Abstract
Background
Sleep problems are common in patients with chronic obstructive pulmonary disease (COPD), but the validity of patient-reported outcome measures (PROMs) that measure sleep dysfunction has not been evaluated. We have reviewed the literature to identify disease-specific and non-disease-specific sleep PROMs that have been validated for use in COPD patients. The review also examined the psychometric properties of identified sleep outcome measures and extracted point and variability estimates of sleep instruments used in COPD studies.
Methods
The online EMBASE, MEDLINE, PsycINFO, and SCOPUS databases for all years to May 2014 were used to source articles for the review. The review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Criteria from the Medical Outcomes Trust Scientific Advisory Committee guidelines were used to evaluate the psychometric properties of all sleep PROMs identified.
Results
One COPD-specific and six non-COPD-specific sleep outcome measures were identified and 44 papers met the review selection criteria. We only identified one instrument, the COPD and Asthma Sleep Impact Scale, which was developed specifically for use in COPD populations. Ninety percent of the identified studies used one of two non-disease-specific sleep scales, ie, the Pittsburgh Sleep Quality Index and/or the Epworth Sleep Scale, although neither has been tested for reliability or validity in people with COPD.
Conclusion
The results highlight a need for existing non-disease-specific instruments to be validated in COPD populations and also a need for new disease-specific measures to assess the impact of sleep problems in COPD.
Introduction
Sleep problems are a common and important, but poorly understood and under-researched, aspect of chronic obstructive pulmonary disease (COPD). After breathlessness and fatigue, sleep disturbance is considered to be the third most common symptom experienced by people with respiratory diseaseCitation1 and is also predictive of exacerbations, respiratory-related emergency hospital visits, and all-cause mortality.Citation2 Insomnia describes any reported difficulty a person has with sleepCitation3 and has four elements: difficulties falling asleep, interrupted sleep, trouble staying asleep, and still feeling tired and worn out even after a usual amount of sleep.Citation3–Citation5 Around 10% of the adult population is affected by insomnia, but the occurrence is much higher in people with COPD, where estimates range between 16% and 75%.Citation6 The benefits of sleep are well known, and long-term interruption of normal sleeping patterns has a detrimental impact on physical, emotional, and social functioning, and is also associated with anxiety, depression, bodily pain, and a wide variety of pre-existing chronic medical conditions.Citation4 In addition to insomnia, narcolepsy (suddenly falling asleep at inappropriate times), restless legs syndrome, and obstructive sleep apnea are the most common sleep disorders found in the general population,Citation6 and people with COPD are disproportionately affected. Restless legs syndrome involves a need to move the legs, usually at night-time, is associated with marked sleep disturbance, and affects 7%–14% of the general population and 29% of patients with COPD.Citation7,Citation8 Obstructive sleep apnea is the periodic interruption of airflow in the upper airway during sleep and affects 3%–7% of the general populationCitation9 and 25%–29% of people with COPD.Citation10 A summary of the occurrence of four common sleep disorders in COPD populations is provided in .Citation8,Citation11–Citation13
Table 1 Summary of the occurrence of common sleep disorders in COPD populations
Given the importance of sleep disorders in COPD, being able to accurately classify their nature and severity is important in the management of COPD. Although self-reported sleep disorders are associated with COPD symptoms and poorer health-related quality of life,Citation14 their relationship with traditional diagnostic markers of lung function (such as forced expiratory volume in one second, forced vital capacity, and oxygen saturation) is weak.Citation15 This emphasizes the need for clinical instruments to accurately assess the impact of the disease and its treatment on a patient’s health and well-being through patient-reported outcome measures (PROMs)Citation16,Citation17 as well as recording changes in physiological function.
Many of the instruments that have measured sleep disturbance in epidemiological studies were originally developed for people with a range of psychological conditions and/or pre-existing sleeping disorders.Citation18,Citation19 However, the validity of these measures cannot be assumed to transfer between clinical populations.Citation19 Thus, the aim of this review was to identify and evaluate the suitability of published measures of sleep disturbance for use in people with COPD in order to make recommendations for best practice for clinical and research purposes. Our objectives were to:
Identify which patient-reported outcome sleep measures have been used in people with COPD
Identify which instruments have been developed and validated specifically for people with COPD
Summarize the evidence for reliability and validity of sleep instruments in COPD patients
Examine associations with sleep disturbance recorded by sleep instruments used in clinical studies of COPD patients.
Materials and methods
Ethical approval was not needed to undertake this review, which was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.Citation20
Search strategy
In this study, we conducted a systematic computerized literature review designed to identify all PROMs concerned with sleep problems experienced by people with COPD. The search included all instruments that had been developed and validated in people with COPD as well as generic instruments that had been developed for use in other disease areas and then administered to adult COPD patients.
Stage 1: Identification of sleep outcome measures used in COPD
The first stage of the search was to identify sleep outcome measures that had been used in COPD. This was conducted using EMBASE, MEDLINE, and PsycINFO electronic databases for all years up to May 2014 using both key words, ie, the Medical Subject Headings (MeSH) “COPD” AND “sleep” and expanded to include all recognized subheadings. All titles, abstracts, and full texts from the identified papers were examined by the lead author (APG) for reference to specific sleep instruments or data indicating that at least one sleep outcome measure had been used. A list of sleep outcome measures was then produced. The reference lists and citations of selected articles were also searched to identify any additional sleep PROMs not found by the electronic database search.
Stage 2: Selection and evaluation of sleep instruments used in COPD
A SCOPUS database search was carried out on each of the detected sleep outcome measures to identify all publications in which the original paper had been cited. The search included the following related terms:
Construct-related terms: sleep problems
Population terms: COPD patients (in the title, abstract, text, or reference section)
Outcome-related terms: development, validation, or psychometric properties of sleep PROMs designed specifically for people with COPD. Sleep outcome measures not specifically designed for people with COPD but used in a COPD patient group whether psychometric data were reported or not
Method-related terms: instrument* OR measure* OR question* OR scale OR assess
Quality assessment terms: valid* or reliab* or evaluat* OR psychometric.
We also screened the reference lists and citations of included articles to identify additional relevant publications.
Eligibility criteria
To be included in the review, all identified articles had to meet the following inclusion criteria: the article described PROMs that either had been specifically designed and validated for use in patients with COPD or included a generic instrument that had been administered to COPD patients; information on at least one measurement property of the outcome measure was reported; the study sample consisted of adults with a clinical diagnosis of COPD; a full text of the original publication was published electronically, in English, in a peer-reviewed journal.
Articles were excluded if reference to COPD and/or sleep only appeared in the text or reference section. Similarly, we excluded all articles with mixed study samples where the results from COPD patients were not reported separately. Review articles, protocols, and case studies were also excluded. Two investigators (APG and JY) read independently all titles, abstracts, and full texts of all the retrieved articles to determine which were eligible for review. Any disagreements were resolved at a consensus meeting.
Methodological quality assessment
The COSMIN (COnsensus-based Standards for the selection of health Measurement INstruments) checklistCitation21 is a standardized tool for evaluating the methodological quality of PROMs. COSMIN checklists are used to evaluate the measurement properties of instruments in terms of their internal consistency, reliability, measurement error, content validity, structural validity, hypothesis testing, cross-cultural validity, criterion validity, and responsiveness to change. As it was anticipated that the number of PROMs that had been developed and validated for use in COPD populations was likely to be very small, rather than using the full COSMIN checklist we used four PROM characteristics recommended by the US Food and Drug AdministrationCitation22 to evaluate the measurement properties of identified sleep PROM instruments in relation to their use in COPD patients, ie, conceptual and measurement model, reliability, validity, and responsiveness to change.
Conceptual model
Identified articles were examined for descriptions of concepts contained within the instrument, including the rationale and process for deriving scale scores from raw scores, identifying and dealing with floor and ceiling effects, and scale variability.
Reliability
Articles were scrutinized for estimates of reliability, including inter-item correlations, test-retest repeatability, internal consistency, and/or kappa statistics.
Validity
Any reference to content, construct, and criterion-related validity were noted. When considering construct validity, we also recorded methods to differentiate between people with different levels of lung function or disease severity, such as the Global Initiative for Chronic Obstructive Lung Disease staging system that classifies people with COPD according to the results of pulmonary tests. Where available, we also collected data regarding the relationships between sleep outcome instruments and other established COPD outcome measures (such as the St George’s Respiratory Questionnaire,Citation23 the Medical Research Council Dyspnea scale,Citation24 and routine clinical tests). Any analyses intended to examine dimensionality using factor analysis or Rasch analysis were noted, along with any assessments of differential item functioning that evaluated group differences in PROM item responses.
Responsiveness to change
All data relating to the ability of the instrument to detect changes over time in terms of sleep disturbance were noted. Where correlations between changes in scores of two measures are reported, these had to relate to predefined hypotheses.
Results
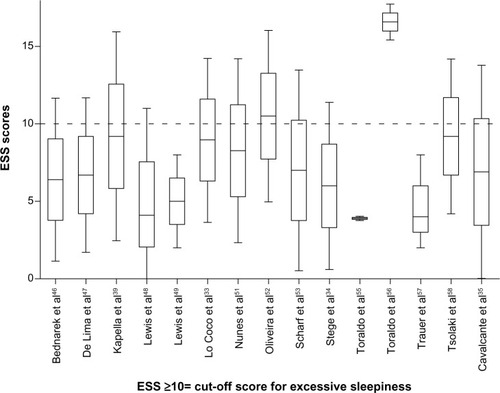
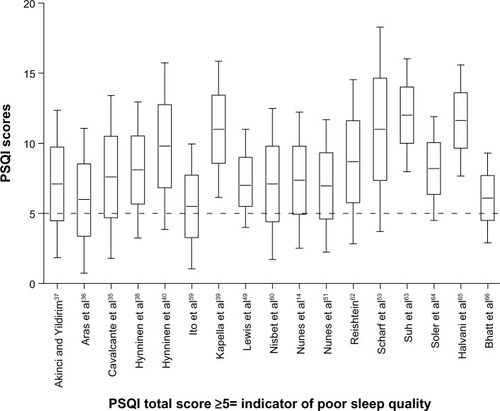
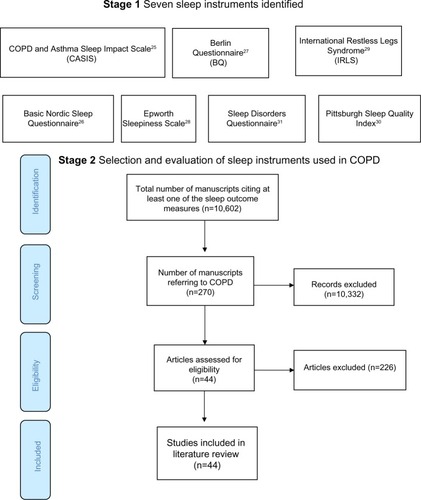
The stage 1 database search identified articles referring to COPD and sleep (Medline 804, EMBASE 2,314, and PsycINFO 59) from which one COPD-specific and six non-disease-specific sleep instruments were identified ().Citation25–Citation31 In stage 2, the SCOPUS search found 10,602 articles citing any of the seven sleep outcome measures, 270 of which referred to COPD. After applying the exclusion criteria, 44 manuscripts were selected for review (). Nearly 90% of the reviewed publications either used the Pittsburgh Sleep Quality Index (PSQI;Citation30 19/44, 43.1%) or the Epworth Sleepiness Scale (ESS; 20/44, 45.5%).Citation28 The 19-item PSQI measures sleep quality in seven domains and the ESS assesses the likelihood of a person dozing off or falling asleep in eight common life situations. Most studies involved patients with moderate-severe COPD recruited from hospital outpatient or specialist respiratory clinics.
Table 2 Number of papers found and excluded or included in the review
Figure 1 Flow diagram showing the total number of studies screened, assessed for eligibility and included in the review.
COPD-specific sleep outcome measures
After assessing the methodological properties of the identified PROMs, only one instrument appeared to have been developed and validated for use in COPD patients, ie, the COPD and Asthma Sleep Impact Scale (CASIS).Citation25
The CASIS is a seven-item measure of sleep impairment during the previous week. Five items relate to disturbance falling asleep or staying awake during the day. The remaining two items concern sleep quality. The items are scored on a five-point scale ranging from 0 if the item never applies, to 4 if the item applies very often. A total raw score is produced from the sum of the seven individual scores which is then linearly transformed to a 0–100 total scale score. A mean CASIS score of 43.3±24.7 was reported in patients with mild COPD. The results of the original psychometric testing of the CASIS (), showed that the scale had good internal consistency (Cronbach’s alpha 0.91), test-retest reproducibility (intraclass coefficient 0.84), and concurrent validity (correlated with the St George’s Respiratory Questionnaire, r=0.68).
Table 3 Psychometric properties of COPD and Asthma Sleep Impact Scale
None of the non-disease-specific sleep scales reported any tests of reliability or validity to justify their use in the COPD population. Significant associations were observed in only 8/20 (40%) of studies where the ESS was compared with other COPD-related outcome measures. For example, the prevalence of daytime sleepiness (ESS >10) was significantly greater in patients diagnosed with insomnia.Citation11 Compared with people who had obstructive sleep apnea/hypopnea syndrome, COPD patients were more likely to be affected by daytime sleepiness.Citation32 Significant differences in mean ESS scores were observed between patients with COPD and restless legs syndrome compared with controls who had restless legs syndrome.Citation33 However, no differences in daytime sleepiness were observed in a study that compared use of temazepam between COPD patients and controls.Citation34 Similarly, no significant differences in ESS scores were detected in patients with and without restless legs syndromeCitation35 ().
Table 4 Summary of studies that used the Epworth Sleepiness Scale
For the PSQI, significant associations were noted in 11/19 (57.9%) of the relevant studies. PSQI scores were found to be significantly higher in patients with restless legs syndrome.Citation36 PSQI total scores also correlated with total scores from the St George’s Respiratory QuestionnaireCitation37,Citation14 and the Fatigue Severity Scale.Citation35 In contrast, no correlation was observed between PSQI and St George’s Respiratory Questionnaire scores in an investigation of factors affecting health status in COPD patients with comorbid anxiety or depression.Citation38 Further, although significant PSQI score reductions were observed in patients receiving a course of cognitive behavioral therapy (where the primary outcome was insomnia),Citation39 no reductions in pre- and post-sleep quality were observed in a randomized controlled trial that compared cognitive behavioral therapy with usual care, where sleep was a secondary outcome measure to anxiety and depressionCitation40 ().
Table 5 Summary of papers that used the Pittsburgh Sleep Quality Index
shows the papers that used the four remaining generic outcome measures in studies of COPD patients.Citation33,Citation35,Citation41,Citation42 With so few studies, there are currently insufficient data to evaluate the utility of these instruments; however, in one study,Citation33 International Restless Leg Study Group scores correlated significantly with ESS scores.
Table 6 Summary of articles using generic sleep measures
Although the results provide some evidence of the validity of measures of sleep disturbance in people with COPD, none of the above sleep measures were specifically evaluated for people with COPD. Similarly, we did not find any articles that provided data on test-retest, intrarater, or inter-rater reliability or responsiveness to change among COPD patient groups.
The point estimates of sleep disturbance from clinical studies of COPD patients using the ESS and the PSQI are shown on and . In 13/15 of the studies using the ESS, the mean/median scores were less than 10, ie, below the accepted cutoff value for excessive daytime sleepiness.Citation28 Most of the observed PSQI scores were above 5, ie, above the cutoff value representing poor quality sleep.Citation30 Point and upper and lower quartile estimates for the ESS and PSQI are displayed on and .
Discussion
Sleep disturbances are an important problem that can seriously impact on physical and mental well-being as well as quality of life for people with COPD. This review identified seven outcome measures that have been used in COPD populations but none has been sufficiently validated to satisfy US Food and Drug Administration requirements to support labeling claims in medical product development. Only one measure, the CASIS, included item response theory modeling when evaluating the psychometric evaluation of the instrument. Incorporating item response theory is now considered to be an essential component in the design and validation of all PROMs.Citation19
The majority of sleep studies in COPD have relied on two general measures of sleep dysfunction, the ESS and the PSQI, and although both of these instruments have been extensively used in a variety of clinical populations, neither has been validated for use in COPD patients.
As far as we are aware, this is the first systematic review of sleep measures in COPD. A strength of this study was the comprehensiveness of our literature search. We believe that we have identified all of the main PROMs of sleep disorders that have been used in COPD populations. Nevertheless, as we did not search all electronic databases or carry out a hand search, there is the possibility that we may have missed some relevant articles, particularly those that appeared in non-English language journals. However, by cross-checking the reference lists of all included papers and that of a recent systematic review of instruments designed to measure sleep dysfunction in adults,Citation15 we believe we have minimized the loss of any important papers.
The review identified only one PROM, ie, the CASIS, which has been specifically designed and validated for use in COPD patients. In each item of the CASIS, patients are advised to “[…] think about the impact of breathing problems/COPD/asthma on your sleep during the past week […]” Most items, however, are general in nature and relate to the frequency of symptoms such as falling asleep, staying awake, and waking up feeling rested. Only one item relates specifically to breathing problems, ie, shortness of breath, coughing, and chest tightness. Further, as these symptoms are all contained within the same item, it is not possible to differentiate patients who may have different severity of symptoms; for example, between patients who wake up at night only with shortness of breath or wake up with both shortness of breath and coughing. Since the publication of the original paper, the CASIS has not been used in any intervention studies, so further evidence is needed to confirm the utility of this instrument in guiding the clinical management of COPD patients and in research.
This review has highlighted the current reliance of sleep research on generic sleep measures and the paucity of disease-specific instruments currently available to assess the patient’s experience of sleep in relation to COPD. By definition, generic measures tend to cover broad aspects such as functional status and perceptions and are more likely to identify aspects that are not disease-related. Because instruments validated in one population may not perform well in specific populations under investigation, separate validation of generic measures in each population is recommended.Citation43 Similarly, given that disease-specific measures are generally more responsive to change, outcomes based solely on generic measures are unlikely to detect treatment-related improvements.Citation44 These deficiencies could call into question findings from previous research on the impact of sleep problems in COPD. The need for validated COPD-specific sleep outcome measures was emphasized in an expert panel meeting held in 2011.Citation45 While appreciating the multifactorial nature of sleep disturbance in COPD, the panel highlighted the need for an instrument to classify patients according to their night or daytime symptoms, which is not possible using existing PROMs for sleep. Development work on new COPD sleep PROMs to address these limitations is currently being carried out by the authors of this review.
Conclusion
This review highlights the complexity of sleep assessment, the inadequacy of non-disease-specific measures to capture problems experienced by people with COPD, and the absence of robust and validated methods of assessing and classifying symptoms associated with disrupted sleep in COPD. In studies using non-disease-specific sleep measures, there is a pressing need for these to be validated with COPD populations and/or for new disease-specific PROMs to be developed.
Acknowledgments
This partnership received financial support from the Knowledge Transfer Partnerships (KTP) program. The project was also supported by the UK Medical Research Council. KTP aims to help businesses to improve their competitiveness and productivity through the better use of knowledge, technology, and skills that reside within the UK knowledge base. KTP is funded by the Technology Strategy Board along with other government funding organizations.
Disclosure
The authors report no conflicts of interest in this work.
References
- KinsmanRAYaroushRAFernandezEDirksJFSchocketMFukuharaJSymptoms and experiences in chronic bronchitis and emphysemaChest1983837557616839816
- OmachiTABlancPDClamanDMDisturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomesSleep Med20121347648322429651
- National Institutes of HealthInformation about sleep2013 Available from: http://science.education.nih.gov/supplements/nih3/sleep/guide/info-sleep.htmAccessed July 9, 2013
- MorphyHDunnKMLewisMBoardmanHFCroftPREpidemiology of insomnia: a longitudinal study in a UK populationSleep20073027428017425223
- LegerDGuilleminaultCDreyfusJPDelahayeCPaillardMPrevalence of insomnia in a survey of 12,778 adults in FranceJ Sleep Res20009354210733687
- KlinkMQuanSFPrevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseasesChest1987915405463829746
- AllenRPEarleyCJRestless legs syndrome: a review of clinical and pathophysiologic featuresJ Clin Neurophysiol20011812814711435804
- KaplanYInonuHYilmazAOcalSRestless legs syndrome in patients with chronic obstructive pulmonary diseaseCan J Neurol Sci20083535235718714805
- PunjabiNMThe epidemiology of adult obstructive sleep apneaProc Am Thorac Soc2008513614318250205
- JelicSDiagnostic and therapeutic approach to coexistent chronic obstructive pulmonary disease and obstructive sleep apneaInt J Chron Obstruct Pulmon Dis2008326927518686735
- BudhirajaRParthasarathySBudhirajaPHabibMPWendelCQuanSFInsomnia in patients with COPDSleep20123536937522379243
- Ali ZohalMYazdiZKazemifarAMDaytime sleepiness and quality of sleep in patients with COPD compared to control groupGlob J Health Sci2013515015523618484
- McNicholasWTChronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular diseaseAm J Respir Crit Care Med200918069270019628778
- NunesDMMotaRMde Pontes NetoOLPereiraEDde BruinVMde BruinPFImpaired sleep reduces quality of life in chronic obstructive pulmonary diseaseLung200918715916319399553
- JonesPMiravitllesMvan der MolenTKulichKBeyond FEV(1) in COPD: a review of patient-reported outcomes and their measurementInt J Chron Obstruct Pulmon Dis2012769770923093901
- National Health ServicePatient reported outcome measures: their role in measuring and improving patient experience2012 Available from: http://patientexperienceportal.org/article/patient-reported-outcome-measures-their-role-in-measuring-and-improving-patient-experienceAccessed April 3, 2014
- RothrockNEHaysRDSpritzerKYountSERileyWCellaDRelative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS)J Clin Epidemiol2010631195120420688471
- American Academy of Sleep MedicineInternational classification of sleep disorders, revised: diagnostic and coding manualChicago, IL, USAAmerican Academy of Sleep Medicine2001 Available from: http://www.esst.org/adds/ICSD.pdfAccessed April 4, 2014
- DevineEBHakimZGreenJA systematic review of patient-reported outcome instruments measuring sleep dysfunction in adultsPharmacoeconomics20052388991216153133
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementBMJ2009339b253519622551
- MokkinkLBTerweeCBPatrickDLInternational consensus on taxonomy, terminology, and definitions of measurement properties: results of the COSMIN studyJ Clin Epidemiol20106373774520494804
- US Department of Health and Human Services Food and Drug AdministrationGuidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims2009 Available from: http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdfAccessed April 30, 2014
- JonesPWQuirkFHBaveystockCMThe St George’s Respiratory QuestionnaireRespir Med199185Suppl B25311759018
- FletcherCMElmesPCFairbairnASWoodCHThe significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working populationBMJ1959225726613823475
- PokrzywinskiRFMeadsDMMcKennaSPGlendenningGARevickiDADevelopment and psychometric assessment of the COPD and Asthma Sleep Impact Scale (CASIS)Health Qual Life Outcomes200979819968881
- PartinenMGislasonTBasic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaintsJ Sleep Res1995415015510607192
- NetzerNCStoohsRANetzerCMClarkKStrohlKPUsing the Berlin Questionnaire to identify patients at risk for the sleep apnea syndromeAnn Intern Med199913148549110507956
- JohnsMWA new method for measuring daytime sleepiness: the Epworth sleepiness scaleSleep1991145405451798888
- WaltersASLeBrocqCDharAInternational Restless Legs Syndrome Study GroupValidation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndromeSleep Med2003412113214592342
- BuysseDJReynoldsCF3rdMonkTHBermanSRKupferDJThe Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and researchPsychiatry Res1989281932132748771
- DouglassABBornsteinRNino-MurciaGThe Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQSleep1994171601678036370
- KarachaliouFKostikasKPastakaCBagiatisVGourgoulianisKIPrevalence of sleep-related symptoms in a primary care population – their relation to asthma and COPDPrim Care Respir J20071622222817660890
- Lo CocoDMattalianoACocoALRandisiBIncreased frequency of restless legs syndrome in chronic obstructive pulmonary disease patientsSleep Med20091057257618996743
- StegeGHeijdraYFvan den ElshoutFJTemazepam 10 mg does not affect breathing and gas exchange in patients with severe normocapnic COPDRespir Med201010451852419910177
- CavalcanteAGde BruinPFde BruinVMRestless legs syndrome, sleep impairment, and fatigue in chronic obstructive pulmonary diseaseSleep Med20121384284722727926
- ArasGKadakalFPurisaSKanmazDAynaciAIsikEAre we aware of restless legs syndrome in COPD patients who are in an exacerbation period? Frequency and probable factors related to underlying mechanismCOPD2011843744322149404
- AkinciACYildirimEFactors affecting health status in patients with chronic obstructive pulmonary diseaseInt J Nurs Pract201319313823432886
- HynninenMJPallesenSNordhusIHFactors affecting health status in COPD patients with co-morbid anxiety or depressionInt J Chron Obstruct Pulmon Dis2007232332818229570
- KapellaMCHerdegenJJPerlisMLCognitive behavioral therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effectsInt J Chron Obstruct Pulmon Dis2011662563522162648
- HynninenMJBjerkeNPallesenSBakkePSNordhusIHA randomized controlled trial of cognitive behavioral therapy for anxiety and depression in COPDRespir Med201010498699420346640
- SaaresrantaTIrjalaKAittokallioTPoloOSleep quality, daytime sleepiness and fasting insulin levels in women with chronic obstructive pulmonary diseaseResp Med200599856863
- ValipourALaviePLothallerHMikulicIBurghuberOCSleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary diseaseSleep Med20111236737221388878
- MatzaLSBoyeKSYurginNValidation of two generic patient-reported outcome measures in patients with type 2 diabetesHealth Qual Life Outcomes200754717672906
- WiebeSGuyattGWeaverBMatijevicSSidwellCComparative responsiveness of generic and specific quality-of-life instrumentsJ Clin Epidemiol200356526012589870
- AgustiAHednerJMarinJMNight-time symptoms: a forgotten dimension of COPDEur Respir Rev20112018319421881146
- BednarekMPlywaczewskiRJonczakLZielinskiJThere is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population studyRespiration20057214214915824523
- De LimaOMOliveira-SouzaRdSantos OdaRMoraesPASáLFNascimentoOJSubclinical encephalopathy in chronic obstructive pulmonary diseaseArq Neuropsiquiatr2007651154115718345421
- LewisCAEatonTEFergussonWWhyteKFGarrettJEKolbeJHome overnight pulse oximetry in patients with COPD: more than one recording may be neededChest20031231127113312684303
- LewisCAFergussonWEatonTZengIKolbeJIsolated nocturnal desaturation in COPD: prevalence and impact on quality of life and sleepThorax20096413313818390630
- McNicholasWTCalverleyPMLeeAEdwardsJCTiotropiumSleepStudy in COPD InvestigatorsLong-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPDEur Respir J20042382583115218993
- NunesDMDe BruinVMLouzadaFMActigraphic assessment of sleep in chronic obstructive pulmonary diseaseSleep Breath20131712513222351160
- OliveiraMGNeryLESantos-SilvaRIs portable monitoring accurate in the diagnosis of obstructive sleep apnea syndrome in chronic pulmonary obstructive disease?Sleep Med2012131033103822841038
- ScharfSMMaimonNSimon-TuvalTBernhard-ScharfBJReuveniHTarasiukASleep quality predicts quality of life in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2011611221311688
- SorianoJBYanezARenomFSet-up and pilot of a population cohort for the study of the natural history of COPD and OSA: the PULSAIB studyPrim Care Respir J20101914014720169290
- ToraldoDMNicolardiGDe NuccioFLorenzoRAmbrosinoNPattern of variables describing desaturator COPD patients, as revealed by cluster analysisChest20051283828383716354851
- ToraldoDMDe NuccioFNicolardiGFixed-pressure nCPAP in patients with obstructive sleep apnea (OSA) syndrome and chronic obstructive pulmonary disease (COPD): a 24-month follow-up studySleep Breath20101411512319756803
- TrauerJMGielenCTrauerTSteinfortCLInability of single resting arterial blood gas to predict significant hypoxaemia in chronic obstructive pulmonary diseaseIntern Med J20124238739421118412
- TsolakiVPastakaCKaretsiEOne-year non-invasive ventilation in chronic hypercapnic COPD: effect on quality of lifeRespir Med200810290491118280131
- ItoKKawayamaTShojiYDepression, but not sleep disorder, is an independent factor affecting exacerbations and hospitalization in patients with chronic obstructive pulmonary diseaseRespirology20121794094922564039
- NisbetMEatonTLewisCFergussonWKolbeJOvernight prescription of oxygen in long term oxygen therapy: time to reconsider the guidelines?Thorax20066177978216769716
- OhEGKimCJLeeWHKimSSCorrelates of fatigue in Koreans with chronic lung diseaseHeart Lung200433132014983134
- ReishteinJLRelationship between symptoms and functional performance in COPDRes Nurs Health200528394715625710
- SuhSEllisRJSollersJJ3rdThayerJFYangHCEmeryCFThe effect of anxiety on heart rate variability, depression, and sleep in chronic obstructive pulmonary diseaseJ Psychosom Res20137440741323597328
- SolerXDiaz-PiedraCRiesALPulmonary rehabilitation improves sleep quality in chronic lung diseaseCOPD20131015616323514215
- HalvaniAMohsenpourFNasirianiKEvaluation of exogenous melatonin administration in improvement of sleep quality in patients with chronic obstructive pulmonary diseaseTanaffos20131291525191456
- BhattSPPetersonMWWilsonJSDurairajLNoninvasive positive pressure ventilation in subjects with stable COPD: a randomized trialInt J Chron Obstruct Pulmon Dis2013858158924293994