Abstract
Introduction
Tiotropium is prescribed for the treatment of chronic obstructive pulmonary disease (COPD) and delivered via HandiHaler® (18 μg once daily) or Respimat® Soft Mist™ inhaler (5 μg once daily). The recent TIOtropium Safety and Performance In Respimat® (TIOSPIR™) study demonstrated that both exhibit similar safety profiles. This analysis provides an updated comprehensive safety evaluation of tiotropium® using data from placebo-controlled HandiHaler® and Respimat® trials.
Methods
Pooled analysis of adverse event (AE) data from tiotropium HandiHaler® 18 μg and Respimat® 5 μg randomized, double-blind, parallel-group, placebo-controlled, clinical trials in patients with COPD (treatment duration ≥4 weeks). Incidence rates, rate ratios (RRs), and 95% confidence intervals (CIs) were determined for HandiHaler® and Respimat® trials, both together and separately.
Results
In the 28 HandiHaler® and 7 Respimat® trials included in this analysis, 11,626 patients were treated with placebo and 12,929 with tiotropium, totaling 14,909 (12,469 with HandiHaler®; 2,440 with Respimat®) patient-years of tiotropium exposure. Mean age was 65 years, and mean prebronchodilator forced expiratory volume in 1 second (FEV1) was 1.16 L (41% predicted). The risk (RR [95% CI]) of AEs (0.90 [0.87, 0.93]) and of serious AEs (SAEs) (0.94 [0.89, 0.99]) was significantly lower in the tiotropium than in the placebo group (HandiHaler® and Respimat® pooled results), and there was a numerically lower risk of fatal AEs (FAEs) (0.90 [0.79, 1.01]). The risk of cardiac AEs (0.93 [0.85, 1.02]) was numerically lower in the tiotropium group. Incidences of typical anticholinergic AEs, but not SAEs, were higher with tiotropium. Analyzed separately by inhaler, the risks of AE and SAE in the tiotropium groups remained lower than in placebo and similarly for FAEs.
Conclusion
This analysis indicates that tiotropium is associated with lower rates of AEs, SAEs, and similar rates of FAEs than placebo when delivered via HandiHaler® or Respimat® (overall and separately) in patients with COPD.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) remains the fourth leading cause of death worldwide, although it is both preventable and treatable. It is a major cause of morbidity and mortality, and its economic and social burden is projected to increase in the coming decades owing to increased risk factors and aging of the population.Citation1 Although characterized by the expiratory airflow (measured by forced expiratory volume in 1 second [FEV1]), COPD is associated with an increased incidence of comorbidities such as cardiovascular (CV) disease, musculoskeletal impairment, and diabetes mellitus, which can affect outcomes and may result in unanticipated adverse events (AEs). In addition, patients are often prescribed a number of medications for the management of the concomitant diseases, as well as their COPD, and therefore it is important to evaluate the long-term safety and efficacy of respiratory treatments.
Tiotropium bromide (SPIRIVA®, Boehringer Ingelheim Pharma GmbH & Co KG, Ingelheim am Rhein, Germany) is an inhaled, once-daily, long-acting anticholinergic bronchodilator indicated for maintenance therapy in patients with COPD,Citation1 and has been shown to improve lung function, health-related quality of life (HRQoL), dyspnea, and exercise tolerance.Citation2–Citation6 Tiotropium has also been shown to reduce the number and risk of exacerbations (including exacerbations leading to hospitalization), and to delay the time to first exacerbation.Citation5,Citation7–Citation9
Tiotropium has been available since 2002 as a single-dose dry-powder formulation delivered via the HandiHaler® (Boehringer Ingelheim Pharma GmbH & Co KG) device (18 μg),Citation10 and since 2007 as an aqueous solution delivered via the multidose Respimat® Soft Mist™ inhaler (SMI) (Boehringer Ingelheim Pharma GmbH & Co KG) (5 μg once daily).Citation11
Tiotropium HandiHaler® and Respimat® have similar efficacy, safety, and pharmacokinetic profiles,Citation5,Citation12–Citation15 and are well established in most countries, with tiotropium HandiHaler® being the most prescribed COPD maintenance treatment worldwide (with more than 31 million patient-years of use).Citation16
Results from the Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT®) trial, which permitted the concomitant use of long-acting β2 agonists (LABAs) and theophylline, demonstrated fewer fatal AEs (FAEs) with tiotropium HandiHaler® versus placebo and no CV safety issues.Citation5,Citation17 In contrast, a post hoc pooled analysis of 6,096 patients in three 1-year trials and one 6-month trial using patient-level data and including vital status data of early discontinued patients showed numerically more deaths with tiotropium Respimat® 5 μg versus placebo, concentrated in patients with known cardiac rhythm disorders at study baseline.Citation11,Citation18
Subsequent meta-analyses based on aggregated data and reviews of the same Respimat® trial data have suggested there might be a significantly increased risk of death with tiotropium Respimat® versus placeboCitation19–Citation22; however, other meta-analyses, based on individual participant data, did not confirm this relationship between tiotropium HandiHaler®23–28 or Respimat®24 and CV or FAEs versus placebo in patients with COPD. Findings from the initial pooled safety analysis of tiotropium Respimat®11,18 drove the initiation of the large TIOtropium Safety and Performance In Respimat® (TIOSPIR™) trial (n=17,135), in which patients were treated over an average period of 2.3 years. The primary endpoint of TIOSPIR™ was noninferiority in all-cause mortality of Respimat® versus HandiHaler®. The TIOSPIR™ trial demonstrated similar safety (including fatal and CV events) and efficacy profiles for tiotropium Respimat® 2.5 and 5 μg, and HandiHaler® 18 μg, including patients with cardiac arrhythmias at baseline.Citation29
The purpose of this report is to describe the findings of all placebo-controlled tiotropium HandiHaler® and tiotropium Respimat® trials available to date, as well as to assist healthcare professionals with their decisions regarding prescribing tiotropium delivered by either HandiHaler® or Respimat® SMI. The safety analysis was determined for HandiHaler® and Respimat® trials together as well as separately.
Methods
Study population
This pooled safety analysis followed a similar methodology to those described by Kesten et alCitation26 and Celli et alCitation24 and describes the risk of AEs by calculating a rate ratio (RR). Data from 35 Phase III and IV tiotropium clinical trials completed as of July 2012 (listed in ) were included. Of these, 28 trials used tiotropium bromide dry powder (delivered via HandiHaler® 18 μg once daily), and 7 trials used tiotropium bromide solution (delivered via Respimat® 5 μg once daily). All placebo-controlled, double-blind, and parallel-group COPD trials of ≥4 weeks’ duration were included in the analysis.
Table 1 Clinical trials included in the pooled analysis
The trials used similar inclusion and exclusion criteria. Patients who had a diagnosis of COPD with FEV1≤70% of forced vital capacity, who were aged ≥40 years and had ≥10 pack-years of smoking history were eligible for inclusion. Exclusion criteria included a diagnosis of asthma, symptomatic prostatic hypertrophy or bladder neck obstruction, narrow-angle glaucoma, and known hypersensitivity to trial medication or components. Patients with significant disease other than COPD that could significantly confound the trial results or preclude trial completion were also excluded. Other exclusion criteria in earlier trial protocols were heart failure resulting in hospitalization in the previous 3 years, cardiac arrhythmia requiring drug treatment, or myocardial infarction (MI) within the past year. Other than these specific criteria, heart failure and ischemic heart disease were not excluded. More recent trials used less stringent exclusion criteria, such as life-threatening cardiac arrhythmia or arrhythmia requiring a change in medication within the last year, heart failure resulting in hospitalization in the past year, and/or MI within the preceding 6 months. In study 205.235 (UPLIFT®), moderate to severe renally impaired patients were excluded, while in all other studies, only severe renally impaired patients were excluded. Written informed consent was obtained from all patients, and all protocols were approved by an ethics committee.
All trials permitted the concomitant use of theophyllines, inhaled corticosteroids (ICS), modest daily doses of oral corticosteroids (provided the dosing was stable), and short-acting β2 agonists. Nine out of 35 trials, including the long-term safety studies 205.235 (UPLIFT®), 205.266, and 205.372, also permitted the use of LABAs as prescribed.
Adverse event reporting
AEs occurring during the period at risk (defined as the period during which the patient received the study drug and up to 30 days thereafter) were reported by the investigator. Definitions of AEs and serious AEs (SAEs) followed the International Conference on Harmonisation guidelines.Citation30 The cause of death was adjudicated by a Clinical Endpoints Committee in studies 205.235 and 205.372 only. Therefore, the investigator-reported preferred terms (PTs) were used for the pooled analysis rather than the adjudicated terms.
Categorization of AEs
All AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 15.0. The dictionary provides individual PTs, and assigns the PTs to so-called system organ classes (SOCs). In some cases, the PTs are assigned to more than one SOC, in which case the “primary” SOC that was most relevant to the AE has been selected. To capture clinical endpoints of interest more comprehensively, and to improve the precision of rate estimates, PTs from different SOCs were combined and referred to as pharmacovigilance (PV) endpoints. Where feasible and appropriate, in order to standardize and facilitate comparability, standardized MedDRA queries (SMQs) have been used, rather than PV endpoints. A similar approach has been used and is described in previous publications.Citation24,Citation26
A composite endpoint of major adverse CV events (MACE) was included in the analysis. The composite endpoint represented FAEs in the SOC cardiac disorders and SOC vascular disorders combined with MI (fatal and nonfatal), stroke (fatal and nonfatal), and the sudden death, sudden cardiac death, and cardiac death PTs. MACE with fatal outcomes are referred to as fatal MACE. The clinical endpoint MI was defined by the sub-SMQ MI (broad), while for the clinical endpoint of “stroke,” a PV endpoint was used in the analysis.
An individual patient may be represented in several PTs but was represented only once when the data were displayed according to a pooled term, ie, SOC, PV endpoint/SMQ, or MACE.
Statistical analysis
In order to adjust for treatment durations in the different clinical trials, incidence rates (IRs) were computed as follows: the number of patients experiencing an event/patient-years at risk. Time at risk was the time from start of treatment to onset of a predetermined event. For patients who did not experience a specific event, the shortest of either the time to death or the time to end of treatment +30 days was used (on-treatment analysis).
To measure the strength of the effect, incidence RRs of tiotropium versus placebo were calculated on the basis of a Cochran–Mantel–Haenszel test stratified by trial. To indicate the stability or precision of the effect estimate, the width of the 95% confidence intervals (CI) was utilized. An RR>1 indicates an increased risk with tiotropium, and an RR<1 indicates a decreased risk with tiotropium.
Results
Study population
Of the 28 HandiHaler® and 7 Respimat® trials included in this analysis (), 11,626 patients were treated with placebo (8,343 with HandiHaler® and 3,283 with Respimat®) and 12,929 with tiotropium (9,647 with HandiHaler® and 3,282 with Respimat®), totaling 14,909 (12,469 with HandiHaler®; 2,440 with Respimat®) patient-years of tiotropium exposure.
Baseline demographics were similar between treatment groups (). The cohort was predominantly male (75.4%) with a mean age of 65 years. The prebronchodilator mean FEV1 was 1.16 L (41% predicted). Baseline concomitant medication use was also similar between treatment groups, with 84.0% of patients receiving respiratory medication of any type, and 48.4% receiving CV medication of any type; 40.7% of patients were receiving LABAs and 54.3% ICS. More patients enrolled into the Respimat® trials were receiving long-acting inhaled anticholinergics at baseline (9.7%) than those enrolled into the HandiHaler® trials (1.7%), probably because these were not available or not commonly used at the time when most HandiHaler® trials were conducted.
Table 2 Patient baseline characteristics
Overall AEs
Overall, 62.6% and 65.5% of patients treated with tiotropium and placebo, respectively, had at least one AE during the trial, and the risk of an AE was significantly reduced with tiotropium (RR [95% CI]: 0.90 [0.87, 0.93]). Tiotropium also significantly reduced the risk of SAEs: 21.7% of tiotropium- and 22.8% of placebo-treated patients had events (RR [95% CI]: 0.94 [0.89, 0.99]). There was a numerically lower risk of death in the tiotropium (4.0%) than in the placebo (4.5%) group (RR [95% CI]: 0.90 [0.79, 1.01]). No increased risk of AEs, SAEs, and FAEs for the tiotropium group was observed in patients with cardiac disorders present at baseline, with all RR<1 (). Similar results were obtained when the analysis was performed by inhaler type (HandiHaler® or Respimat®) (). No significantly increased risk of AEs, SAEs, and FAEs for the tiotropium HandiHaler® and Respimat® groups of all patients or patients with cardiac disorders, cardiac arrhythmia, or renal disorders present at baseline was observed, with the exception of the previously described increase in FAEs in patients with cardiac arrhythmia at baseline in the tiotropium Respimat® group (RR [95% CI]: 3.25 [1.23, 8.60]).Citation11,Citation18 (For some of the subgroups, there was at least one trial with patients in only one treatment group, and thus no statistics could be derived [no RR shown for the subgroups]).
Table 3 IRs (per 100 patient-years) and RRs for all AEs, by subgroup
Cardiovascular and respiratory AEs
Pooled CV and respiratory terms based on clinical categories of public health interest, or on possible pathophysiologic associations suggested by either previous published reports or potential biologic mechanisms, are reported in the present analysis, with AEs and SAEs shown in and , respectively.
Table 4 IRs (per 100 patient-years) and RRs for cardiac, vascular, and respiratory AEs
Table 5 IRs (per 100 patient-years) and RRs for cardiac, vascular, and respiratory SAEs
In total, 8.0% of patients had at least one cardiac AE during the study, and 6.2% of patients had at least one vascular AE (). Tiotropium was not associated with an increased risk of cardiac AEs (RR [95% CI]: 0.93 [0.85, 1.02]) or vascular AEs (RR [95% CI]: 0.96 [0.87, 1.07]) (). There was no indication of an increased risk of MI or stroke (RR [95% CI]: 0.85 [0.67, 1.09] or RR [95% CI]: 1.02 [0.79, 1.32]). There was no increased risk associated with tiotropium for ischemic heart disease and hypertension (RR [95% CI]: 1.01 [0.85, 1.19] and RR [95% CI]: 0.93 [0.82, 1.06]). Respiratory, thoracic, and mediastinal disorders were common (41.9% of patients) and were associated with a significantly decreased risk with tiotropium (RR [95% CI]: 0.81 [0.78, 0.85]), including for COPD exacerbations and respiratory failure (RR [95% CI]: 0.78 [0.75, 0.81] and RR [95% CI]: 0.81 [0.67, 0.97]). Similar results were obtained when the HandiHaler® and Respimat® groups were analyzed separately, with no increased risk of cardiac, vascular, and respiratory, thoracic, and mediastinal disorders or stroke in the tiotropium groups except for an increased risk of ischemic heart disease in the tiotropium Respimat® group (RR [95% CI]: 1.61 [1.04, 2.49]) ().
Overall, 4.4% of patients had at least one cardiac SAE during the study, and 1.3% of patients had at least one vascular SAE (). Tiotropium was associated with a decreased risk for cardiac SAEs (RR [95% CI]: 0.86 [0.76, 0.97]) and a similar risk for vascular SAEs (RR [95% CI]: 1.05 [0.84, 1.32]) () compared with placebo. Tiotropium was not associated with an increased risk for the SAEs of ischemic heart disease, MI, or stroke (RR [95% CI]: 0.85 [0.67, 1.08], RR [95% CI]: 0.87 [0.68, 1.13], or RR [95% CI]: 1.07 [0.80, 1.42], respectively). Respiratory, thoracic, and mediastinal disorder SAEs were common (occurring in 13% of patients), and were associated with a significantly decreased risk with tiotropium (RR [95% CI]: 0.84 [0.78, 0.91]), including for COPD exacerbations and respiratory failure (RR [95% CI]: 0.83 [0.76, 0.90] and RR [95% CI]: 0.77 [0.63, 0.94]). Numerical differences for tiotropium Respimat® and HandiHaler® versus placebo could be observed for single terms; however, overall similar results were obtained for SAEs occurring when the HandiHaler® and Respimat® groups were analyzed separately ().
Major adverse CV events
MACE and fatal MACE are shown in . There was no evidence of increased risk for MACE (RR [95% CI]: 0.87 [0.75, 1.01]) or fatal MACE (including death unknown, RR [95% CI]: 0.90 [0.74, 1.10]) for the tiotropium group. Similarly, no increased risk was observed with the tiotropium HandiHaler® and Respimat® groups separately ().
Table 6 IRs (per 100 patient-years) and RRs for MACE, by inhaler type
Anticholinergic events
Potential anticholinergic AEs and SAEs are depicted in and , respectively. The PV endpoints shown are a summary of AEs for presumed, potential, or hypothetical events that may be a consequence of anticholinergic pharmacology.
Table 7 IRs (per 100 patient-years) and RRs for potential anticholinergic AEs
Table 8 IRs (per 100 patient-years) and RRs for potential anticholinergic SAEs
Dry mouth (RR [95% CI]: 2.35 [1.99, 2.77]), constipation (RR [95% CI]: 1.28 [1.06, 1.54]), and urinary retention (RR [95% CI]: 1.56 [1.03, 2.36]) occurred more frequently with tiotropium versus placebo, with dry mouth being the most common AE, occurring in 4% of patients in the tiotropium group (). Gastrointestinal obstruction, dyspepsia, dysuria, and gastroesophageal reflux also increased with tiotropium use. The incidence of glaucoma or the worsening of an existing glaucoma at baseline was not increased with tiotropium, as shown by an RR close to 1 (RR [95% CI]: 0.97 [0.62, 1.52]). There was no increased risk for metabolism and nutrition, nervous system, and psychiatric AEs (). Potential anticholinergic SAEs were not associated with an increased risk with tiotropium (). Similar results were obtained for anticholinergic AEs and SAEs occurring when the HandiHaler® and Respimat® groups were analyzed separately (data not shown).
Fatal events
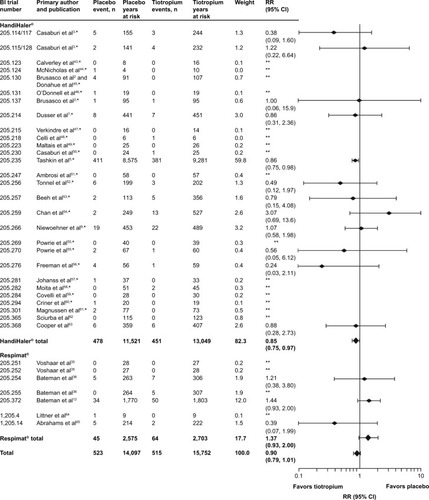
As noted earlier, there was a numerically lower risk of death in the tiotropium group (RR [95% CI]: 0.90 [0.79, 1.01]). depicts all-cause FAEs by trial. Most individual trials were associated with a wide CI, a few of which exhibited higher IRs for the tiotropium group.
Figure 1 Number of events, time at risk, and RRs for all-cause FAEs.
Abbreviations: BI, Boehringer Ingelheim; CI, confidence interval; FAE, fatal adverse event; RR, rate ratio.
depicts the most common causes of death according to SOC (with a frequency of ≥3% of total FAEs within any of the treatment arms in either study pool, ie, HandiHaler®, Respimat®, or both combined) and PT (with a frequency of ≥10 FAEs within the pooled analysis). Respiratory, thoracic, and mediastinal disorders were the most common cause of death (occurring in 1.2% of patients), and were associated with a reduced overall risk for tiotropium (RR [95% CI]: 0.76 [0.61, 0.96]). None of the SOCs were associated with a significantly increased risk for tiotropium. When the HandiHaler® and Respimat® groups were analyzed separately, neither tiotropium HandiHaler® nor tiotropium Respimat® was associated with significantly increased FAEs according to SOC, while the risk associated with tiotropium HandiHaler® was additionally decreased for respiratory, thoracic, and mediastinal disorders as well as for cardiac disorders ().
Table 9 IRs (per 100 patient-years) and RRs for FAEs according to SOC and PTTable Footnotea
Discussion
Long-term safety evaluation of treatment is essential for prescribing physicians. The present safety analysis reflects the large safety database of placebo-controlled studies with tiotropium, a long-established (over 10 years) treatment for COPD. This analysis of 24,555 patients, which adds to the last comprehensive safety review of 2009 (17,014 patients),Citation26 includes data with Respimat® – in all, 28 tiotropium HandiHaler® and 7 tiotropium Respimat® trials (compared with 26 HandiHaler® trials from the previous safety review). The recent TIOSPIR™ trial did not show any relevant differences between tiotropium HandiHaler® and tiotropium Respimat®, in particular relating to safety. This led to the development of the present pooled safety analysis, and provides the justification for the pooling of both inhaler types. Additionally, the pooled studies had similar entry criteria, assessment procedures, and data collection methods; the patient baseline characteristics were also comparable between the HandiHaler® and Respimat® trials.
This pooled safety analysis shows that IRs for overall AEs and SAEs were significantly lower in the tiotropium group compared with those in the placebo group, while the incidence of FAEs was numerically lower in the tiotropium group. In the tiotropium HandiHaler® group, IRs were significantly lower for all AEs, while for the tiotropium Respimat® group, AEs were significantly lower and SAEs and FAEs were comparable to the placebo group. Overall, these results demonstrate that both tiotropium Respimat® and tiotropium HandiHaler® do not increase the risk of an AE. Furthermore, there was no evidence to show an increased risk of cardiac or vascular events, including MACE, with either tiotropium HandiHaler® or tiotropium Respimat®. A higher incidence of ischemic heart disease for tiotropium versus placebo was seen in the Respimat® group but not in the HandiHaler® group; however, the IRs were lower in the placebo Respimat® group than in the placebo HandiHaler® group (IR: 1.25 versus 1.89), and no significant difference in the risk of MI was seen between the Respimat® and HandiHaler® groups in the TIOSPIR™ trial.
FAEs by SOC and PT did not reveal an imbalance between the placebo and tiotropium groups, either pooled or by inhaler type. As expected, known anticholinergic AEs (such as dry mouth, constipation, intestinal obstruction, and urinary retention) were more frequent in the tiotropium versus placebo groups, although SAEs were not. The large patient database size and large tiotropium exposure in terms of patient years provide a robust estimate of the IRs for these effects and allow a sound assessment of risks and benefits with tiotropium HandiHaler® and Respimat® in patients with COPD.
We have carried out further analysis on patients with renal impairment. The results were presented at the European Respiratory Society Annual Congress 2014.Citation31 The safety of tiotropium was analyzed in trials where creatinine clearance was reported at baseline. Incidence RRs of AEs, SAEs, and FAEs with tiotropium HandiHaler® or Respimat® versus placebo showed no association with mild to moderately impaired renal function (10,805 patients were evaluable: n=4,282 in HandiHaler® trials; n=6,523 in Respimat® trials). Results for severe renal impairment were, however, limited owing to low patient numbers (n=52).
The results reported here support the 2009 safety analysis,Citation26 which showed a reduced risk of overall AEs, SAEs, and FAEs with tiotropium HandiHaler®. The safety results within the Kesten et alCitation26 analysis were also consistent with those seen in the UPLIFT® trial (with the analyses for HandiHaler® trials being driven predominantly by the data from UPLIFT®, based on large patient numbers and 4-year trial length), and indicated a reduction in cardiac AEs and MACE associated with tiotropium, and no increased risk of vascular AEs. Our analysis presents a numerically reduced risk of cardiac AEs with tiotropium in both inhalers. The risk of respiratory disorders was significantly reduced in both the Kesten et alCitation26 and our analyses, while the recognized anticholinergic effects were higher with tiotropium use. A limitation of comparing the results of the present analysis with those of the Kesten et alCitation26 pooled analysis is that the latter included all but two of the HandiHaler® trials included in our pooled analysis, so that its results are driven mainly by the findings from the HandiHaler® trials (28 HandiHaler® trials versus 7 Respimat® trials).
The results from this safety review support the recent findings of the largest randomized, double-blind, parallel-group trial performed to date in COPD, involving 17,135 patients – the TIOSPIR™ trial.Citation29 TIOSPIR™ showed that tiotropium Respimat® (2.5 or 5 μg once daily) had a similar safety profile and exacerbation efficacy as tiotropium HandiHaler® (18 μg once daily) (risk of death HR [95% CI]: 0.96 [0.84, 1.09] for Respimat® 5 μg and 1.00 [0.87, 1.14] for Respimat® 2.5 μg; risk of first exacerbation HR [95% CI]: 0.98 [0.93, 1.03] for Respimat® 5 μg). Our analysis, similar to earlier analyses, found an increased risk of FAEs in patients with cardiac arrhythmia at baseline for the tiotropium Respimat® group. This finding may be due to an unusually low IR in the placebo Respimat® group (1.83), compared with an IR of 6.08 in the active treatment group. The corresponding IRs in the HandiHaler® database were higher (placebo: 6.80, tiotropium: 5.24). Direct comparison of tiotropium HandiHaler® and Respimat® in the TIOSPIR™ trial, which was powered to assess the all-cause mortality noninferiority, did not indicate a mortality imbalance for patients with cardiac arrhythmias at baseline (RR [95% CI]: 0.81 [0.58, 1.12] for tiotropium Respimat® 5 μg versus HandiHaler®).Citation29 Furthermore, the accompanying editorial by JenkinsCitation32 concluded that both HandiHaler® and Respimat® have equal safety profiles.
The findings of our analysis contrast with retrospective analyses regarding CV events and inhaled anticholinergics, which suggested that CV deaths were increased with inhaled anticholinergic, in particular with ipratropium.Citation33,Citation34 Limitations to these meta-analyses have been described previously,Citation24 and include improper design due to lack of data, errors in article identification and data extraction, as well as improper accounting for differential trial discontinuation. A mortality imbalance was seen with tiotropium Respimat® in a pooled analysis of three 1-year trials and one 6-month trial,Citation11,Citation18 and with subsequent meta-analyses.Citation19–Citation22 These meta-analyses were based on the same trials as the present review, but were carried out differently. In the Singh et alCitation22 and Karner et alCitation20 meta-analyses, which included five randomized, controlled trials (NCT0239473,Citation35 NCT0240435,Citation35 NCT0168844,Citation36 NCT0168831,Citation36 and NCT0387088), tiotropium Respimat® was associated with an increased risk of FAEs. These analyses were limited due to imprecise estimates owing to fairly low event rates and were performed on published aggregated on-treatment data, including the higher 10 μg dose, rather than using individual patient data, possibly leading to bias.Citation21 The Dong et alCitation19 meta-analysis, which also associated tiotropium Respimat® with a higher risk of FAEs, in particular for CV death, in patients with severe COPD and in those at a higher daily dose (10 μg), only used three Respimat® trials (NCT0168844,Citation36 NCT0168831,Citation36 and NCT0387088Citation12). All of the trials included in these meta-analyses were also included in the current analysis, which totaled seven Respimat® trials (all placebo-controlled trials available to date). The high number of patients and patient-years of exposure to tiotropium included in our review, and especially the use of individual patient data, ensures the robustness of the analysis.
Although most prospective, randomized, double-blind, clinical trials with tiotropium HandiHaler® and Respimat® in COPD have been conducted by Boehringer Ingelheim and are included in this analysis, a few other studies have been carried out. The Investigating New Standards for Prophylaxis In Reduction of Exacerbations (INSPIRE) study observed an increase in mortality in the tiotropium (HandiHaler®) group compared with the salmeterol and fluticasone groups. However, the interpretation beyond the primary exacerbation endpoint should be done with caution as the withdrawal of the run-in medication, salmeterol and oral prednisolone (30 mg), at the time of randomization may also have affected the outcome and results of this trial.Citation37,Citation38 Additional studies of tiotropium HandiHaler® were performed in an open-label fashion, for which patient-level data were not available.Citation39,Citation40
Pooling clinical data into meta-analyses includes such limitations as differences in trial populations, trial design, and physician diagnostic reporting. However, the present safety review reduced these limitations by analyzing the data on a patient level and controlling for differences in exposure between treatment groups, therefore reducing bias and confounding. In addition, the analysis of patient-level data also allows grouping of AEs, such as SMQs, PV endpoints, and subgroup analyses defined by potential risk factors. Potential problems in combining studies have been minimized because the trials were conducted using consistent inclusion and exclusion criteria, the same diagnostic criteria, and nearly identical approaches to data collection. A potential limitation may arise from the accuracy of causes of death reported by investigators, as only a few trials had an adjudication committee (studies 205.235 and 205.372). However, for consistency with data capture for AEs, SAEs, and other trials, the investigator-reported causes were used for the pooled analysis rather than the adjudicated terms from the clinical endpoints committee.
In our analysis, some HandiHaler® trials (such as UPLIFT® and 205.266) as well as the Respimat® trials had relatively broad inclusion criteria (including patients with stable cardiac disorders), and allowed concomitant respiratory medication including ICS, LABAs, and theophylline. Therefore, the patient population analyzed here generally reflects real-world heterogeneous populations and phenotypes of patients with COPD, as far as is possible in randomized, controlled trials. The absolute rates of FAEs observed for the groups reported in this analysis (IR/100 patient-years: 3.71 and 3.27 for the placebo and tiotropium groups, respectively) are comparable with those of mortality in general in COPD for which observed mortality IRs are 1.7–2.2 for Global Initiative for Chronic Obstructive Lung Disease (GOLD) Stage 2 patients and 3.07–4.29 for GOLD Stage 3 and 4 patients.Citation41,Citation42 Furthermore, the increased size of the safety database, pooling 35 studies to include 12,929 tiotropium-treated patients, providing 14,909 patient-years’ exposure to tiotropium, provides a robust dataset.
In summary, the present analysis describes the pooled safety data for tiotropium HandiHaler® (28 trials) and Respimat® (7 trials), as well as by inhaler type, in 24,555 patients and 14,909 patient-years of exposure to tiotropium. The results indicate that tiotropium, given via either HandiHaler® or Respimat®, does not increase the overall risks of AEs, SAEs, FAEs, or CV events. Given the evidence, provided by the TIOSPIR™ trial, that both have an equal safety profile, and given their established efficacy in terms of bronchodilation, reduction in exacerbations, and improvement in HRQoL, physicians may consider tiotropium in either formulation depending on availability and their preference or that of the patient.
Acknowledgments
The authors would like to thank Dr Inge Leimer and Dr Katrin Kupas for their assistance with the statistical analysis. Writing assistance was provided by Sarah J Petit and Natalie Dennis of PAREXEL, funded jointly by Boehringer Ingelheim and Pfizer. The abstract of this paper was presented at the American Thoracic Society International Conference 2014 as a poster presentation with interim findings. The poster’s abstract was published within “Conference Abstracts” of ATS Journals: D44. COPD: Treatment from Bronchodilators to Other Pharmacological Interventions, May 1, 2014, A5973-A5973; http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A5973. The actual paper, however, has never been published.
Supplementary material
Table S1 PV endpoint definition
Disclosure
Dr David MG Halpin has received personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, GSK, Intermune, Novartis, and Pfizer, and nonfinancial support from Boehringer Ingelheim and Novartis. Dr Ronald Dahl has received consulting fees, lecture fees, and grant support from Boehringer Ingelheim and Novartis. Dr Chistoph Hallmann and Dr Achim Mueller are employees of Boehringer Ingelheim. Dr Donald Tashkin has received grants or personal fees for consulting, speaking, or acting on the advisory board from Astra Zeneca, Boehringer Ingelheim, Forest, GlaxoSmithKline, Novartis, Pfizer, Sunovion, and Theravance. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary diseaseGOLD updated 2014 Available from: http://www.goldcopd.com/uploads/users/files/GOLD_Report_2014_Oct30.pdfAccessed December 15, 2014
- BrusascoVHodderRMiravitllesMKorduckiLTowseLKestenSHealth outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax20035839940412728159
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J20021921722411866001
- TashkinDPCooperCBThe role of long-acting bronchodilators in the management of stable COPDChest200412524925914718448
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med20083591543155418836213
- VinckenWvan NoordJAGreefhorstAPDutch/Belgian Tiotropium Study GroupImproved health outcomes in patients with COPD during 1 yr’s treatment with tiotropiumEur Respir J20021920921611871363
- DusserDBravoMLIaconoPThe effect of tiotropium on exacerbations and airflow in patients with COPDEur Respir J20062754755516507855
- HalpinDMenjogeSVielKPatient-level pooled analysis of the effect of tiotropium on COPD exacerbations and related hospitalisationsPrim Care Respir J20091810611319407916
- NiewoehnerDERiceKCoteCPrevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trialAnn Intern Med200514331732616144890
- Boehringer IngelheimSpiriva 18 microgram inhalation powder, hard capsule. Summary of Product Characteristics (SPC), Electonic Medicines Compendium (EMC) Available from: http://www.medicines.org.uk/emc/medicine/10039/SPC/Spiriva+18+microgram+inhalation+powder%2c+hard+capsule/Accessed July 19, 2013
- Boehringer IngelheimSpiriva Respimat 2.5 microgram solution for inhalation. Summary of Product Characteristics (SPC), Electonic Medicines Compendium (EMC) Available from: http://www.medicines.org.uk/emc/medicine/20134/SPCAccessed July 19, 2013
- BatemanEDTashkinDSiafakasNA one-year trial of tiotropium Respimat plus usual therapy in COPD patientsRespir Med20101041460147220620037
- IchinoseMFujimotoTFukuchiYTiotropium 5microg via Respimat and 18microg via HandiHaler; efficacy and safety in Japanese COPD patientsRespir Med201010422823619969446
- van NoordJACornelissenPJAumannJLPlatzJMuellerAFogartyCThe efficacy of tiotropium administered via Respimat Soft Mist inhaler or HandiHaler in COPD patientsRespir Med2009103222919022642
- HohlfeldJMSharmaAvan NoordJAPharmacokinetics and pharmacodynamics of tiotropium solution and tiotropium powder in chronic obstructive pulmonary diseaseJ Clin Pharmacol201454440541424165906
- Boehringer IngelheimBoehringer Ingelheim submits application in Europe to extend the indication for the use of tiotropium Respimat® to the treatment of asthma in adults aged 18 years and over [press release archive: asthma]Ingelheim, GermanyBoehringer Ingelheim20131113 Available from: http://www.boehringer-ingelheim.com/news/news_releases/press_releases/2013/13_november_2013_tiotropium.htmlAccessed December 15, 2014
- CelliBDecramerMKestenSLiuDMehraSTashkinDPUPLIFT Study InvestigatorsMortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200918094895519729663
- Boehringer IngelheimTiotropium (Spiriva) Respimat: evaluation of fatal eventsIngelheim, GermanyBoehringer Ingelheim Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/Pooled%20analysis/PA_205.372_251_252_254_255_U10-3255-01.pdfAccessed January 30, 2014
- DongYHLinHHShauWYWuYCChangCHLaiMSComparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trialsThorax201368485623042705
- KarnerCChongJPoolePTiotropium versus placebo for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20127CD00928522786525
- LokeYKSinghSRisks associated with tiotropium in chronic obstructive pulmonary disease: overview of the evidence to dateTher Adv Drug Safe20123123131
- SinghSLokeYKEnrightPLFurbergCDMortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trialsBMJ2011342d321521672999
- BarrRGBourbeauJCamargoCARamFSTiotropium for stable chronic obstructive pulmonary disease: a meta-analysisThorax20066185486216844726
- CelliBDecramerMLeimerIVogelUKestenSTashkinDPCardiovascular safety of tiotropium in patients with COPDChest2010137203019592475
- KestenSJaraMWentworthCLanesSPooled clinical trial analysis of tiotropium safetyChest20061301695170317166984
- KestenSCelliBDecramerMLeimerITashkinDTiotropium HandiHaler in the treatment of COPD: a safety reviewInt J Chron Obstruct Pulmon Dis2009439740920037679
- RodrigoGJCastro-RodriguezJANanniniLJPlaza MoralVSchiaviEATiotropium and risk for fatal and nonfatal cardiovascular events in patients with chronic obstructive pulmonary disease: systematic review with meta-analysisRespir Med20091031421142919556116
- SalpeterSRDo inhaled anticholinergics increase or decrease the risk of major cardiovascular events?: a synthesis of the available evidenceDrugs2009692025203319791824
- WiseRAAnzuetoACottonDTIOSPIR InvestigatorsTiotropium Respimat inhaler and the risk of death in COPDN Engl J Med20133691491150123992515
- ICH harmonised tripartite guidelines. Clinical safety data management: definitions and standards for expidited reporting E2AInternational Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human UseOctober 271994
- TashkinDPMetzdorfNHallmanCKoenen-BergmannMKupasKDahlRSafety of tiotropium in renally impaired patientsEur Respir J201444Suppl 58 Abstract 923
- JenkinsCRMore than just reassurance on tiotropium safetyN Engl J Med2013369161555155624131181
- LeeTAPickardASAuDHBartleBWeissKBRisk for death associated with medications for recently diagnosed chronic obstructive pulmonary diseaseAnn Intern Med200814938039018794557
- SinghSLokeYKFurbergCDInhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysisJAMA20083001439145018812535
- VoshaarTLapidusRMaleki-YazdiRA randomized study of tiotropium Respimat Soft Mist inhaler vs ipratropium pMDI in COPDRespir Med2008102324117996436
- BatemanESinghDSmithDEfficacy and safety of tiotropium Respimat SMI in COPD in two 1-year randomized studiesInt J Chron Obstruct Pulmon Dis2010519720820714373
- WedzichaJACalverleyPMSeemungalTAHaganGAnsariZStockleyRAINSPIRE InvestigatorsThe prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromideAm J Respir Crit Care Med2008177192617916806
- KeatingGMTiotropium bromide inhalation powder: a review of its use in the management of chronic obstructive pulmonary diseaseDrugs20127227330022217233
- ChurchABeeraheeMBrooksJMehtaRShahPDose response of umeclidinium administered once or twice daily in patients with COPD: a randomised cross-over studyBMC Pulm Med201414224393134
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group studyLancet Respir Med2013119920924429126
- ShavelleRMPaculdoDRKushSJManninoDMStraussDJLife expectancy and years of life lost in chronic obstructive pulmonary disease: findings from the NHANES III follow-up studyInt J Chron Obstruct Pulmon Dis2009413714819436692
- ManninoDMDohertyDEBuistSAGlobal initiative on obstructive lung disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) studyRespir Med200610011512215893923
- CalverleyPMLeeATowseLvan NoordJWitekTJKelsenSEffect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary diseaseThorax20035885586014514937
- McNicholasWTCalverleyPMLeeAEdwardsJCLong-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPDEur Respir J20042382583115218993
- DonohueJFvan NoordJABatemanEDA 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterolChest2002122475512114338
- O’DonnellDEFlügeTGerkenFEffects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J20042383284015218994
- VerkindreCBartFAguilaniuBThe effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary diseaseRespiration20067342042716484769
- CelliBZuWallackRWangSKestenSImprovement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumesChest20031241743174814605043
- MaltaisFHamiltonAMarciniukDImprovements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPDChest20051281168117816162703
- CasaburiRKukafkaDCooperCBWitekTJJrKestenSImprovement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPDChest200512780981715764761
- AmbrosinoNFoglioKBalzanoGPaggiaroPLLessiPKestenSTiotropium Multicentric Italian Study GroupTiotropium and exercise training in COPD patients: effects on dyspnea and exercise toleranceInt J Chron Obstruct Pulmon Dis2008377178019281092
- TonnelABPerezTGrosboisJMVerkindreCBravoMLBrunMTIPHON study groupEffect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPDInt J Chron Obstruct Pulmon Dis2008330131018686739
- BeehKMBeierJBuhlRStark-LorenzenPGerkenFMetzdorfNATEM-StudiegruppeEfficacy of tiotropium bromide (Spiriva) in patients with chronic-obstructive pulmonary disease (COPD) of different severitiesPneumologie20066034134616761228
- ChanCKMaltaisFSigouinCHaddonJMFordGTSAFE Study GroupA randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary diseaseCan Respir J20071446547218060091
- PowrieDJWilkinsonTMDonaldsonGCEffect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPDEur Respir J20073047247817504798
- FreemanDLeeAPriceDEfficacy and safety of tiotropium in COPD patients in primary care–the SPiRiva Usual CarE (SPRUCE) studyRespir Res200784517605774
- JohanssonGLindbergARombergKNordstromLGerkenFRoquetABronchodilator efficacy of tiotropium in patients with mild to moderate COPDPrim Care Respir J20081716917518536860
- MoitaJBarbaraCCardosoJTiotropium improves FEV1 in patients with COPD irrespective of smoking statusPulm Pharmacol Ther20082114615117693107
- CovelliHBhattacharyaSCassinoCConoscentiCKestenSAbsence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary diseasePharmacotherapy2005251708171816305289
- CrinerGJSharafkhanehAPlayerREfficacy of tiotropium inhalation powder in african-american patients with chronic obstructive pulmonary diseaseCOPD20085354118259973
- MagnussenHBugnasBvanNJSchmidtPGerkenFKestenSImprovements with tiotropium in COPD patients with concomitant asthmaRespir Med2008102505617920256
- SciurbaFCSiafakasNTroostersTThe efficacy of safety of tiotropium HandiHaler®, 18 ìg, once daily plys prn salbutamol versus placebo plus prn salbutamolin COPD subjects naïve to maintenance therapyAmerican Thoracic Society International Conference2011 May 13–18Denver, CO Abstract 21005
- CooperCBCelliBRJardimJRTreadmill endurance during 2-year treatment with tiotropium in patients with COPD: a randomized trialChest201314449049723558890
- LittnerMRvanNJMoroni-ZentgrafPSigmundRJosephEKarpelJPhase IIB dose-finding study of BEA2180 via Respimat® in patients with chronic obstructive pulmonary disease (COPD)Respirology201217Suppl 244
- AbrahamsRMoroni-ZentgrafPRamsdellJSchmidtHJosephEKarpelJSafety and efficacy of the once-daily anticholinergic BEA2180 compared with tiotropium in patients with COPDRespir Med201310785486223490224
