Abstract
Background
High-sensitivity cardiac troponin T (hs-cTnT) in serum is a useful marker of acute myocardial injury, yet information is limited in patients with chronic obstructive pulmonary disease. We aimed to explore the association between hs-cTnT levels and cardiac and pulmonary dysfunction in patients with stable chronic obstructive pulmonary disease and at-risk individuals.
Methods
We examined community-dwelling adults with/without chronic obstructive pulmonary disease, with a life-long smoking history, current symptoms of dyspnea during exertion, prolonged coughing, and/or sputum. Serum hs-cTnT concentrations were measured, and subjects underwent pulmonary function tests, high-resolution computed tomography of the chest, an echocardiogram, and a 6-minute walking test.
Results
Eighty-six stable patients were identified (mean age 65.5 years; predicted forced expiratory volume in 1 second [FEV1% predicted] 75.0%). Their overall mean hs-cTnT level was 0.008 ng/mL. Logarithmically transformed hs-cTnT levels significantly and positively correlated with age, smoking index, serum high-sensitivity C-reactive protein levels, right ventricle systolic pressure, low attenuation area percentage, and brain natriuretic peptide levels (range r=0.231–0.534, P=0.000 to P=0.042). Further, logarithmically transformed hs-cTnT values significantly and negatively correlated with forced vital capacity, FEV1% predicted, diffusion capacity, arterial oxygen tension, and 6-minute walking distance (range r= −0.482 to −0.377, P=0.000 to P=0.002). Multivariate analyses showed that hs-cTnT values varied independently according to the following three parameters: high-sensitivity C-reactive protein levels (B=0.157, β=0.450, t=3.571, P=0.001), age (B=0.008, β=0.352, t=2.789, P=0.009), and right ventricular systolic pressure (B=0.008, β=0.280, t=2.202, P=0.035).
Conclusion
Even in patients with stable chronic obstructive pulmonary disease, the serum troponin T concentration was controlled by at least three major factors, ie, systemic inflammation, advancing age, and right cardiac overload.
Introduction
Previous studies have indicated that ischemic heart disease is a major cause of death in patients with chronic obstructive pulmonary disease (COPD),Citation1,Citation2 suggesting that acute myocardial infarction should be differentiated from or identified in coexistence with an acute exacerbation of COPD. Since clinical symptoms of acute exacerbation of COPD and acute myocardial infarction are often similar, differential diagnosis may be difficult.
Cardiac injury is defined as disruption of the normal cardiac myocyte membrane integrity, resulting in loss of its intracellular contents into the extracellular space (such as blood). This loss results in detectable levels of a variety of biologically active cytosolic and structural proteins in the blood, including troponins, creatine kinase, myoglobin, heart-type fatty acid-binding proteins, and lactate dehydrogenase.Citation3 Therefore, serum troponin T (TnT), released with cardiac stress,Citation4 is considered a useful biomarker for cardiac injury. A study using pacing and blood sampling from the coronary sinus and peripheral blood showed that high-sensitivity cardiac troponin T (hs-cTnT) increases in both the coronary sinus and in peripheral blood, regardless of whether lactate levels in the coronary sinus are elevated.Citation5 These findings could suggest that hs-cTnT is released because of cardiac stress and repeated injury. Alternatively, another hypothesis purports that the elevations could represent partially reversible myocyte injury. Other proposed sources of troponin release include apoptosis, normal myocyte turnover, cellular release of proteolytic troponin breakdown products, increased cell wall permeability, and formation and release of membranous blebs.Citation6 Available data suggest that hs-cTnT increases in acute exacerbationCitation7–Citation10 and even in stable COPD.Citation11 Other data based on necropsy findings show that left ventricular hypertrophy occurs in 10%–90% of COPD cases.Citation12 This finding strongly suggests that left ventricular hypertrophy and dysfunction frequently occur in COPD. Several potential mechanisms have been proposed for left ventricular failure in COPD. Although hs-cTnT has been proven to be a useful biomarker for differential diagnosis in patients hospitalized with a severe COPD exacerbation,Citation13 it is not clear whether hs-cTnT levels change in patients with stable COPD. In this study, we explored the association between hs-cTnT levels and cardiac and pulmonary dysfunction in patients with stable COPD and in individuals at risk of COPD, in order to elucidate the meaning of hs-cTnT values in patients with stable COPD in a clinical setting.
Materials and methods
Study population
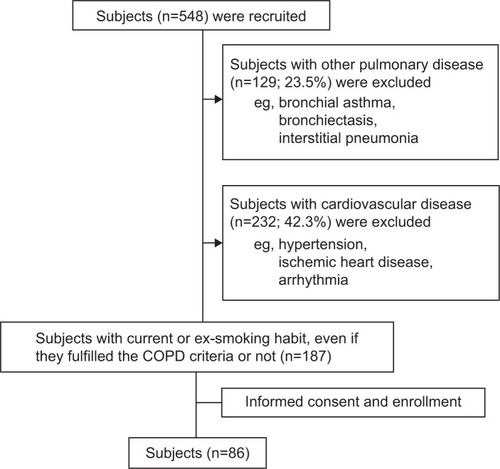
This prospective study examined 548 subjects with a life-long history of smoking and current symptoms of dyspnea during exertion, prolonged coughing, and/or sputum. Patients with any cardiovascular disease (n=232) or other respiratory disease (n=129), such as bronchiectasis or lung fibrosis, were carefully excluded as mentioned below, and 187 subjects with COPD-related symptoms were examined. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Tripartite Guideline for Good Clinical Practice. Before the studies were initiated, the protocol was reviewed and approved by the ethics committee of the Nippon Medical School (approval number 18-11-31). All patients were required to provide written, informed consent before enrollment. Finally, 86 subjects were enrolled with informed consent in the present study, and they were also confirmed to have been in a stable condition for at least 3 months.
Exclusion criteria
Subjects suffering from any one of the following pulmonary and cardiovascular diseases were excluded: bronchial asthma, bronchiectasis, interstitial pneumonia, hypertension, ischemic heart disease, any type of arrhythmia, and congestive heart failure. Detailed information is provided in Figure S1.
Measurements
All subjects underwent chest roentgenography from both directions, routine blood chemistry, electrocardiogram, pulmonary function tests, blood gas analysis, echocardiogram, 6-minute walking test (6MWT), and high-resolution computed tomography (HRCT) of the chest. An electrocardiogram was performed to identify obvious heart disease such as arrhythmia and previous myocardial infarction. Serum samples were taken simultaneously and were preserved at −80°C for hs-cTnT measurements.
Pulmonary function tests
The pulmonary tests were performed by well-trained technicians according to the American Thoracic Society guidelines,Citation14 using specialized equipment for lung function testing with computer processing (Chestac 55, Chest Co., Tokyo, Japan). Subjects were diagnosed with COPD according to a value of <0.70 for the post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio, as indicated by the Global Initiative for Chronic Obstructive Lung Disease.Citation15 Subjects not meeting the COPD criteria were classified into the “at-risk” group.
HRCT parameters
We performed helical HRCT scans (details in Supplementary materials). Percentage of low attenuation area (LAA%) for the upper, middle, and lower zones of bilateral lung fields and overall mean values were calculated as previously reported.Citation16
Echocardiography
Echocardiography was performed using a diagnostic ultrasound system (Aplio SSA-770A, Toshiba Medical Systems Co., Tokyo, Japan; details in Supplementary materials).
Serum troponin measurement
Serum hs-cTnT levels were measured using an electrochemiluminescence immunoassay (BML, Inc., Kawagoe, Japan). The lower limit of detection for hs-cTnT was 0.003 ng/mL, and the standard value was below 0.014 ng/mL.
Statistical analysis
The following parameters were not normally distributed and were therefore transformed logarithmically: hs-cTnT concentration, FEV1% predicted, percent diffusing capacity of the lung for carbon monoxide/alveolar volume (%DLco/VA), high-sensitivity C-reactive protein (hs-CRP), and walking distance in the 6MWT. The associations between hs-cTnT levels and other parameters were tested using Pearson’s correlation analyses and single linear regression analysis. Among the parameters, the following showed significant differences by Pearson’s analysis: age, smoking habit, FVC, FEV1% predicted, %DLco/VA, partial pressure of oxygen (PaO2), serum hs-CRP level, right ventricular systolic pressure (RVSP), walking distance, and LAA% for the upper and lower lung fields. Therefore, we performed multivariate, stepwise backward analyses for hs-cTnT serum concentrations.
The data were analyzed using PASW Statistics version 18 for Windows software (SPSS Inc, Chicago, IL, USA). All P-values were two-tailed, and a P-value of <0.05 was considered to be statistically significant.
Results
The study design is shown in . Among the total of 548 subjects, 86 were selected as the patient group who either had or were at risk of COPD. In total, 361 subjects were excluded after various examinations because of either cardiac comorbidities or other pulmonary diseases ( and S1).
Figure 1 Study flow chart. Subjects (n=548) were recruited, and subjects with other pulmonary disease and/or any cardiovascular disease were excluded (see Supplementary materials). A total of 86 subjects were enrolled in the study.
The basic characteristics of the subjects are shown in . The mean age was 65.5 years. Based on the COPD criteria, 54 subjects were diagnosed with COPD, 32 subjects were diagnosed as being at risk of COPD, and the mean FEV1% predicted was 75.0%. The numbers of patients for each Global Initiative for Chronic Obstructive Lung Disease (2004)Citation15 classification were as follows: 13, 22, 17, and 2 for class I, II, III, and IV, respectively. Mean hs-CRP and hs-cTnT serum levels were 0.182 mg/dL and 0.008 ng/mL, respectively. Eight patients had hs-cTnT levels below the level of detection (0.003 ng/mL). In addition, hs-cTnT levels in seven subjects with COPD and in one subject in the at-risk group were over the normal limit of 0.014 (range 0.016–0.027) mg/mL.
Table 1 Baseline patient characteristics
The results of chest HRCT, echocardiography, and the 6MWT are shown in . The percentage of subjects with emphysema was 71.4% (all, n=60; COPD, n=51; at-risk, n=9). Mean RVSP was 33.7 mmHg. The 6MWT revealed 21 subjects with hypoxia, whose mean minimum oxygen saturation was 82.5% (range 71%–89%), and whose mean delta saturation of pulse oximetry oxygen (ΔSpO2; defined as the difference between the initial value at rest and the minimum value during the test) was 10.4% (range 6%–24%).
Table 2 Results of HRCT, echocardiography, and 6-minute walking test
The correlations between logarithmically transformed hs-cTnT levels and demographic data, results of the various pulmonary function tests, arterial blood gas, and other laboratory data are shown in . The hs-cTnT level (logged) was significantly and positively correlated with age (r=0.534, P=0.000), smoking habit (r=0.280, P=0.042), brain natriuretic peptide (r=0.334, P=0.004), hs-CRP (logged; r=0.517, P=0.000), RVSP (r=0.008, P=0.317), and LAA% for the upper (r=0.231, P=0.042) and lower (r=0.286, P=0.011) lung fields. On the other hand, there was significant and negative correlation with FVC (r= −0.388, P=0.000), FEV1% predicted (logged; r= −0.377, P=0.001), %DLco/VA (logged; r= −0.431, P=0.000), PaO2 (r= −0.385, P=0.002), and walking distance (logged; r= −0.482, P=0.000).
Table 3 Association between hs-cTnT levels (logged) and demographic and cardiopulmonary parameters in all subjects
The associations between hs-cTnT levels (logged) and demographic and cardiopulmonary parameters in a linear regression analysis model are shown in . Age was the parameter best fitting to hs-cTnT values (logged; ).
Figure 2 Association between log(hs-cTnT) and demographic and cardiopulmonary parameters in single linear regression analysis.
Abbreviations: FEV1% predicted, predicted forced expiratory volume in 1 second; RVSP, right ventricular systolic pressure; hs-CRP, high-sensitivity C-reactive protein; hs-cTNT, high-sensitivity cardiac troponin T.

To determine the variables affecting the hs-cTnT values (logged), multiple regression analysis with a stepwise estimation model was used on parameters that appeared to be significantly correlated with hs-cTnT values (logged) in Pearson’s correlation analyses. Multivariate analysis revealed that hs-cTnT levels were independently affected by the following three parameters: hs-CRP (logged; regression coefficient B=0.157, standardized regression coefficient β=0.450, t=3.571, P=0.001), age (B=0.008, β=0.352, t=2.789, P=0.009), and RVSP (B=0.008, β=0.280, t=2.202, P=0.035). There was no multicollinearity among these variables. Thus, hs-CRP values (logged), age, and RVSP were independent variables affecting hs-cTnT values (logged; R2=0.506; ). We also checked whether this multivariate model for hs-cTnT would be accurate for both a subgroup of COPD and at-risk subjects. Hs-CRP and age, but not RVSP, were associated with hs-cTnT in this model for these two subgroups (Tables S1 and S2).
Table 4 Multiple regression analysis for hs-cTnT levels (logged, stepwise estimation model)
Discussion
In the present study, we have shown that hs-cTnT in the serum of patients with stable COPD and in at-risk subjects can be explained by three independent factors, ie, hs-CRP, age, and RVSP. In addition to strict selection criteria aimed at including only COPD patients and exclude those with any cardiac or pulmonary comorbidities, all subjects were confirmed to have been in a stable condition for at least 3 months prior to the study.
We used the fixed FEV1/FVC ratio to define airflow obstruction since the use of FEV1% predicted is commonly used in clinical practice; however, using FEV1% predicted might result in overdiagnosis of COPD in elderly individuals.Citation17 Therefore, from the onset of the study, we recruited patients who had a life-long history of smoking and continuous respiratory symptoms, such as cough, expectoration, and/or dyspnea in daily exercise. These patients were then assigned to one of two groups, ie, COPD or at-risk.Citation15 Interestingly, 42.3% of the original 548 subjects who were recruited into this study and had COPD-related symptoms also had some cardiovascular comorbidity. Currently, evidence regarding the role of hs-cTnT in subjects with COPD is of high interest; hs-cTnT levels increase in acute exacerbation of COPD, and elevation of hs-cTnT levels is associated with a poor prognosis.Citation7–Citation10 An increased hs-cTnT concentration was observed among some patients with stable COPD, and was significantly and positively associated with serum interleukin-6 concentration and the presence of pathologic Q waves, and negatively associated with FEV1.Citation11 The reason for the increase in hs-cTnT levels in stable COPD is still subject to speculation and is the subject of several hypotheses described herein.
First, Neukamm et alCitation11 hypothesized that the hs-cTnT increase is induced by systemic inflammation, which is common in the pathogenesis of COPD, cardiovascular diseases, and hypoxemia. Consistent with this hypothesis, they reported that higher hs-TnT levels are associated with higher interleukin-6 concentrations, which are thought to reflect systemic inflammation.Citation11 Here, we have further demonstrated that hs-cTnT is closely associated with hs-CRP, reflective of systemic inflammation. The previously reported association between increased hs-cTnT and decreased FEV1Citation11 was also observed in this study. Moreover, our data demonstrate a close association between increased hs-cTnT and decreased FVC, FEV1% predicted, and %DLco/VA. Thus, local inflammation in the lungs of COPD patients might be transmitted to the entire body, including the heart,Citation18 and induce cardiomyopathy. If a medication, eg, an inhaled corticosteroid, could reduce systemic inflammation,Citation19 it could also inhibit occurrence of cardiac comorbidities in COPD subjects with high hs-cTnT values.
Second, it was interesting to observe that reduced PaO2 was associated with increased hs-cTnT (logged; r= −0.38, P=0.002). Since higher hs-cTnT was associated with more severe airflow obstruction and hypoxemia in COPD subjects without cardiac comorbidities, patients with more severe COPD might have latent and progressive cardiomyopathy with a relatively low hs-cTnT value, but higher than in non-COPD subjects, possibly due to prolonged hypoxemia. We might be able to predict whether some COPD patients will have cardiac comorbidity in the near future by measuring hs-cTnT and prevent the occurrence by long-term oxygen supplementation. If this hypothesis is correct, the current criteria used for long-term oxygen therapyCitation20 should be amended, as they are primarily based on data at rest. In the same vein, attention should be paid to cardiac injury by hypoxemia, which occurs not only at rest but also during exercise. Since we only observed correlation between hs-cTnT in the serum and PaO2, a causal relationship between hs-cTnT and PaO2 remains to be elucidated.
Third, cardiovascular risk factors, morbidity, and mortality are reportedly associated with increased hs-cTnT levels among the aged population, particularly in men.Citation21,Citation22 The mean age of the recruited subjects in this study was 65.5 years, and 91.9% were men. Multivariate analyses showed that age was one of the independent factors that explained increased hs-cTnT, consistent with recent data by Van Remoortel et al.Citation23 Therefore, COPD is characterized by multi-morbidity, and is independently caused by age, smoking, physical inactivity, systemic inflammation, and obesity. Hence, older male subjects with COPD might be at a higher risk for cardiac injury, as previously observed.Citation21,Citation22 Notably, in the present study, only 34.1% of patients remained after excluding those who had cardiac and/or pulmonary comorbidity. This observation implies that most patients, who might normally be suspected of having COPD or of being at risk, may in fact be suffering from other such comorbidities. This deduction generally describes the nature of chronic disease in the elderly, and it is consistent with recent data by Van Remoortel et al.Citation23 Interestingly, the results of the present study might suggest that cardiovascular comorbidities do not occur in severe COPD alone and also that cardiac injury occurs in stable or even in mild COPD. In this regard, serum hs-cTnT is a promising biomarker of cardiovascular dysfunction or injury in patients with COPD. However, we have not tested whether hs-cTNT is a sensitive marker for cardiac injury in COPD, and it would be interesting to examine the COPD patients with cardiac injury to investigate this matter in the future. Clearly, this hypothesis should also be tested on a large population with consideration of potential ethnic differences.
Our study has some limitations defined herein. First, there are limitations inherent in the sample size. Before starting this study, we determined that the required sample size was 85 to detect a simple correlation between hs-cTnT and continuous variables related to COPD (eg, FEV1% predicted) with a correlation coefficient (r) of 0.3, 80% power (β=0.2), and a significance level of 5% (α=0.05).Citation24 Because 86 subjects were included, the sample size was large enough to accurately calculate and test simple correlations. However, we also performed multiple regression analyses, in which the sample size for this type of study could be smaller. Second, all subjects enrolled in this study were recruited from a secondary referral medical clinic. This recruitment did not allow us to evaluate the effects of medications, since most patients had already been administered medication prior to their referral to our clinic. Third, recent reports indicated that hs-cTnT levels were higher in patients with obstructive sleep apnea syndrome (OSAS) and that hs-cTnT is significantly associated with apnea and hypopnea index.Citation25,Citation26 Randby et alCitation26 suspect that increased activity of the sympathetic nervous system, increased heart rates, and intermittent increase of blood pressure induce elevated hs-cTnT levels in patients with OSAS. We did not exclude possible OSAS comorbidity by polysomnography; this association requires further investigation in patients with stable COPD. Concomitant COPD and OSAS, termed the overlap syndrome, is not rare and affects at least 1% of the general population.Citation27 Fourth, future studies should examine a non-smoker cohort with COPD.
Conclusion
In conclusion, the increased hs-cTnT concentration was explained by three independent factors, ie, hs-CRP, age, and right cardiac overload, in patients with stable COPD and at-risk subjects.
Supplementary materials
Study design
The study design is shown in Figure S1. The subjects were recruited from March 2005 to December 2013 at the Respiratory Care Clinic, which is a secondary referral facility affiliated with the Nippon Medical School in Tokyo, Japan. From a total of 548 subjects, 86 who either had or were at risk of chronic obstructive pulmonary disease (COPD) were selected as the patient group, and any patients with cardiac and/or pulmonary comorbidities after various examinations were carefully excluded (details in the following section on Exclusion criteria).
Exclusion criteria
Subjects with only COPD were examined in this study, excluding any cardiac and pulmonary comorbidities. Subjects suffering from any one of these pulmonary and/or cardiovascular diseases were excluded: bronchial asthma, bronchiectasis, interstitial pneumonia, sinobronchial syndrome, active or old pulmonary tuberculosis, non-tuberculous mycobacteriosis, abnormal chest shadow suggestive of a lung tumor, resolved pneumothorax, lymphangioleiomyomatosis, lung cancer, diffuse panbronchiolitis, hypersensitivity pneumonitis, asbestosis, pulmonary hypertension, pneumonia, chronic eosinophilic pneumonia, chronic pulmonary thromboembolism, hypertension, ischemic heart disease, any type of arrhythmia, congestive heart failure, atrial septal aneurysm, ventricular septal aneurysm, hypertrophic cardiomyopathy, valvular disease, atrial septal defect, ventricular septal defect, tetralogy of Fallot, and genetic α1 anti-trypsin deficiency. Thus, careful attention was paid to exclude all these cases with cardiopulmonary comorbidities in order to focus on subjects with only COPD or those with a life-long smoking history who exhibited clinical symptoms similar to COPD, whom we considered at-risk subjects.
Pulmonary function tests
The tests were performed by well-trained technicians according to the American Thoracic Society guidelinesCitation1 using specialized equipment for lung function testing with computer processing (Chestac 55; Chest Co., Tokyo, Japan). The standards of the Japanese Respiratory SocietyCitation2 were used as the reference values for post-bronchodilator forced expiratory volume in 1 second and forced vital capacity.
High-resolution computed tomography
We performed helical high-resolution computed tomography scans at 1.25 mm collimation, 0.8 second scan time (rotation time), 120 kV, and 100–600 mA using a Light Speed Pro16 CT Scanner (GE Co., Tokyo, Japan).
Echocardiography
The same experienced echocardiographer performed the procedure on all subjects. The examination was recorded in the standard parasternal and apical views during normal breathing at end-expiration. All measurements were obtained according to the standards of the American Society of Echocardiography.Citation3 The left ventricular internal end-diastolic and end-systolic diameters were measured over five consecutive cycles. Systolic pulmonary artery pressure was calculated using the modified Bernoulli equation with an estimated right atrial pressure of 10 mmHg.
Multiple regression analysis for hs-cTnT levels (logged) in the COPD group and at-risk group
We determined whether this multivariate model for high-sensitivity cardiac troponin T (hs-cTnT) would be accurate for both a subgroup of COPD and at-risk subjects. High-sensitivity C-reactive protein and age, but not right ventricular systolic pressure, were associated with hs-cTnT using this model for these two subgroups (Tables S1 and S2).
Association between hs-cTnT levels (logged) and demographic and cardiopulmonary parameters in subjects with median or higher hs-TnT levels and in subjects with lower hs-TnT levels
The hs-cTnT level was low in the entire population. Thus, we also performed simple correlation analyses between hs-cTnT and clinical parameters (Table S3), and performed stepwise regression analysis on logged hs-cTnT values (Table S4) again in the subjects whose hs-cTnT was greater than the median (0.007). Although the population was relatively small in this analysis, we were still able to observe an association between hs-cTnT and C-reactive protein.
Figure S1 Study flow chart. Subjects (n=548) were recruited, and those with other pulmonary diseases (n=129; eg, bronchial asthma and bronchiectasis) and/or any cardiovascular diseases (n=232; eg, hypertension and ischemic heart disease) were all excluded. Some subjects had multiple comorbidities. A total of 86 subjects were enrolled in the study.
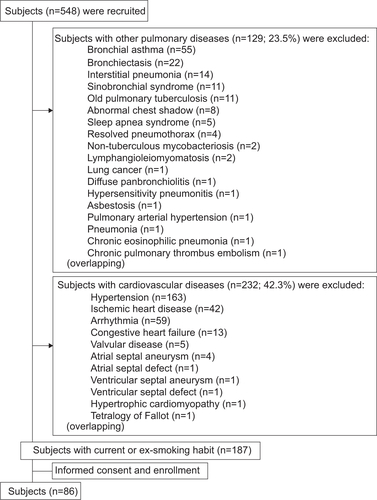
Table S1 Multiple regression analysis for hs-cTnT levels (logged) in the group with chronic obstructive pulmonary disease
Table S2 Multiple regression analysis for hs-cTnT levels (logged) in the at-risk group
Table S3 Association between hs-cTnT levels (logged) and demographic and cardiopulmonary parameters in subjects with median or higher level of hs-TnT and in subjects with lower levels of hs-TnT
Table S4 Multiple regression analysis for hs-cTnT levels (logged) in subjects with median or higher levels of hs-TnT (stepwise estimation model)
References
- Global Initiative for Chronic Obstructive Lung Disease Executive Committee2004 update: Workshop Report, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary diseaseMedical Communications Resources, Inc Updated2004 Available from: http://www.goldcopd.org/Accessed October 19, 2014
- Japanese Respiratory SocietyThe predicted values of spirometry and arterial blood gas analysis in JapaneseJ Jpn Respir Soc200139117 Japanese
- CheitlinMDArmstrongWFAurigemmaGPAmerican College of Cardiology, American Heart Association, American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography)Circulation200310891146116212952829
Disclosure
The authors report no conflicts of interest in this work.
References
- PatelARHurstJRExtrapulmonary comorbidities in chronic obstructive pulmonary disease: state of the artExpert Rev Respir Med20115564766221955235
- DecramerMJanssensWMiravitllesMChronic obstructive pulmonary diseaseLancet201237998231341135122314182
- SinghVMartinezclarkPPascualMShawESO’NeillWWCardiac biomarkers – the old and the new: a reviewCoron Artery Dis201021424425620351549
- RøyslandRKravdalGHøisethADCardiac troponin T levels and exercise stress testing in patients with suspected coronary artery disease: the Akershus Cardiac Examination (ACE) 1 studyClin Sci (Lond)20121221259960622239123
- TurerATAddoTAMartinJLMyocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a high sensitive assay: insights from a coronary sinus sampling studyJ Am Coll Cardiol201157242398240521658559
- HickmanPEPotterJMAroneyCCardiac troponin may be released by ischemia alone, without necrosisClin Chim Acta20104115–631832320036224
- BrekkePHOmlandTHolmedalSHSmithPSøysethVTroponin T elevation and long-term mortality after chronic obstructive pulmonary disease exacerbationEur Respir J200831356357018032444
- HøisethADNeukammAKarlssonBDOmlandTBrekkePHSøysethVElevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary diseaseThorax201166977578121653926
- HøisethADOmlandTHagveTABrekkePHSøysethVDeterminants of high-sensitivity cardiac troponin T during acute exacerbation of chronic obstructive pulmonary disease: a prospective cohort studyBMC Pulm Med2012122222651225
- SøysethVBhatnagarRHolmedahlNHAcute exacerbation of COPD is associated with fourfold elevation of cardiac troponin THeart201399212212623024006
- NeukammAMHøisethADHagveTASøysethVOmlandTHigh-sensitivity cardiac troponin T levels are increased in stable COPDHeart201399638238723315609
- ThurlbeckWMWrightJLDisease in the other organThurlbeckWilliam MichaelWrightJoanne LynneThurlbeck’s Chronic Airflow Obstruction2nd edLondon, UKBC Decker Inc1999
- BhattSPDransfieldMTChronic obstructive pulmonary disease and cardiovascular diseaseTransl Res2013162423725123727296
- [No authors listed]Standardization of spirometry, 1994 update. American Thoracic SocietyAm J Respir Crit Care Med19951523110711367663792
- Global Initiative for Chronic Obstructive Lung Disease2004 update: Workshop Report, Global Strategy for Diagnosis, Management and Prevention of COPD Available from: http://www.goldcopd.org/Accessed October 19, 2014
- KamioKIshiiTMotegiTDecreased serum transforming growth factor-β1 concentration with aging is associated with the severity of emphysema in chronic obstructive pulmonary diseaseGeriatr Gerontol Int20131341069107523441714
- HardieJABuistASVollmerWMEllingsenIBakkePSMørkveORisk of over-diagnosis of COPD in asymptomatic elderly never-smokersEur Respir J20022051117112212449163
- BarnesPJCelliBRSystemic manifestations and comorbidities of COPDEur Respir J20093351165118519407051
- ZervasESamitasKGagaMInhaled corticosteroids in COPD: pros and consCurr Drug Targets201314219222423256718
- BudweiserSJörresRAPfeiferMTreatment of respiratory failure in COPDInt J Chron Obstruct Pulmon Dis20083460561819281077
- deFilippiCRde LemosJAChristensonRHAssociation of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adultsJAMA2010304222494250221078811
- EggersKMAl-ShakarchiJBerglundLHigh-sensitive cardiac troponin T and its relations to cardiovascular risk factors, morbidity, and mortality in elderly menAm Heart J2013166354154824016505
- Van RemoortelHHornikxMLangerDRisk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary diseaseAm J Crit Care Med201418913038
- LachinJMIntroduction to sample size determination and power analysis for clinical trialsControl Clin Trials198122931137273794
- InamiTSeinoYOtsukaTLinks between sleep disordered breathing, coronary atherosclerotic burden, and cardiac biomarkers in patients with stable coronary artery diseaseJ Cardiol201260318018622525967
- RandbyANamtvedtSKEinvikGObstructive sleep apnea is associated with increased high-sensitivity cardiac troponin T levelsChest2012142363964622406957
- ZamarrónCGarcía PazVMoreteEdel Campo MatíasFAssociation of chronic obstructive pulmonary disease and obstructive sleep apnea consequencesInt J Chron Obstruct Pulmon Dis20083467168219281082
