
- GrossNJSkorodinMSRole of the parasympathetic system in airway obstruction due to emphysemaN Engl J Med19843114214256749189
- SchockenDDRothGSReduced beta-adrenergic receptor concentrations in ageing manNature1977267856858197415
- VogelmeierCHedererBGlaabTTiotropium versus salmeterol for the prevention of exacerbations of COPDN Engl J Med20113641093110321428765
- VogelmeierCRamos-BarbonDJackDIndacaterol provides 24-hour bronchodilation in COPD: a placebo-controlled blinded comparison with tiotropiumRespir Res20101113520920365
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global strategy for diagnosis, management, and prevention of chronic obstructive lung disease [updated 2013] Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdfAccessed December 9, 2013
- VestboJAndersonJACalverleyPMAdherence to inhaled therapy, mortality and hospital admission in COPDThorax20096493994319703830
- HanJDaiLZhongNIndacaterol on dyspnea in chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized placebo-controlled trialsBMC Pulm Med2013132623617268
- HatajiONaitoMItoKWatanabeFGabazzaECTaguchiOIndacaterol improves daily physical activity in patients with chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis201381523293514
- MrozRMMinarowskiLChyczewskaEIndacaterol add-on therapy improves lung function, exercise capacity and life quality of COPD patientsAdv Exp Med Biol2013756232822836615
- KornSKerwinEAtisSAmosCOwenRLassenCINSIST study groupIndacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week studyRespir Med201110571972621367594
- FeldmanGJImproving the quality of life in patients with chronic obstructive pulmonary disease: focus on indacaterolInt J Chron Obstruct Pulmon Dis20138899623431038
- SegretiAFioriECalzettaLThe effect of indacaterol during an acute exacerbation of COPDPulm Pharmacol Ther201326663063423578980
- DonohueJFFogartyCLötvallJOnce-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropiumAm J Respir Crit Care Med201018215516220463178
- MahlerDABuhlRLawrenceDMcBryanDEfficacy and safety of indacaterol and tiotropium in COPD patients according to dyspnoea severityPulm Pharmacol Ther20132634835523434446
- BuhlRDunnLJDisdierCBlinded 12-week comparison of once-daily indacaterol and tiotropium in COPDEur Respir J20113879780321622587
- MahlerDAD’UrzoABatemanEDConcurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparisonThorax20126778178822544891
- JiangFMLiangZAZhengQLWangRCLuoJLiCTSafety and efficacy of 12-week or longer indacaterol treatment in moderate-to-severe COPD patients: a systematic reviewLung201319113514623306410
- LareauSCYawnBPImproving adherence with inhaler therapy in COPDInt J Chron Obstruct Pulmon Dis2010540140621191434
- RestrepoRDAlvarezMTWittnebelLDMedication adherence issues in patients treated for COPDInt J Chron Obstruct Pulmon Dis2008337138418990964
