 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Impaired peripheral oxygenation (IPO)-related variables readily achieved with cardiopulmonary exercise testing (CPET) represent cardiovascular dysfunction. These variables include peak oxygen uptake ( predicted, anaerobic threshold
predicted,
rate slope <8.6 mL/watt, oxygen pulse <80% predicted, and ventilatory equivalents for O2 and CO2 at nadir of >31 and >34, respectively. Some of these six variables may be normal while the others are abnormal in patients with chronic obstructive pulmonary disease (COPD). This may result in confusion when using the interpretation algorithm for diagnostic purposes. We therefore hypothesized that patients found to have abnormal values for all six variables would have worse cardiovascular function than patients with abnormal values for none or some of these variables.
Methods
In this cross-sectional comparative study, 58 COPD patients attending a university teaching hospital underwent symptom-limited CPET with multiple lactate measurements. Patients with abnormal values in all six IPO-related variables were assigned to an IPO group while those who did not meet the requirements for the IPO group were assigned to a non-IPO group. Cardiovascular function was measured by two-dimensional echocardiography and
, and respiratory dynamics were compared between the two groups.
Results
Fourteen IPO and 43 non-IPO patients were entered into the study. Both groups were similar with regard to left ventricular ejection fraction and right ventricular morphology (P>0.05 for both). At peak exercise, both groups reached a similar heart rate level and
. The IPO patients had an unfavorable dead space to tidal volume ratio, mean inspiratory tidal flow, and shallow breathing (P<0.05–P<0.001).
Conclusion
Our IPO and non-IPO patients with COPD had similar cardiovascular performance at rest and at peak exercise, indicating that IPO variables are non-specific for cardiovascular function in these patients. COPD patients with full IPO variables have more deranged ventilatory function.
Introduction
Impaired peripheral oxygenation (IPO) of exercising muscles is caused by impaired circulation and/or mitochondrial function and a low arterial oxygen content, such that oxygen cannot flow adequately to myocytes.Citation1 The mechanisms of IPO are quite different from those of hypoxemia, mostly due to respiratory pathology. IPO can seriously limit patients’ ability to perform certain activities of daily living, and the impact of related symptoms can be quite significant.Citation2
Clinicians tend to screen for the causes of exertional dyspnea using non-invasive cardiopulmonary exercise testing (CPET).Citation1,Citation3–Citation5 IPO-related variables obtained from CPET include peak oxygen uptake
predicted, anaerobic threshold
predicted,
rate slope <8.6 mL/watt, oxygen pulse <80% predicted, and ventilatory equivalents for O2 and CO2 at nadirs of >31 and >34, respectively.Citation1
These six individual IPO variables represented cardiovascular parameters, but are reported to be non-specific in discriminating chronic obstructive pulmonary disease (COPD) with and without chronic circulatory changesCitation6–Citation8 using invasive pulmonary artery catheterization.Citation7,Citation8 However, at present, it is not clear how we can effectively interpret the algorithm since some of the six variables may be normal while others are abnormal. We hypothesized that patients with all six variables found to be abnormal would have worse cardiovascular function than those with none or some having abnormal values. Our approach in this study was oriented toward interpretation of the CPET report, and we found that this approach may be more useful when evaluating IPO-related variables with regard to their clinical implications.
Materials and methods
Study design
In this cross-sectional comparative study, the patients were divided into three groups based on six variables obtained from CPET. Patients with abnormal values for all six variables were assigned to a full IPO group, those with normal values for all six variables were assigned to a non-IPO group, and the remaining patients were assigned to an intermediate IPO group. Two-dimensional echocardiography, changes (Δ) in lactate, Δ in oxygen uptake
, a parameter of cardiovascular function,Citation9 and arterial pH values were compared across the groups. Arterial pH values should be lower in patients with cardiovascular dysfunction than in those without cardiovascular dysfunction performing at the same exercise intensity or cardiovascular stress level. A comparison of respiratory dynamics was performed across the groups. The institutional review board of Chung Shan Medical University Hospital approved the study (approval number CS11144) and all participants provided their written informed consent. This trial is registered with the number CSH-2012-C-23 at Chung Shan Medical University Hospital, Taichung, Taiwan.
Subjects
Global Initiative for Chronic Obstructive Lung Disease criteria were used to diagnose COPD.Citation10 Adult patients with COPD who underwent lung function tests were enrolled only if their forced expired volume in one second (FEV1) was <80% of the predicted value and their FEV1/forced vital capacity ratio was <70%. If they agreed, they performed symptom-limited incremental CPET with arterial blood gas analysis and lactate measurements. All patients were clinically stable and had had no significant changes in medication in the month prior to performing the tests. Patients were excluded if they had significant comorbidities, such as left ventricular failure, renal failure, cancer, significant anemia, peripheral arterial occlusive disease, uncontrolled diabetes mellitus, or hypertension, or if they were participating in any physical training program during the study period.
Protocols and measurements
Pulmonary function testing
FEV1, total lung capacity, and residual volume were measured by spirometry and plethysmography (6200 Autobox DL, Yorba Linda, CA, USA, or MasterScreen™ Body, Carefusion, Würzburg, Germany) at body temperature, ambient atmospheric pressure, and fully saturated, using the best of three technically satisfactory readings.Citation11–Citation13 The diffusing capacity for carbon monoxide (DLCO) was measured by the single-breath technique. Direct maximum voluntary ventilation (MVV) was calculated from a 12-second maneuver of rapid and deep breathing as recommended for patients with COPD.Citation14 All lung function data were obtained after inhaling 400 μg of fenoterol HCl.
Maximum cardiopulmonary exercise testing
After acclimating to a computer-controlled and electronic-brake cycle ergometer (Medical Graphics, St Paul, MN, USA) and following a 2-minute rest period, each patient began a 2-minute period of unloaded cycling followed by a ramp-pattern exercise test to the limit of tolerance. Work rate was selected at a slope of 5–20 watts per minute based on a derived protocol formula.Citation15 Twelve-lead electrocardiography,
,
, minute ventilation, pulse rate, and oxyhemoglobin saturation were continuously measured. Blood pressure was recorded at the end of each minute and at the point where the patient reported having reached peak exercise. A dyspnea score was obtained using the Borg scale by asking the patients about their dyspnea levels while they were performing the ramp-pattern exercise at the end of each minute and at peak exercise. Please refer to Chuang and LinCitation16 for the anaerobic threshold, oxyhemoglobin saturation, calibrations of the pneumotachograph and O2 and CO2 analyzers, and
prediction equations.
The
achieved by patients was the highest recorded value averaged over the last 15 seconds of loaded exercise and designated as
or
.Citation15
Maximum exercise effort achieved was a prerequisite for final analysis.Citation4,Citation5,Citation17 Each criterion at peak exercise, such as respiratory exchange ratio ≥1.09, heart rate ≥85% of predicted maximum, pH ≤7.35, bicarbonate (HCO3−) concentration ≤21 mEq/L, change in HCO3− concentration between rest and peak exercise ≥4 mEq/L, and change in lactate concentration between rest and peak exercise ≥4 mEq/L represented one point. The maximum effort level was scored from 1 to 6 points. The accumulated points, representing the effort level of exercise, were compared across the groups.
Exercise intensity or cardiovascular stress level was defined as follows:
Ventilatory limitation was defined as either <30% or <11–15 L/min breathing reserve, calculated as follows:Citation1,Citation4
Two-dimensional echocardiography
Two-dimensional echocardiography (iE33, Philips, Seattle, WA, USA) was performed within 4 weeks before or after CPET by two experienced cardiologists who were blinded to the clinical data, lung function, and CPET reports. If there were acute exacerbations of COPD in the time between the two tests, one of the tests would be postponed. Parasternal, apical, and subcostal studies were conducted, and the definition of cor pulmonale was used according to previous reports.Citation20,Citation21
Arterial blood sampling and lactate determination
Brachial artery blood samples were drawn via an arterial catheter connected to a pressure transducer within the last 15 seconds of each minute after the start of exercise to the peak of exercise.Citation22 Whole blood lactate was also analyzed (YSI Inc, Yellow Springs, OH, USA).
At the peak of exercise, the dead space to tidal volume ratio (VD/VT) was calculated using a standard formula.Citation23
The slopes of lactate as a function of
calculated using linear regression
were compared across the groups.
Statistical analysis
The data are shown as the mean ± standard deviation or median (interquartile range). Analysis of variance was initially considered for comparing the means across the groups; however, there were no patients in the non-IPO group. Therefore, only two groups were established. Thus, the unpaired-t-test or Mann–Whitney U-test was used to compare the means between the two independent groups. Fisher’s Exact test for contingency tables was used to compare the stages of COPD between the two groups. A P<0.05 was considered to be statistically significant, and P<0.1 but P>0.05 was considered to have a trend to be significant.Citation24 All statistical procedures were performed using SAS software package version 9.3 (SAS Institute Inc, Cary, NC, USA).
Results
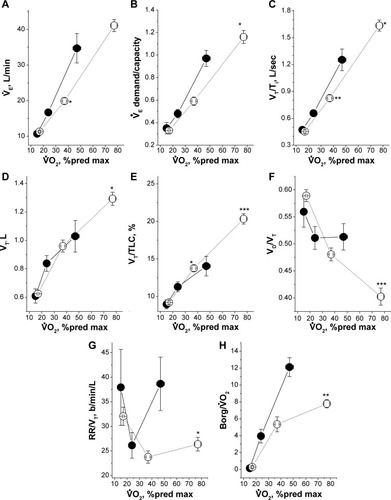
Fifty-eight consecutive male patients of mean age 64.6±6.1 years were enrolled in the study. After excluding one patient who did not reach the maximum exercise level when performing CPET, 14 patients were assigned to the IPO group, no patients were assigned to the non-IPO group, and the other 43 patients were assigned to the intermediate IPO group (). For the sake of simplicity, the latter two groups were deemed to be non-IPO groups. presents the distribution of the six variables regarding the abnormal values for the non-IPO groups. The IPO and non-IPO groups had similar stages of COPD severity. The IPO group had more hyperinflated lungs and lower DLCO (P=0.05 to P<0.01; ). Both groups performed at a similar level of maximum exercise effort.
Table 1 Variables related to impaired peripheral oxygenation and selected clinical characteristics and lung function test data in patients with COPD
Table 2 Distribution of the six variables regarding IPO criteria for the non-IPO group (n=43Table Footnote*)
No patient experienced an acute exacerbation in the time interval between echocardiography and CPET. shows that the two-dimensional echocardiographic findings, including left ventricular ejection fraction and right ventricular morphology, were similar between the two groups (all P>0.05).
Table 3 Two-dimensional echocardiography results
The heart rate percentage predicted maximum at peak exercise was similar between the two groups (IPO group: 77%±14% versus non-IPO group: 82%±10%, P=0.26). pH levels were higher at anaerobic threshold and peak exercise in the IPO group (P=0.007 and P=0.0007, respectively), with a smaller decrease in HCO3− concentration and increase in lactate concentration (3.6±0.5 mmol/L versus 5.7±0.3 mmol/L, P=0.02, and 1.4±1.8 mmol/L versus 2.9±1.8 mmol/L, P=0.003, respectively). The slopes of
were similar between the two groups (2.4 [error 0.15] versus 2.3 [error 0.1] mmol/L, not statistically significant).
The IPO group had a rapid increase in
but slower expansion of tidal volume in response to exercise (, both P<0.05). The IPO group had a significantly lower
demand/capacity ratio (P<0.05) with slower inspiratory tidal flow and lower VT/total lung capacity expansion at both anaerobic threshold and peak exercise (, P<0.01 and P<0.05, and both P≤0.001, respectively) and higher VD/VT, rapid shallow breathing index, and
in the IPO group at peak exercise (, P<0.001, P<0.05, and P<0.01, respectively).
Figure 1 Findings in 14 patients with COPD complicated by IPO (solid line and solid circle symbol) and 43 patients with COPD complicated by non-IPO (dashed line and open circle symbol) performing incremental cardiopulmonary exercise testing.
Abbreviations: COPD, chronic obstructive pulmonary disease; IPO, impaired peripheral oxygenation; max, maximum; pred, predicted.
Discussion
In this study, COPD patients with full IPO and those with non-IPO had similarly impaired forced vital capacity, FEV1, and COPD severity (). Both groups had different exercise capacity with very different cardiovascular exercise variables (), but were similar in terms of cardiovascular function measured by two-dimensional echocardiography and
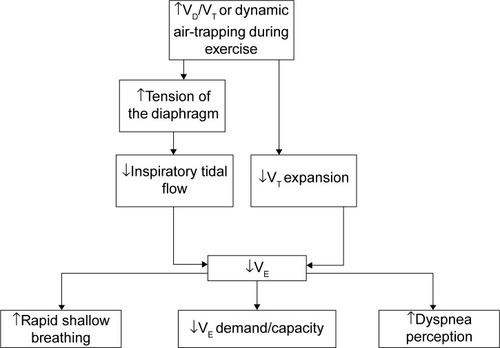
(). These findings suggest that cardiovascular variables on CPET cannot predict cardiac function morphologically. We therefore further differentiated the mechanism of full IPO and non-IPO during exercise. We found that patients with IPO had much poorer airflow and lung expansion and a greater perception of breathlessness ().
Left ventricular performance has been reported not to be impaired in COPD patients.Citation25 A previous study reported that there were no differences in oxygen uptake, work rate, oxygen pulse, and heart rate at peak exercise between patients with and without pulmonary arterial hypertension.Citation8 The authors concluded that ventilatory limitation itself was the primary factor causing exercise intolerance in COPD patients. Circulatory impairment is not usually a limiting factor for exercise intolerance in patients with COPD unless complications of severe pulmonary hypertension are involved.Citation26 The cardiovascular response to exercise was constrained, but not impaired, by dynamic hyperinflation or intrathoracic pressure swings limiting ventilation in COPD patients.Citation27–Citation29 A study using multiple regression analysis showed that ventilatory inefficiency was not caused by oxygen uptake, work rate, oxygen pulse, or circulatory power at peak exercise, but was related to expiratory flow limitations and dynamic hyperinflation,Citation30 which is consistent with other reports.Citation31,Citation32 Although all of the variables found in previous reports were comprehensive, they cannot be directly used when interpreting a CPET report. Our approach to the present study was oriented toward interpretation of the CPET.
We did not use pulmonary arterial hypertension as a study criterion because of the invasiveness of pulmonary arterial catheterization. Instead, we attempted to use the six non-invasive variables relating to circulatory function to categorize our COPD patients. We found that the circulatory function of the IPO patients was similar to that of the non-IPO patients according to a two-dimensional echocardiography study and by utilizing the slope of
, which is a marker of cardiovascular impairment.Citation30 In addition, relatively low changes in HCO3− and lactate concentrations and higher pH levels at peak exercise in the IPO group did not support cardiovascular limitations as being the primary factor of exercise limitation.
Resting FEV1, DLCO, peak inspiratory pressure, and exertional maximum
play a pivotal role in exercise intolerance in patients with COPD.Citation33,Citation34 In the present study, the IPO group showed a lower
demand/capacity, probably due to the slower inspiratory tidal flow not able to adequately increase
further in response to exercise, thereby reaching the ventilatory limit at an earlier stage. The slower inspiratory tidal flow and poorer tidal volume expansion might be due to higher tension of the diaphragm caused by increased VD/VT when approaching peak exercise (). This notion is supported by studies in lung volume reduction surgery and bronchodilator use showing decreases in VD/VT resulting in airflow improvement.Citation31,Citation32 Increased VD/VT contributes to dynamic hyperinflation,Citation35 thereby resulting in more rapid shallow breathing and a greater perception of dyspnea (). In the present study, a strong statistical power of 0.98 for VD/VT was estimated, given 14 subjects with a mean VD/VT of 0.51±0.09 in the IPO group and of 0.4±0.09 in the non-IPO group, and the probability of type I error of 0.05.
Figure 2 Flow chart showing the deductive mechanism of ventilatory dysfunction and perception of dyspnea at peak exercise in patients with chronic obstructive pulmonary disease complicated with impaired peripheral oxygenation.
Abbreviations: VD/VT, dead space to tidal volume ratio;
Study limitations
There are a number of limitations in this study that are worthy of note. First, our COPD cohort was comprised exclusively of males, and as such the results cannot be extrapolated to females. However, it should be noted that the incidence of COPD is relatively low in Taiwanese females. Second, IPO includes impairment of cardiac, hemoglobin, and/or peripheral vascular function, and as a result, IPO cannot be fully evaluated by two-dimensional echocardiography. Further study of the peripheral circulation using near-infrared spectroscopy during exercise might be helpful.Citation36 Moreover, cardiac function at rest as evaluated by two-dimensional echocardiography may not represent cardiac function during exercise. However, performing an echocardiographic examination while the subject is exercising is technically difficult. Stress echocardiography using pharmacological agents is feasible; however, cardiac performance using this modality is different from that seen during exercise. Third, the patient grouping in this study is not precise, given that some patients in the non-IPO group had abnormal values in some of the variables relevant to IPO. Since none of our 57 patients had all six variables in the normal range, 1,000 patients or more would be required to identify 20 with normal values in all six variables. Fourth, one may argue that using
alone instead of the six IPO-related variables might draw similar conclusions to the study. However, this is another issue, and
alone cannot represent IPO. Finally, this study did not evaluate intrapulmonary shunt during exercise, although this may not be an issue given that there was no difference in partial pressure of arterial oxygen at peak exercise between the two groups (IPO 68.6±13.1 mmHg versus non-IPO group 71±15.1 mmHg, not statistically significant).Citation37
Conclusion
Although the six variables used herein are related to IPO or circulatory function, they are inconsistent with the findings of two-dimensional echocardiography and
in patients with COPD. Further analysis shows that the mechanisms of exercise intolerance in COPD-IPO patients are primarily due to derangements in airflow and/or dead space ventilation. Given the strength of our findings, this study might help with the interpretation of CPET reports for COPD patients.
Acknowledgments
This study was supported in part by the Chung Shan Medical University Hospital Research Program (CSHRP 11144, CSH-2012-C-023) and the Chang Gung Medical Research Program (CMRP 443). The authors thank Fen-Chiung Lin, Chang Gung Memorial Hospital, Taoyuan, Taiwan, Republic of China, and Hsiu-Ching Yu, Chung Shan Medical University Hospital Taichung, Taiwan, Republic of China, for performing the two-dimensional echocardiography.
Disclosure
The authors report no conflicts of interest in this work.
References
- WassermanKHansenJESueDYStringerWWWhippBJPrinciples of interpretation: a flowchart approachWassermanKPrinciples of Exercise Testing and Interpretation4th edPhiladelphia, PA, USALippincott Williams & Wilkins2005
- LouvarisZKortianouEASpetsiotiSIntensity of daily physical activity is associated with central hemodynamic and leg muscle oxygen availability in COPDJ Appl Physiol (1985)2013115679480223845982
- ChuangMLChangHCLimKEVintchJRGas exchange detection of right-to-left shunt in dyspneic patients: report of three casesInt J Cardiol2006108111711916516706
- McNichollDMMegarryJMcGarveyLPRileyMSHeaneyLGThe utility of cardiopulmonary exercise testing in difficult asthmaChest201113951117112321292756
- ZavalaDManual of Exercise Testing: A Training Handbook2nd edIowa City, IA, USAUniversity of Iowa Press1987
- American Thoracic SocietyAmerican College of Chest PhysiciansATS/ACCP statement on cardiopulmonary exercise testingAm J Respir Crit Care Med2003167221127712524257
- HolverdaSBogaardHJGroepenhoffHPostmusPEBoonstraAVonk-NoordegraafACardiopulmonary exercise test characteristics in patients with chronic obstructive pulmonary disease and associated pulmonary hypertensionRespiration200876216016717960052
- PynnaertCLamotteMNaeijeRAerobic exercise capacity in COPD patients with and without pulmonary hypertensionRespir Med2010104112112619577458
- WassermanKHansenJESueDYStringerWWWhippBJPathophysiology of disorders limiting exerciseWassermanKPrinciples of Exercise Testing and Interpretation4th edPhiladelphia, PA, USALippincott Williams & Wilkins2005
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (revised 2011) Available from: www.goldcopd.orgAccessed December 18, 2014
- American Thoracic Society/European Respiratory SocietyATS/ERS statement on respiratory muscle testingAm J Respir Crit Care Med2002166451862412186831
- MillerMRCrapoRHankinsonJGeneral considerations for lung function testingEur Respir J200526115316115994402
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- WassermanKHansenJESueDYStringerWWWhippBJClinical exercise testingWassermanKPrinciples of Exercise Testing and Interpretation4th edPhiladelphia, PA, USALippicott Williams & Wilkins2005
- ChuangMLLeeCHLinIFUsing the oxygen-cost diagram in ramp-slope selection for dyspneic patientsIntern Med201049141325133220647644
- ChuangMLLinIFClinical characteristics and lung function in chronic obstructive pulmonary disease complicated with impaired peripheral oxygenationIntern Emerg Med20149663364024062273
- WassermanKHansenJESueDYStringerWWWhippBJNormal valuesWassermanKPrinciples of Exercise Testing and Interpretation4th edPhiladelphia, PA, USALippincott Williams & Wilkins2005
- O’DonnellDEVoducNFitzpatrickMWebbKAEffect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary diseaseEur Respir J2004241869415293609
- YangKLTobinMJA prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilationN Engl J Med199132421144514502023603
- BertoliLManteroACiceroSLAlpagoRRizzatoGBelliCUsefulness of two-dimensional echocardiography in the assessment of right heart in chronic obstructive lung diseaseProgress in Respiratory Research19852091100
- DanchinNCornetteAHenriquezATwo-dimensional echocardiographic assessment of the right ventricle in patients with chronic obstructive lung diseaseChest19879222292332956068
- ChuangMLLinIFVintchJRHoBSChaoSWKerJJSignificant exercise-induced hypoxaemia with equivocal desaturation in patients with chronic obstructive pulmonary diseaseIntern Med J200636529430116650194
- WassermanKHansenJESueDYStringerWWWhippBJCalculations, formulas, and examplesWassermanKPrinciples of Exercise Testing and Interpretation4th edPhiladelphia, PA, USALippincott Williams & Wilkins2005
- RosnerBHypothesis testing: one-sample inferenceFundamentals of Biostatistics7th edBoston, MA, USABrooks/Cole Cengage Learning2011
- OliverRMFlemingJSWallerDGRight ventricular function at rest and during exercise in chronic obstructive pulmonary disease. Comparison of two radionuclide techniquesChest1993103174808417941
- BoerrigterBGBogaardHJTripPVentilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertensionChest201214251166117422556320
- ComeCEDivoMJSan Jose EsteparRLung deflation and oxygen pulse in COPD: results from the NETT randomized trialRespir Med2012106110911921843930
- Montes de OcaMRassuloJCelliBRRespiratory muscle and cardiopulmonary function during exercise in very severe COPDAm J Respir Crit Care Med19961545128412898912737
- TzaniPAielloMEliaDDynamic hyperinflation is associated with a poor cardiovascular response to exercise in COPD patientsRespir Res20111215022074289
- CaviedesIRDelgadoISotoRVentilatory inefficiency as a limiting factor for exercise in patients with COPDRespir Care201157458358922005099
- BendittJOLewisSWoodDEKlimaLAlbertRKLung volume reduction surgery improves maximal O2 consumption, maximal minute ventilation, O2 pulse, and dead space-to-tidal volume ratio during leg cycle ergometryAm J Respir Crit Care Med19971562 Pt 15615669279240
- BertonDCBarbosaPBTakaraLSBronchodilators accelerate the dynamics of muscle O2 delivery and utilisation during exercise in COPDThorax201065758859320627914
- DillardTAPiantadosiSRajagopalKRDeterminants of maximum exercise capacity in patients with chronic airflow obstructionChest19899622672712752808
- LoRussoTJBelmanMJElashoffJDKoernerSKPrediction of maximal exercise capacity in obstructive and restrictive pulmonary diseaseChest19931046174817548252956
- MahutBChevalier-BidaudBPlantierLDiffusing capacity for carbon monoxide is linked to ventilatory demand in patients with chronic obstructive pulmonary diseaseCOPD201291162122292594
- MohlerER3rdLechGSuppleGEWangHChanceBImpaired exercise-induced blood volume in type 2 diabetes with or without peripheral arterial disease measured by continuous-wave near-infrared spectroscopyDiabetes Care20062981856185916873792
- ShaikhZFKellyJLShrikrishnaDPatent foramen ovale is not associated with hypoxemia in severe chronic obstructive pulmonary disease and does not impair exercise performanceAm J Respir Crit Care Med2014189554054724450410
