Abstract
Despite a number of studies on biomarkers in chronic obstructive pulmonary disease (COPD), only a few disease-related markers have been identified, yet we still have no satisfactory markers specific to innate immune system and neutrophil activation, which is essential in airway inflammation in COPD. Recent biological studies indicated that lipocalins (LCNs) might be involved in airway inflammation and innate immunity; however, results from available studies on the association of LCNs with COPD are not consistent. We carried out a multicenter prospective observational cohort study to investigate the differences in serum levels of LCN1 and LCN2 between subjects with COPD (n=58) and healthy controls (n=29). Several validated inflammatory markers, including C-reactive protein, tumor necrosis factor-α, interleukin-6, and interleukin-8, were measured. The correlation of LCN1 and LCN2 with clinical features such as smoking habits, lung function, symptoms, and disease category was also analyzed. When comparing with healthy controls, serum levels of LCN1 (66.35±20.26 ng/mL versus 41.16±24.19 ng/mL, P<0.001) and LCN2 (11.29±3.92 ng/mL versus 6.09±5.13 ng/mL, P<0.001) were both elevated in subjects with COPD after adjusting for age, sex, smoking habits, and inflammatory biomarkers. Smoking history and tobacco exposure, as quantified by pack-year, had no impact on systemic expressions of LCN1 and LCN2 in our study. Blood levels of LCN1 and LCN2, respectively, were negatively correlated to COPD Assessment Test and Modified Medical British Research Council score (P<0.001). Disease category by Global Initiative for Chronic Obstructive Lung Disease grade 1–4 or group A–D was not associated with levels of LCNs. Patient-reported exacerbations and body mass index were also tested, but no relationship with LCNs was found. In summary, serum concentrations of LCN1 and LCN2 were both elevated in patients with COPD, with their levels correlating to COPD Assessment Test and Modified Medical British Research Council score. These findings warrant large-scale and longitudinal studies to validate LCNs as circulating biomarkers for COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality both worldwide and in the People’s Republic of China, and causes a huge and growing economic and social burden.Citation1–Citation3 It is characterized by chronic inflammation and irreversible airflow obstruction, which involves structural changes of the lung.Citation4 The heterogeneity of COPD is well acknowledged, but the underlying pathogenesis is still not fully understood.Citation5 Circulating biomarkers are easily accessible and would facilitate a deeper understanding of disease mechanism. Blood biomarkers that distinguish subtypes, stratify disease severity and category, predict disease progression, and monitor treatment response would be particularly valuable.Citation6–Citation8
Despite a number of studies on biomarkers in COPD, only a few disease-related markers have been identified. Inflammatory markers, including C-reactive protein (CRP), fibrinogen, tumor necrosis factor (TNF)-α, soluble TNF receptor-1, interleukin-6 (IL-6), and IL-8, are reported to be elevated in COPD and related to lung function and acute exacerbation (AE).Citation9–Citation13 Pneumoproteins, including Clara cell secretory protein and surfactant protein D, have recently been identified to be associated with COPD and its clinical phenotypes.Citation14–Citation16 These markers, however, are not specific to innate immune system and neutrophil activation, which is essential in airway inflammation in COPD and acute exacerbation of COPD, although IL-6 and IL-8 were acknowledged to be involved in this process. Therefore, it is rational to look into candidate markers from the perspective of innate immune molecules in airways.
Lipocalins (LCNs) are a family of proteins that are involved in the transport of steroids and lipids into cells. Recent studies have suggested that LCNs serve as mediators in airway inflammation. In the LCNs family, LCN1 and LCN2 have been shown to play a role in pulmonary disease and infection.Citation17 LCN1 is expressed in tears, lingual sweat, and lower airway glands, and it can be detected in bronchoalveolar lavage fluid (BALF) and sputum.Citation18,Citation19 It is believed that LCN1 is involved in innate immune response against bacterial and fungal infections. It can also inhibit oxidation induced by lipid peroxidation products in vitro.Citation20 LCN2, also named neutrophil gelatinase-associated lipocalin, is constitutively expressed in neutrophilic granules and thus could serve as a marker for neutrophil activity.Citation21 It is currently widely used to detect and monitor acute renal failure, indicating a potential value in its application in COPD.Citation22 In addition, LCN2 might serve as a mediator in the inflammation network. It can be unregulated in airway structural and inflammatory cells by stimuli like lipopolysaccharide, TNF-α, IL-1, and IL-17.Citation17 An increased mortality due to Escherichia coli infection in LCN2 knockout mice suggested an antimicrobial function by sequestrating iron.Citation23 Most excitingly, Nicholas et alCitation18 discovered a reduced sputum level of LCN1, and Eagan et alCitation24 reported an increased plasma concentration of LCN2 in COPD patients recently. Taking these facts together, LCNs might have a potential role as a more specific indicator of innate immunity and neutrophil inflammation. However, the relationship of LCN1 and LCN2 with COPD and disease presentation and severity is still to be validated.
To address this issue, we undertook a multicenter prospective observational cohort study to investigate the differences in systemic levels of LCN1 and LCN2 between subjects with COPD and healthy controls. The association of LCN1 and LCN2 with clinical features such as tobacco exposure, lung function, symptoms, disease category, and inflammatory markers was also analyzed.
Methods
Ethics statement
This study was approved by the Ethics Committee of Zhongshan Hospital of Fudan University, Shanghai, People’s Republic of China, and has been registered in the Chinese Clinical Trials Registry (ChiCTR-OCC-11001621). Written informed consent was obtained from all participants.
Definitions
The diagnosis of COPD was according to the Global initiative for chronic Obstructive Lung Disease (GOLD) definition. Briefly, COPD was considered to be present if postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio was less than 0.70.Citation1 The grading of COPD was based on FEV1/%pred.Citation1 COPD severity was also evaluated according to an A, B, C, or D classification proposed by GOLD.Citation1
Nonsmokers were defined as never smokers or ex-smokers with less than 5 pack-years of tobacco exposure and having quit for at least 10 years; otherwise, the subjects were classified as smokers.
Study population and subject enrollment
Participants in this study were recruited from Zhongshan Hospital of Fudan University (a comprehensive teaching hospital) and Dahua Hospital (a district hospital) in Shanghai, and Changshou People’s Hospital (a district hospital) in Chongqing, People’s Republic of China, since December 2011. The last subject was enrolled in October 2012 when the estimated sample size was achieved. Healthy subjects without COPD were recruited as controls.
Participants who had a primary diagnosis of congestive heart failure, bronchiectasis, bronchial asthma, active pulmonary tuberculosis, obstructive bronchiolitis, pneumosilicosis, interstitial lung disease, pleural effusion, diffuse panbronchiolitis, or a history of pneumonectomy were excluded from the study. Patients who had airflow limitation due to abnormalities in large airways were also excluded. Those who had a previous exacerbation of COPD within 4 weeks of admission were excluded.
Subjects with COPD were predominantly recruited from respiratory outpatient clinics and discharged patients from a respiratory ward. Adults aged 45 years and over with a history of spirometry-defined physician-diagnosed stable COPD were screened for further enrollment.
Study procedure and data collection
For all participants, clinical data, including age, sex, weight, height, smoking status (never smoker, ever smoker, or current smoker), quantity of tobacco smoking exposure (pack-years), history of respiratory diseases, respiratory symptoms, number of exacerbations in the previous year, comorbid diseases, and family history, were recorded into a case report form. Spirometry was performed according to international guidelines using a calibrated Jaeger® MasterScreen™ PFT spirometer (Jaeger, North Rhine-Westphalia, Germany) before and after inhalation of 400 mcg of salbutamol via spacer. Serum was collected at the time of the study visit for measurement of CRP and biomarkers. All the blood samples were collected in the early morning after fasting for at least 8 hours and before taking medications. Subjects with COPD were also asked to complete a COPD Assessment Test (CAT) and a modified Medical British Research Council (mMRC) dyspnea questionnaire.
Quantification of biomarkers of interest in serum samples
Commercial enzyme-linked immunosorbent assay kits from USCN Life Science Inc. (Wuhan, People’s Republic of China) were used for measuring IL-6, IL-8, TNF-α, LCN1, and LCN2. The level of protein of interest in serum was determined according to the manufacturer’s instructions. Specifically for LCN1 and LCN2, the sensitivity is 25 pg/mL and the detection range is 78–5,000 pg/mL. The samples were diluted to fit into the linear detection range as needed. The percentage of coefficient of variance of LCN1 and LCN2 was 4.6% and 5.7%, respectively.
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). Group-wise comparisons using all subjects were performed, and correlation analyses were carried out in COPD patients only. The D’Agostino–Pearson omnibus normality test was performed on continuous data. All continuous data were expressed and graphed as mean ± standard deviation. For data normally distributed, unpaired t-tests or one-way analysis of variance followed by Bonferroni post-test was performed to determine whether the differences between the groups were statistically significant. Otherwise, comparisons were made using the Mann–Whitney test or Kruskal–Wallis analysis followed by Dunn’s multiple comparison test. Correlation analyses were carried out using Pearson or Spearman methods, depending on the normality of the data distribution. For categorical variables, a chi-squared test and Spearman rank correlation analysis were used. Multiple logistic regressions were used for multivariate analysis. Differences were considered significant at the level of P<0.05.
Results
Basic characteristics
In this study, 58 patients with COPD and 29 healthy controls were recruited and tested. The demographic data, smoking habits, grade stratified by postbronchodilator FEV1, and disease category in the context of GOLD A–D are described in and . Subjects with COPD smoked more heavily than controls. Although the percentage of males was higher in the COPD group, the difference was not statistically significant. There was no statistically significant difference in serum creatinine levels or glomerular filtration rate between the two groups.
Table 1 Basic characteristics of the study population (data expressed as mean ± standard deviation)
Table 2 Severity distribution of subjects with COPD
Inflammatory biomarkers
As shown in , serum levels of CRP, IL-6, IL-8, and TNF-α were all significantly elevated in patients with COPD compared with in subjects without COPD.
Table 3 Comparisons of inflammatory biomarkers between COPD and controls (data expressed as mean ± standard deviation)
Lipocalins
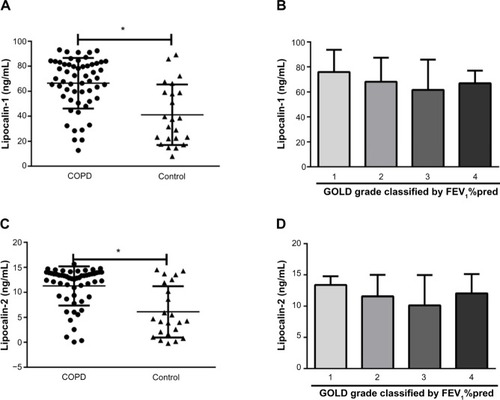
As compared with non-COPD controls, serum LCN1 was elevated in subjects with COPD (66.35±20.26 ng/mL versus [vs] 41.16±24.19 ng/mL, P<0.001), and levels of LCN2 were also higher (11.29±3.92 ng/mL vs 6.09±5.13 ng/mL, P<0.001) (). LCN1 and LCN2 were significantly higher in the COPD group than in both nonsmoker controls and smoker controls (P<0.001). In multiple logistic regression models, the differences in both LCN1 and LCN2 between the two groups remained significant after adjusting for age, sex, smoking habit, smoking quantity (pack-year), and inflammatory biomarkers, including CRP, IL-6, IL-8, and TNF-α.
Figure 1 Comparisons of lipocalins between COPD and control. (A) Serum levels of lipocalin-1. (B) Serum levels of lipocalin-1 in patients with GOLD grade 1–4 COPD. (C) Serum levels of lipocalin-2. (D) Serum levels of lipocalin-2 in COPD patients with different disease grade. Data are presented as mean ± standard deviation.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GOLD, Global initiative for chronic Obstructive Lung Disease; %pred, percentage predicted.
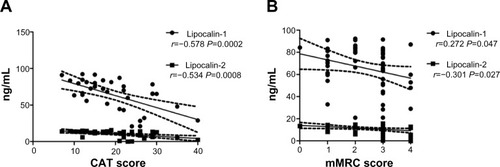
In our cohort, smoking history and exposure, as quantified by pack-year, seemed to have no impact on systemic expressions of LCN1 and LCN2. In subgroup analysis of both controls and patients with COPD, no differences in either LCN1 or LCN2 were found between smokers and nonsmokers (data not shown). No relationship between pack-year and levels of LCN1 and LCN2 was observed in COPD subjects ().
Table 4 Correlation analysis between serum levels of lipocalins and disease features in patients with COPD
In patients with COPD, bivariate associations between specific disease characteristics and serum levels of LCN1 and LCN2 were investigated. Results are shown in and . Serum levels of LCNs were negatively correlated to CAT and mMRC score. After adjustment for age, sex, smoking habit, and inflammatory marker, CAT and mMRC scores were still significantly associated with levels of LCN1 and LCN2, respectively. Levels of LCNs in patients of grade 2 and 3 seemed to be lower than in patients of grade 1, but the differences were not statistically significant (). Neither FEV1/%pred nor FEV1/FVC% was related to levels of LCNs. For COPD patients, disease category by GOLD grade 1–4 or group A–D was not associated with level of LCNs. Patient-reported AEs, body mass index, and blood concentration of TNF-α were also tested, but no relationship with LCNs was found.
Discussion
Our study confirms that circulating levels of LCN1 and LCN2 were both increased in patients with COPD. It is the first study testing serum LCN1 and LCN2 simultaneously in a Chinese population. As compared with the available data in a similar topic,Citation18,Citation24,Citation25 our study involved all of the disease categories as defined by GOLD. In addition to lung function, disease severity was also evaluated using GOLD grouping criteria updated in 2011.Citation1 In this context, symptoms were quantified, and this study provided the first piece of information on the negative correlation of serum LCNs with CAT and mMRC score. Participants were recruited prospectively and no data were missing. Considering the role of LCNs in immunity and inflammation, systemic LCN1 and LCN2 were tested along with several validated inflammatory markers to exclude potential confounding factors.
In our study, serum LCN1 was elevated in COPD patients as expected. Our finding was in line with several previous human studies on LCN1. LCN1 was found to be significantly upregulated in saliva, nasal lavage fluid, and BALF in heavy smokers compared with in nonsmokers.Citation19,Citation26 It was also increased in bronchial secretions of cystic fibrosis patients compared with healthy subjects.Citation27 On the other hand, the only available study on COPD reported a reduction of LCN1 in induced sputum in patients when comparing with healthy smokers, with its levels correlating to FEV1/FVC%.Citation18 The authors suggested that a decrease in local LCN1 might impair epithelial immune defense and increase risk for infections. Simultaneous measurements of LCN1 in blood and sputum could help to elucidate the controversy.
In previous studies, LCN2 has been found to be elevated in BALF,Citation28 induced sputum,Citation29 and blood,Citation24,Citation25 and this is consistent with our results. Research by Eagen et al.Citation24 revealed that LCN2 levels were increased in patients who reported frequent AEs requiring oral steroids, while decreased in those with GOLD grade 3–4 compared with stage 2 and those using inhaled steroids.Citation24 We also note a decrease in the levels of LCN2 in GOLD stage 2–3 patients, but the differences did not reach statistical significance. LCN2 might have multifaceted functions of inflammation enhancement and infection protection, thus explaining the inverted decrease in an advanced stage of disease. In addition, airway bacterial colonization or exacerbation induced by infection might have a mutual impact on LCN expressions. A longitudinal follow-up of local and systemic levels of LCN2 would clarify the dynamic expression of LCN2 in disease progression and make it a candidate biomarker for disease monitoring. At the same time, LCN2 has been reported to be involved in the pathogenesis of allergic airway inflammation and could be increased in patients with asthma and airway hypersensitivity, which could be overlapped in patients with COPD.Citation17
One of the most striking findings of our study was the relationship of LCNs to symptomatic scores. The only study looking into this issue is by Eagan et al.Citation24 The authors reported that respiratory symptoms, which were defined as chronic cough, phlegm, or dyspnea, were not significantly associated with plasma levels of LCN2. Instead of traditional evaluation methods, we adopted CAT and mMRC dyspnea score, with convenience and validity confirmed in Chinese patients, to quantify symptoms, and showed that more symptomatic patients had lower serum levels of LCNs. Eagan et alCitation24 reported that frequent exacerbations, another key component of COPD subtype, were related to higher levels of LCN2. Combined with our data, it indicates that LCNs could be potential indicators for defining subtypes. Further research on biological functions of LCNs is warranted to connect these changes to underlying mechanisms, and studies should be performed to include other parameters of disease features.
This study has limitations. First, it is a study with moderate size, and sample sizes across disease categories were unbalanced, which affected the power to detect associations with modest effect. Furthermore, this study was performed as a cross-sectional evaluation, and therefore alterations in LCNs are correlative but not predictive. Besides, the medications for patients were not investigated, and the influence of inhaled corticosteroid on systemic levels of LCNs could not be measured.
Conclusion
We distinguished significant associations of circulating LCN1 and LCN2 with COPD and found a negative relationship with symptoms in patients with COPD. Large-scale and longitudinal studies are required to confirm our findings and validate the value of LCNs as biomarkers for COPD.
Acknowledgments
This study is supported by funding from Zhuoxue Talents Scheme of Fudan University, Shanghai Leading Talent Projects (No 036, 2010), Shanghai Committee of Science and Technology (Number 12411950100), and Shanghai Xuhui District Committee of Science and Technology (Number SHXH2013).
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung DiseaseGOLD workshop report: global strategy for diagnosis, management and prevention of COPD [updated 2013] Available from: http://www.goldcopd.org/Guidelines/guidelines-global-strategy-for-diagnosis-management-2003-3.htmlAccessed April 1, 2014
- ZhongNWangCYaoWPrevalence of chronic obstructive pulmonary disease in China: a large, population-based surveyAm J Respir Crit Care Med200717675376017575095
- FangXWangXBaiCCOPD in China: the burden and importance of proper managementChest201113992092921467059
- BalkissoonRLommatzschSCarolanBChronic obstructive pulmonary disease: a concise reviewMed Clin North Am2011951125114122032431
- HanMKAgustiACalverleyPMChronic obstructive pulmonary disease phenotypes: the future of COPDAm J Respir Crit Care Med201018259860420522794
- BarnesPJChowdhuryBKharitonovSAPulmonary biomarkers in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200617461416556692
- TzortzakiEGLambiriIVlachakiEBiomarkers in COPDCurr Med Chem2007141037104817439401
- WoodruffPGNovel outcomes and end points: biomarkers in chronic obstructive pulmonary disease clinical trialsProc Am Thorac Soc2011835035521816991
- Pinto-PlataVMMullerovaHTosoJFC-reactive protein in patients with COPD, control smokers and non-smokersThorax200661232816143583
- EaganTMUelandTWagnerPDSystemic inflammatory markers in COPD: results from the Bergen COPD Cohort StudyEur Respir J20103554054819643942
- DickensJAMillerBEEdwardsLDCOPD association and repeatability of blood biomarkers in the ECLIPSE cohortRespir Res20111214622054035
- CelliBRLocantoreNYatesJInflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20121851065107222427534
- Garcia-RioFMiravitllesMSorianoJBSystemic inflammation in chronic obstructive pulmonary disease: a population-based studyRespir Res2010116320500811
- LomasDASilvermanEKEdwardsLDSerum surfactant protein D is steroid sensitive and associated with exacerbations of COPDEur Respir J2009349510219164344
- SinDDMillerBEDuvoixASerum PARC/CCL-18 concentrations and health outcomes in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20111831187119221216880
- WinklerCAtochina-VassermanENHolzOComprehensive characterisation of pulmonary and serum surfactant protein D in COPDRespir Res2011122921396106
- DittrichAMMeyerHAHamelmannEThe role of lipocalins in airway diseaseClin Exp Allergy20134350351123600540
- NicholasBLSkippPBartonSIdentification of lipocalin and apolipoprotein A1 as biomarkers of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20101811049106020110559
- LindahlMStahlbomBTagessonCNewly identified proteins in human nasal and bronchoalveolar lavage fluids: potential biomedical and clinical applicationsElectrophoresis1999203670367610612294
- LechnerMWojnarPRedlBHuman tear lipocalin acts as an oxidative-stress-induced scavenger of potentially harmful lipid peroxidation products in a cell culture systemBiochem J200135612913511336644
- SeveusLAminKPetersonCGHuman neutrophil lipocalin (HNL) is a specific granule constituent of the neutrophil granulocyte. Studies in bronchial and lung parenchymal tissue and peripheral blood cellsHistochem Cell Biol19971074234329208334
- DevarajanPReview. Neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injuryNephrology (Carlton)20101541942820609093
- FloTHSmithKDSatoSLipocalin 2 mediates an innate immune response to bacterial infection by sequestrating ironNature200443291792115531878
- EaganTMDamasJKUelandTNeutrophil gelatinase-associated lipocalin: a biomarker in COPDChest201013888889520495108
- CockayneDAChengDTWaschkiBSystemic biomarkers of neutrophilic inflammation, tissue injury and repair in COPD patients with differing levels of disease severityPLoS One20127e3862922701684
- JessieKPangWWHajiZProteomic analysis of whole human saliva detects enhanced expression of interleukin-1 receptor antagonist, thioredoxin and lipocalin-1 in cigarette smokers compared to non-smokersInt J Mol Sci2010114488450521151451
- RedlBWojnarPEllemunterHIdentification of a lipocalin in mucosal glands of the human tracheobronchial tree and its enhanced secretion in cystic fibrosisLab Invest199878112111299759656
- BetsuyakuTNishimuraMTakeyabuKNeutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysemaAm J Respir Crit Care Med19991591985199110351949
- KeatingsVMBarnesPJGranulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjectsAm J Respir Crit Care Med19971554494539032177