Abstract
Background
Nutritional depletion is an important manifestation of chronic obstructive pulmonary disease (COPD), which has been related to systemic inflammation. It remains unclear to what degree airway inflammation contributes to the presence or progression of nutritional depletion.
Objectives
To determine whether airway inflammation and lung bacterial colonization are related to nutritional status or predict progressive weight loss and muscle atrophy in patients with COPD.
Methods
Body composition using dual energy X-ray absorptiometry, indices of airway inflammation, and bacterial colonization were measured in 234 COPD patients. Systemic inflammation was assessed from serum C reactive protein (CRP) and circulating total and differential leukocyte counts. Nutritional depletion was defined as a body mass index (BMI) less than 21 kg/m2 and/or fat-free mass index (FFMI) less than 15 or 17 kg/m2 in women and men, respectively. FFMI was calculated as the fat-free mass (FFM) corrected for body surface area. Measurements were repeated in 94 patients after a median 16-month follow-up. Regression analysis was used to assess the relationships of weight change and FFM change with indices of bacterial colonization and airway and systemic inflammation.
Results
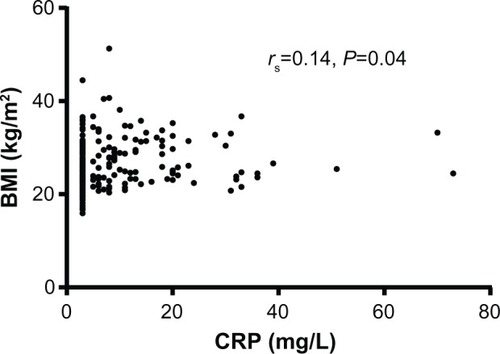
Nutritional depletion occurred in 37% of patients. Lung function was worsened in patients with nutritional depletion compared to those without (forced expiratory volume in 1 second 1.17 L versus 1.41 L, mean difference 0.24, 95% confidence interval 0.10 to 0.38, P<0.01). There were no differences in airway inflammation and bacterial colonization in patients with and without nutritional depletion. At baseline, BMI correlated positively with serum CRP (rs=0.14, P=0.04). Change in weight and change in FFM over time could not be predicted from baseline patient characteristics.
Conclusion
Nutritional depletion and progressive muscle atrophy are not related to airway inflammation or bacterial colonization. Overspill of pulmonary inflammation is not a key driver of muscle atrophy in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a significant cause of morbidity and mortality worldwide.Citation1 Systemic manifestations of COPD include weight loss, which is an independent predictor of mortality and morbidity.Citation2–Citation5 Nutritional depletion in the context of COPD can be defined as reduced body mass index (BMI) and/or reduction in fat-free mass index (FFMI); muscle atrophy has been identified as the most important component of nutritional depletion, often occurring in the presence of a preserved body mass index.Citation2 Muscle atrophy and weight loss in COPD has been associated with an increase in systemic inflammation, measured by tumor necrosis factor-alpha,Citation6,Citation7 although elevated body mass in COPD has also been associated with increased systemic inflammation.Citation8 The inflammatory stimuli driving the systemic manifestations of COPD are uncertain,Citation6–Citation9 and it is unclear whether nutritional depletion is related to pulmonary inflammation or bacterial overgrowth in the airways.
We hypothesized that in patients with COPD, nutritional depletion, defined as a low BMI and/or low FFMI, is related to airway inflammation and that accelerated weight loss is associated with increased inflammatory parameters.
Methods
Study design
This was a prospective cohort study performed at Glenfield Hospital, Leicester, UK, between November 2006 and October 2012. Study design, inclusion, and exclusion criteria have been described previously.Citation10,Citation11 Patients aged over 40 with a physician diagnosis of COPD were recruited from hospital clinics and from local advertising. COPD was defined according to the Global Initiative for Obstructive Lung Disease criteria (GOLD),Citation12 and all subjects demonstrated airflow obstruction with a postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity ratio of less than 0.7. The study was approved by the Leicestershire, Northamptonshire, and Rutland ethics committee. All patients gave informed written consent.
Methods
At study entry, baseline demographics were collected, including smoking history and exacerbation frequency in the previous year. Full lung function and reversibility testing was performed according to American Thoracic Society/European Respiratory Society standards.Citation13 Disease specific health status was assessed using the Chronic Respiratory Disease questionnaire (McMasters University, Hamilton, Canada).Citation14 Spontaneous or induced sputum was collected and processed to measure differential cell counts, bacterial culture, colony forming units, and total bacterial load measured by the abundance of 16S ribosomal unit encoding genes.Citation10,Citation15–Citation17
Venous blood was taken to measure peripheral blood differential cell counts and serum C reactive protein (CRP). Body composition was assessed using dual energy X-ray absorptiometry (DEXA, Lunar Prodigy, GE Healthcare, Little Chalfont, UK).Citation18 Fat-free mass (FFM) was calculated from the sum of lean mass and bone mineral content and normalized for body surface area to derive the FFMI. Muscle atrophy was defined as FFMI less than 15 kg/m2 and 17 kg/m2 in women and men, respectively, as per previous definitions.Citation19
Patients were also classified as underweight if the BMI was less than 21 kg/m2, normal weight if the BMI was between 21 and 25 kg/m2, overweight if the BMI was greater or equal to 25 but less than 30 kg/m2, and obese if the BMI was found to be equal or greater than 30 kg/m.Citation20–Citation22 The threshold of 21 kg/m2, below which patients were classified as underweight, was chosen because mortality has been shown to be increased below this value in COPD populations.Citation21,Citation22 In this study, nutritional depletion was defined as the presence of a low BMI and/or low FFMI.
Repeated measures of body composition, airway inflammation, pulmonary function, and health status were available in 94 patients. Patients were divided into three groups according to their change in weight over the study period: significant weight loss (greater than 5% of the total body weight), minimal weight change (less than 5% weight loss and less than 5% weight gain of total body weight), and weight gain (greater than 5% of the total body weight).
Statistical analysis was performed using PRISM version 6 (GraphPad, La Jolla, CA) and SPSS version 20 (IBM Corporation, Armonk, NY, USA). Parametric data were expressed as mean [standard error of the mean (SEM)] and nonparametric data were expressed as median (interquartile range). Log transformed data were presented as geometric mean [95% confidence interval (CI)]. Correlation of indices was measured using the Pearson correlation (r) or Spearman rank correlation (rs) coefficients for parametric and nonparametric data. For comparison of unpaired parametric or nonparametric groups, the Student’s t-test and the Mann–Whitney test were used. For comparison of three or more groups, the one-way analysis of variance and Kruskal–Wallis test were used for parametric and nonparametric data. The chi-squared test (χ2) was used to compare proportions. Changes in DEXA measurements between baseline and follow-up were assessed using the paired t-test and Wilcoxon matched pairs for parametric and nonparametric data, respectively. No adjustments for multiple comparisons were made. Multiple regression was used to assess the relationship of the dependent variables change in weight and change in FFM (Δ weight, Δ FFM) with explanatory (independent) variables. The independent baseline variables inputted into the regression analysis were i) FEV1% predicted, ii) exacerbation frequency, iii) sputum neutrophil%, and iv) CRP. Smoking status was also entered as a binary variable, as smoking cessation has been shown to be an independent predictor of FFM gain using bioelectrical impedance analysis (BIA).Citation4 Collinearity diagnostics determined that the model showed no violation and the residuals observed normality. A P-value of <0.05 was taken as the threshold of significance for all statistical testing.
Results
Two hundred and thirty-four patients (156 men) entered the study for a baseline visit. Of these patients, 5% were categorized as GOLD I, 45% were GOLD II, 37% were GOLD III, and 13% were GOLD IV. BMI classification identified that 11% were underweight, 29% were normal weight, 34% were overweight, and 27% were obese. Nutritional depletion was present in 86 (37%) patients; 20 patients had both a low BMI and low FFMI, six had a low BMI and a normal FFMI, and 60 had a normal BMI and low FFMI (). Patients with nutritional depletion had worsened lung function and elevated peripheral blood neutrophil counts. The clinical characteristics of depleted and nondepleted patients are shown in . There were no significant correlations between BMI, FFMI, and markers of airway inflammation or airway colonization at baseline. BMI correlated weakly with serum CRP (rs=0.14, P=0.04; ). In patients with nutritional depletion, there were no differences in airway or systemic inflammation in those with low or normal BMI or low or normal FFMI.
Table 1 Baseline characteristics of patients divided into groups according to presence or absence of nutritional depletion
Figure 1 Body composition of patients within cohort; low BMI defined as BMI <21 kg/m2, and low FFMI defined as <15 or <17 kg/m2 in females and males, respectively.
Abbreviations: BMI, body mass index; FFMI, fat-free mass index.
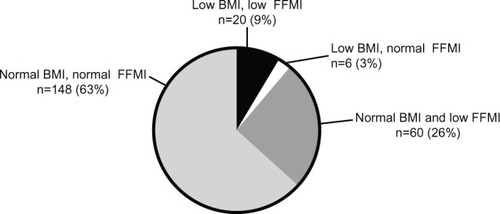
Figure 2 Correlation graph between BMI and CRP.
Longitudinal observations
In 94 patients (64 men) with repeated DEXA scans with a median follow-up of 16 months, men, but not women, had a small but significant reduction in FFM (men: change in FFM 0.7 kg, 95% CI 0.2 to 1.1, P=0.01; women: 0.1 kg, 95% CI −0.5 to 0.7, P=0.73), Significant weight loss of greater than 5% of the total body weight occurred in 15 (16%) patients; 17 (18%) demonstrated significant weight gain. The clinical characteristics according to weight change classifications are shown in . Baseline blood neutrophil count correlated with gain in FFM over the study period (r=0.22, P=0.04). There were no differences in lung function decline over time in patients that lost, gained, or did not change their weight during the study. Multiple regression analyses showed that neither change in weight nor change in FFM could be predicted from baseline variables ().
Table 2 Patients followed up (n=94)
Table 3 Beta coefficients and P-values for baseline variables used in multivariate model identifying predictors of weight and fat-free mass loss over study period
Of patients with a significant gain in body weight (>5%, n=17), only one patient changed group from the nutritionally deplete to the no nutritional depletion group between baseline and follow-up assessment. One patient had significant weight gain, but actually changed from the no nutritional depletion to the nutritionally deplete group due to a drop in their FFMI below the threshold for nutritional depletion classification. Of patients with a significant loss of body weight (>5%, n=15), no patient changed nutritional classification group between baseline and follow-up.
Thirty-three patients died during the study. Patients that died were older at baseline (mean ± SEM age 73±1 versus 68±1 years, P<0.01) and had reduced gas transfer (mean ± SEM DLCO% 41±3 versus 58±2, P<0.01). There was no significant difference in FFMI (mean ± SEM FFMI 17.4±0.4 versus 17.4±0.2 kg/m2, P=0.90) or BMI (mean ± SEM BMI 25.6±0.9 versus 27.3±0.4 kg/m2, P=0.08), in those that died compared to those that survived. There were also no significant differences in exacerbation frequency, pulmonary, or systemic inflammation in those that died compared to those that survived.
Discussion
We have shown that systemic and pulmonary inflammation is independent of skeletal muscle changes in patients with chronic obstructive pulmonary disease. Furthermore, loss of skeletal muscle was not associated with accelerated airway inflammation or lung function decline.
Several previous studies have explored relationships between body composition and systemic inflammation in COPD, but data exploring relationships between airway inflammation and body composition are scarce.Citation23 To our knowledge, this is the first cross-sectional and longitudinal study to explore relationships between airway bacterial load and body composition in COPD. While data from the ECLIPSE study have shown that the subgroup of COPD patients with persistent systemic inflammation has an elevated BMI and FFMI,Citation24 measurements of airway colonization and their potential significance were not performed. Two small studies have produced conflicting results for associations between airway bacterial colonization and body composition in other respiratory diseases; increased colonization rates have been observed in obese patients with resectable lung carcinoma,Citation25 whereas body composition differences have not been observed between asthmatics with and without bacterial colonization.Citation26 While it remains debated whether systemic inflammation in COPD is the result of pulmonary overspill, our findings so far would suggest that there is pulmonary and systemic inflammatory independence.Citation27 Moreover, this would be in keeping with the more recent concepts that muscle mass loss in COPD is due to deconditioning, exacerbations, and hypoxia.Citation28,Citation29 It may be that the role of inflammation in muscle mass loss is more closely linked to infective exacerbations, an aspect that is beyond the scope of this study.
Previous COPD studies have found that skeletal muscle depletion prevalence ranges between 10% and 40%,Citation3,Citation30,Citation31 which is consistent with our findings. We could not demonstrate any relationship between gas transfer rate of decline and weight loss, but this may reflect the short follow-up period in our study. In a study by Hopkinson et al, functional residual capacity, maintenance prednisolone, and continued smoking were identified as independent predictors of FFM decline.Citation4 In our study, we could not identify clinical predictors of muscle loss, although smoking status had a trend to independence, similar to the findings by Hopkinson et al.Citation4 The contrast in observations may reflect body composition differences employed using bioelectrical impedance and DEXA imaging.
One limitation in this study was that our follow-up period was relatively short. Nevertheless, our longitudinal observations are the largest set in a COPD population using DEXA analysis for measurement of body composition and skeletal muscle. Another limitation is that potential confounding factors including physical activity and metabolic syndrome features were not measured. Physical activity has been shown to be an independent predictor of CRP, IL-6, and fibrinogen in COPD,Citation32 and we accept that further longitudinal studies investigating skeletal muscle loss and systemic and airway inflammation should include detailed analysis of physical activity and muscle strength.
In our study, nutritional depletion was associated with elevated peripheral leukocyte and neutrophil counts. Our observations may reflect corticosteroid use, or overspill of systemic inflammation into muscle; however, whether there is a link between systemic and muscle inflammation remains unclear. It is interesting to note, however, that although neutrophil infiltration has been observed in the vastus lateralis muscle of COPD patients,Citation33,Citation34 muscle cytokine levels are not consistently elevated.Citation35–Citation37 In this study we also demonstrated that CRP positively correlated with BMI. In population studies CRP has been shown to be predictive of weight gain,Citation38 and it has been postulated that obesity is a proinflammatory state leading to increased circulating levels of IL-6 and CRPCitation39 which may explain our findings. Furthermore, conflicting data exist regarding systemic inflammation and body composition in COPD, with some authors finding increased and others decreased systemic inflammation in COPD patients with muscle depletion.Citation8,Citation40,Citation41
Conclusion
Nutritional depletion and progressive muscle atrophy are not related to airway inflammation, further suggesting that overspill of pulmonary inflammation is not a key driver of nutritional depletion in COPD.
Author contributions
SM, VM, MP, HP, and KH were involved in data collection and interpretation. BLB, MRB, MCS, IDP, and CEB were involved in the design of the study, data collection, and interpretation. MB was involved in the study design, volunteer recruitment, data collection, data interpretation, data analysis, and had full access to the data and is responsible for the integrity of the data and final decision to submit. All authors contributed to the writing of the manuscript and have approved the final version for submission.
Acknowledgments
The study was funded by the Medical Research Council (UK) and the COPD MAP (MRC/ABPI) Inflammation and Immunology Initiative. This report is independent research arising from a postdoctoral fellowship held by MB supported by the National Institute for Health Research and a senior fellowship held by CEB supported by the Wellcome Trust. This article presents independent research funded by the National Institute for Health Research. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The research was performed in laboratories part funded by the European Regional Development Fund (ERDF 05567). The authors would like to thank the volunteers for taking part and Mrs B Hargadon, Mrs M Shelley, Mrs Sarah Terry, and Miss Amisha Singapuri for their assistance in volunteer characterization.
Disclosure
IDP has received consultancy fees from AstraZeneca, GlaxoSmithKline, and Novartis. CEB has received grant support and consultancy fees from AstraZeneca, MedImmune, Roche, and GlaxoSmithKline. MB has received travel support from Almirall, Boerhinger Ingelheim, and GlaxoSmithKline. The other authors report no conflicts of interest in this work.
References
- World Health OrganizationThe global burden of disease: 2004 update2008
- EidAAIonescuAANixonLSInflammatory response and body composition in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20011648 Pt 11414141811704588
- MostertRGorisAWeling-ScheepersCWoutersEFScholsAMTissue depletion and health related quality of life in patients with chronic obstructive pulmonary diseaseRespir Med200094985986711001077
- HopkinsonNSTennantRCDayerMJA prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary diseaseRespir Res200782517355636
- VestboJPrescottEAlmdalTBody mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart StudyAm J Respir Crit Care Med20061731798316368793
- Di FranciaMBarbierDMegeJLOrehekJTumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19941505 Pt 1145314557952575
- TakabatakeNNakamuraHAbeSThe relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20001614 Pt 11179118410764309
- EaganTMAukrustPUelandTBody composition and plasma levels of inflammatory biomarkers in COPDEur Respir J20103651027103320413541
- EaganTMGabazzaECD’Alessandro-GabazzaCTNF-α is associated with loss of lean body mass only in already cachectic COPD patientsRespir Res2012134822708547
- BafadhelMMcKennaSTerrySAcute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkersAm J Respir Crit Care Med2011184666267121680942
- BafadhelMMcKennaSTerrySBlood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trialAm J Respir Crit Care Med20121861485522447964
- RabeKFHurdSAnzuetoAGlobal Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- MillerMRCrapoRHankinsonJATS/ERS Task ForceGeneral considerations for lung function testingEur Respir J200526115316115994402
- GuyattGMeasuring health status in chronic airflow limitationEur Respir J1988165605643049146
- Health Protection AgencyInvestigation of bronchoalveolar lavage, sputum and associated specimensNational Standard Method BSOP2009572.3127
- PizzichiniEPizzichiniMMEfthimiadisAIndices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurementsAm J Respir Crit Care Med19961542 Pt 13083178756799
- PyeAStockleyRAHillSLSimple method for quantifying viable bacterial numbers in sputumJ Clin Pathol19954887197247560197
- MillerAStraussBJMolSDual-energy X-ray absorptiometry is the method of choice to assess body composition in COPDRespirology200914341141819353776
- SchutzYKyleUUPichardCFat-free mass index and fat mass index percentiles in Caucasians aged 18–98 yInt J Obes Relat Metab Disord200226795396012080449
- World Health OrganisationPhysical status: The use and interpretation of anthropometryReport of a WHO expert committee19958541452
- CelliBRCoteCGMarinJMThe body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med2004350101005101214999112
- LandboCPrescottELangePVestboJAlmdalTPPrognostic value of nutritional status in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199916061856186110588597
- IschakiEPapatheodorouGGakiEPapaIKoulourisNLoukidesSBody mass and fat-free mass indices in COPD: relation with variables expressing disease severityChest2007132116416917505043
- AgustíAEdwardsLDRennardSIEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsPersistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotypePLoS One201275e3748322624038
- IoanasMAngrillJBaldoXBronchial bacterial colonization in patients with resectable lung carcinomaEur Respir J200219232633211866014
- ZhangQIllingRHuiCKBacteria in sputum of stable severe asthma and increased airway wall thicknessRespir Res2012133522513083
- SindenNJStockleyRASystemic inflammation and comorbidity in COPD: a result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidenceThorax2010651093093620627907
- ManWDKempPMoxhamJPolkeyMISkeletal muscle dysfunction in COPD: clinical and laboratory observationsClin Sci (Lond)2009117725126419681758
- ManWDKempPMoxhamJPolkeyMIExercise and muscle dysfunction in COPD: implications for pulmonary rehabilitationClin Sci (Lond)2009117828129119689433
- VermeerenMACreutzbergECScholsAMCOSMIC Study GroupPrevalence of nutritional depletion in a large out-patient population of patients with COPDRespir Med200610081349135516412624
- ScholsAMBroekhuizenRWeling-ScheepersCAWoutersEFBody composition and mortality in chronic obstructive pulmonary diseaseAm J Clin Nutr2005821535916002800
- WatzHWaschkiBBoehmeCClaussenMMeyerTMagnussenHExtrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional studyAm J Respir Crit Care Med2008177774375118048807
- MenonMKHouchenLSinghSJMorganMDBraddingPSteinerMCInflammatory and satellite cells in the quadriceps of patients with COPD and response to resistance trainingChest201214251134114222459782
- BarreiroEFerrerDSanchezFInflammatory cells and apoptosis in respiratory and limb muscles of patients with COPDJ Appl Physiol (1985)2011111380881721636562
- BarreiroEScholsAMPolkeyMIENIGMA in COPD projectCytokine profile in quadriceps muscles of patients with severe COPDThorax200863210010717875568
- CrulTSpruitMAGayan-RamirezGMarkers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patientsEur J Clin Invest2007371189790417883420
- Montes de OcaMTorresSHDe SanctisJMataAHernándezNTálamoCSkeletal muscle inflammation and nitric oxide in patients with COPDEur Respir J200526339039716135718
- DuncanBBSchmidtMIChamblessLEFolsomARCarpenterMHeissGFibrinogen, other putative markers of inflammation, and weight gain in middle-aged adults – the ARIC study. Atherosclerosis Risk in CommunitiesObes Res20008427928610933303
- DandonaPAljadaABandyopadhyayAInflammation: The link between insulin resistance, obesity and diabetesTrends Immunol20042514714698276
- GakiEKontogianniKPapaioannouAIAssociations between BODE index and systemic inflammatory biomarkers in COPDCOPD20118640841322149400
- PoulainMDoucetMDrapeauVMetabolic and inflammatory profile in obese patients with chronic obstructive pulmonary diseaseChron Respir Dis200851354118303100
