Abstract
Background
Presence of chronic obstructive pulmonary disease (COPD) in heart failure (HF) has prognostic and therapeutic implications. Exact prevalence estimates are lacking because most previous studies estimated the prevalence of COPD among HF patients while unstable and in the presence of pulmonary congestion.
Methods
Community-dwelling patients with an established diagnosis of HF and in a stable phase of their disease were invited for spirometry. COPD was defined according to the Global initiative for chronic Obstructive Lung Disease (GOLD) classification and considered present if the ratio of the post-bronchodilator forced expiratory volume in 1 second and forced vital capacity was below 0.7.
Results
Thirty of the 106 patients with HF (mean age 76 [standard deviation] 11.9 years, 57% male) had COPD (prevalence 28.3% [95% confidence interval (CI) 19.7%–36.9%]), with similar rates among those with HF and a reduced ejection fraction (18 individuals; prevalence 28.6% [95% CI 20.0%–37.2%]) and HF with preserved ejection fraction (12 individuals; prevalence 27.9% [95% CI 19.4–36.4]). Twenty-one (70%) of the 30 participants were newly detected cases of COPD.
Conclusion
More than a quarter of the patients with HF concomitantly have COPD, with the large majority being previously unrecognized. Coexistence of COPD should be considered more often in these patients.
Introduction
Heart failure (HF) and chronic obstructive pulmonary disease (COPD) are both common in the elderly, and often coexist.Citation1 Diagnosing COPD in HF is challenging because clinical features overlap, and dyspnea and fatigue are common symptoms.Citation1,Citation2 Both share smoking as an important risk factor, and they have a chronic progressive disease trajectory with systemic effects, and require complex treatment regimens.Citation3–Citation5 The prognosis of patients with both disorders is worse than those with only one of the diseases.Citation6,Citation7 Importantly, bronchodilators may improve symptoms of COPD, but possibly cause cardiac side effects.Citation8,Citation9 Under-diagnosis of one disease in the presence of another is an important issue,Citation10,Citation11 but also there is a risk of over-diagnosing COPD in patients with HF if these patients are not stable and have some pulmonary congestion.Citation12 Pulmonary congestion is common in those hospitalized for an exacerbation of HF, and this may mimic COPD clinically, but also spirometrically. Spirometry at discharge in that situation would show – caused by pulmonary congestion – a larger reduction in forced expiratory volume in 1 second (FEV1) than in forced vital capacity (FVC), resulting in a risk of over-diagnosing COPD.Citation13,Citation14 When patients with HF are stable and euvolemic, the FEV1/FVC allows to adequately detect COPD, although, both the FEV1 and the FVC are reduced to a similar extent.Citation15 Diagnosing COPD at the right moment is therefore key in patients with HF.
Earlier reports on the prevalence of co-morbid COPD in HF often relied on previous documentation of COPD, or on spirometry results performed in HF patients when unstable.Citation10,Citation16,Citation17 Prevalence estimates ranged from 9% to 52%.Citation2 Data in a representative sample of patients with HF in a stable phase of their disease, also including patients with preserved ejection fraction (PEF), are still lacking.Citation1
The aim of our study was to provide a valid prevalence estimate of COPD in a representative sample of patients with HF while in a stable condition (ClinicalTrials.gov identifier NCT1662323).
Methods
Design and study population
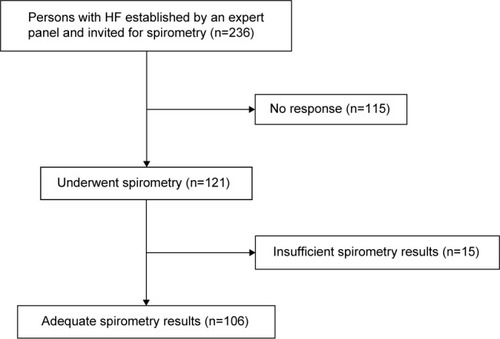
In a cross-sectional study we enrolled patients from 30 general practices from the vicinity of Amersfoort, the Netherlands. The study and recruitment period was from November 2010 until October 2011. In total 70,000 persons were enlisted in these practices. In the Netherlands all citizens are registered with a general practitioner (GP), irrespective of cooperative care by a hospital specialist, including those living in a home for the elderly, but excluding those living in a nursing home or hospice. Patients were eligible if they had a diagnosis of HF confirmed by an expert panel consisting of two cardiologists and a GP specializing in HF using all diagnostic information, including echocardiography. The panel based the diagnosis of HF on the criteria of the European Society of Cardiology, that is, suggestive symptoms and objective evidence of cardiac structural or functional cardiac abnormality related to ventricular dysfunction at rest detectable with echocardiography. For HF with reduced ejection fraction (HF-REF) the left ventricular ejection fraction should be <45%. For HF with preserved ejection fraction (HF-PEF) the left ventricular ejection fraction should be ≥45%, in addition to diastolic dysfunction identified by echocardiography.Citation18 Disagreement between panel members was resolved by majority. We invited 236 patients with a confirmed diagnosis of HF for spirometry, and 121 (51.2%) consented to participate ().
The study was approved by the Regional Medical Ethics Committee of the Meander Medical Center, Amersfoort, the Netherlands. All participants gave written informed consent.
Measurements
Between November 2010 and June 2011 baseline characteristics of the participants were extracted from the medical files of the participating GPs. Participants underwent spirometry in the primary care setting performed by trained personnel. Spirometry was performed with SPIDA software (Spida 5) and microloop. Before and after 400 micrograms of salbutamol, the FVC and FEV1 were measured. COPD was considered present if the post-bronchodilator FEV1/FVC ratio was below 0.7, as defined by the Global initiative for chronic Obstructive Lung Disease (GOLD) criteria.Citation5 Severity of pulmonary obstruction was expressed as the proportion of the predicted FEV1 (the latter based on “normal” values as derived from the population at large with the same age, sex, and height) and derived from the forced spirometry.Citation19 The FEV1 as percentage predicted was subdivided into four categories: ≥80, 50–79, 30–49, ≤30. The quality of the flow-volume curves was assessed by an experienced physician.
Data analysis
We compared HF patients who decided to participate with nonparticipants. In the participants we compared those with and without COPD. In those with COPD, we compared “already known” with “newly detected”. Finally, we compared patients with HF-REF and HF-PEF. Differences between groups were assessed with the chi-square or Fisher’s exact tests for categorical variables, and independent student’s t-test for continuous variables. The prevalence of COPD was calculated with the binominal 95% confidence intervals (95% CIs). All data were analyzed with SPSS version 20.0.
Results
Two-hundred and thirty-six patients with established HF were invited for spirometry. Baseline characteristics of the participants (n=121) were not significantly different from the nonparticipants (n=115) (Table S1). In 15 patients spirometry results were of insufficient quality, leaving 106 for this analysis (). The mean age of the 106 participants was 76.0 (standard deviation [SD] 11.9) years, and 57% were male. The prevalence of COPD was 28.3% (95% CI 19.7%–36.9%), with similar rates for those with a REF (28.6% [95% CI 20.0%–37.2%]) and PEF (27.9% [95% CI 19.4%–36.4%]).
shows patient characteristics of all 106 patients. On average, patients with COPD were older than those without COPD. Mean FEV1 % predicted was 65.8 (SD 19.5) and 73.9 (SD 10.9) for those with newly detected COPD and without COPD, respectively. Seventy percent of the subjects with COPD was previously undiagnosed by their GP. None of the patients with COPD had a history of asthma. shows the patient characteristics of newly detected cases vs HF patients without COPD. In we compared patients with HF-REF and HF-PEF. The mean post-dilator FEV1 % predicted was approximately 80% for both HF-REF and HF-PEF patients. Patients with HF-REF were more often male than those with HF-PEF.
Table 1 Characteristics of the 106 patients with established heart failure divided into those with and without newly detected, or already known COPD
Table 2 Characteristics of the patients with heart failure and a new diagnosis of COPD vs those without COPD
Table 3 Characteristics and spirometry results of the 106 patients with established HF, divided in those with HF-REF and HF-PEF
Discussion
In our study among stable community-dwelling HF patients the prevalence of COPD was 28.3%, and in 70% this was a new diagnosis of COPD. Prevalence rates were comparable for the subgroups of HF-REF and HF-PEF.
Our results are in line with previous studies reporting prevalence rates of 9%–52% in patients with HF.Citation2 These previous studies were performed in hospitalized patients with an over-representation of HF-REF, and spirometry was performed while these patients were recently pulmonary fluid overloaded. We could therefore provide a more valid point estimate of the COPD prevalence of the HF population at large than previous studies. A recent study among 118 elderly patients with stable HF and a smoking history of ≥10 pack years also showed a prevalence rate of 30% of COPD, similar to our study, and also a similarly high number of newly detected cases of COPD (64%).Citation20 In this study COPD was also defined according to the GOLD criteria. Moreover, patients were investigated while stable, approximately 3 months after being hospitalized for HF. Separate prevalence rates for HF-REF and HF-PEF, however, were lacking.Citation20
The prevalence of COPD in patients with HF in our study is approximately 1.5 times higher than the expected estimate of 20% in elderly from the population at large.Citation21
We found that the post-bronchodilator FEV1 in patients with HF was on average 80% of predicted in patients with the same age and length. This is in line with a previous study showing that HF in the absence of COPD may cause a reduction in FEV1 of approximately 20%, caused by HF itself.Citation15 Thus, classifying severity of COPD based on FEV1 % predicted may overestimate the severity of obstruction in patients with HF. Because both FVC and FVC as % predicted are reduced approximately 20%, the ratio FEV1/FVC is not affected.Citation15
A strength of our study is the inclusion of a representative sample of patients with HF in a stable phase of their disease, and we could present prevalence rates for patients with HF-REF and HF-PEF separately.
A limitation is the relatively small sample size and the lack of body box measurements. Measurements of the total lung capacity and the residual volume would have been helpful to refine the diagnosis of COPD, especially in cases with spirometry results around the critical cut-off point of FEV1/FVC 0.7.Citation22 Importantly, however, previous studies reporting on the prevalence of COPD in HF did not apply pulmonary function tests other than spirometry. Recently, some authors advocated age- and sex-related thresholds for the FEV1/FVC lower limit of normal to define COPD.Citation19 We did not use one of the suggested lower limit of normal methods, because application in our study would reduce the possibility to compare our results with previous studies. Finally, some COPD cases may in fact represent persistent asthma, or “mixed cases”, however, none of the COPD cases in earlier years had been labeled with asthma by the GP. We therefore preferred to use the GOLD-definition for COPD (a FEV1/FVC <0.7). Knowledge of co-morbid COPD in patients with HF has clinical implications because for the management of breathlessness there is room for pulmonary inhalation drugs. We have, however, realized that some authors suggest that both beta-mimetics and anti-muscarinic agents may harm the heart. HF drugs may be prescribed in patients with COPD, including cardio selective β-blockers. It is, however, important to uptitrate slowly.Citation23
Conclusion
COPD is common in patients with HF, both HF-REF and HF-PEF, and remains undetected in the majority of patients. Selective screening of patients with HF when in a stable phase of their disease should be considered.
Author contributions
FHR conceived the study idea. AWH, FHR, AM, and MJV designed the study. MJV did the data acquisition, and MJV, BDB, and NPZ did the statistical analysis. MJV, and BDB drafted the manuscript and all authors contributed to the revision of the manuscript. FHR supervised the study and is guarantor of the study.
Acknowledgments
We wish to thank the participating patients and GPs, and Sjaane Hilligehekken, Sanne Rooymans, and Sanne Hillebrand for performing the pulmonary function tests. The study was conducted with an unrestricted research grant from the health insurance company Agis Achmea.
Supplementary material
Table S1 Characteristics of the 236 patients with established heart failure who were invited for spirometry, divided into participants and nonparticipants
Disclosure
The authors report no conflicts of interest in this work.
References
- RuttenFHCramerMJLammersJWGrobbeeDEHoesAWHeart failure and chronic obstructive pulmonary disease: An ignored combination?Eur J Heart Fail20068770671116531114
- HawkinsNMPetrieMCJhundPSChalmersGWDunnFGMcMurrayJJHeart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiologyEur J Heart Fail200911213013919168510
- McMurrayJJAdamopoulosSAnkerSDAuricchioABohmMDicksteinKESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESCEur Heart J201233141787184722611136
- Le JemtelTHPadelettiMJelicSDiagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failureJ Am Coll Cardiol200749217118017222727
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- IversenKKKjaergaardJAkkanDThe prognostic importance of lung function in patients admitted with heart failureEur J Heart Fail201012768569120395261
- BoudesteinLCRuttenFHCramerMJLammersJWHoesAWThe impact of concurrent heart failure on prognosis in patients with chronic obstructive pulmonary diseaseEur J Heart Fail200911121182118819887495
- AuDHUdrisEMFanVSCurtisJRMcDonellMBFihnSDRisk of mortality and heart failure exacerbations associated with inhaled beta-adrenoceptor agonists among patients with known left ventricular systolic dysfunctionChest200312361964196912796175
- SalpeterSRCardiovascular safety of beta(2)-adrenoceptor agonist use in patients with obstructive airway disease: a systematic reviewDrugs Aging200421640541415084142
- IversenKKKjaergaardJAkkanDChronic obstructive pulmonary disease in patients admitted with heart failureJ Intern Med2008264436136918537871
- RuttenFHCramerMJGrobbeeDEUnrecognized heart failure in elderly patients with stable chronic obstructive pulmonary diseaseEur Heart J200526181887189415860516
- BrennerSGuderGBerlinerDAirway obstruction in systolic heart failure – COPD or congestion?Int J Cardiol201316831910191623369673
- DimopoulouIDaganouMTsintzasOKTzelepisGEEffects of severity of long-standing congestive heart failure on pulmonary functionRespir Med199892121321132510197224
- PetermannWBarthJEntzianPHeart failure and airway obstructionInt J Cardiol19871722072093679602
- GuderGRuttenFHBrennerSThe impact of heart failure on the classification of COPD severityJ Card Fail201218863764422858080
- KjollerEKoberLIversenKTorp-PedersenCTrace Study GroupImportance of chronic obstructive pulmonary disease for prognosis and diagnosis of congestive heart failure in patients with acute myocardial infarctionEur J Heart Fail200461717715012921
- MacchiaAMonteSRomeroMD’EttorreATognoniGThe prognostic influence of chronic obstructive pulmonary disease in patients hospitalised for chronic heart failureEur J Heart Fail20079994294817627878
- Boonman-de WinterLJRuttenFHCramerMJHigh prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetesDiabetologia20125582154216222618812
- QuanjerPHTammelingGJCotesJEPedersenOFPeslinRYernaultJCLung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory SocietyEur Respir J Suppl1993165408499054
- BoschettoPFuciliAStendardoMOccurrence and impact of chronic obstructive pulmonary disease in elderly patients with stable heart failureRespirology201318112513022985248
- HalbertRJNatoliJLGanoABadamgaravEBuistASManninoDMGlobal burden of COPD: systematic review and meta-analysisEur Respir J200628352353216611654
- GuderGBrennerSAngermannCEGOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-studyRespir Res20121311322309369
- GuderGBrennerSStorkSHoesARuttenFHChronic obstructive pulmonary disease in heart failure: accurate diagnosis and treatmentEur J Heart Fail201416121273128225345927