Abstract
Introduction
Few data are available in regards to the prevalence of pulmonary hypertension (PH) in the broad spectrum of COPD. This study was aimed at assessing the prevalence of PH in a cohort of COPD patients across the severity of airflow limitation, and reporting the hemodynamic characteristics at rest and during exercise.
Methods
We performed a retrospective analysis on COPD patients who underwent right-heart catheterization in our center with measurements obtained at rest (n=139) and during exercise (n=85). PH was defined as mean pulmonary artery pressure (mPAP) ≥25 mmHg and pulmonary capillary wedge pressure <15 mmHg. Exercise-induced PH (EIPH) was defined by a ratio of ΔmPAP/Δcardiac output >3.
Results
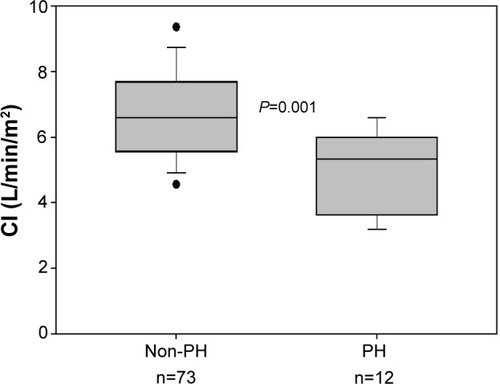
PH was present in 25 patients (18%). According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification, PH prevalence in GOLD 2 was 7% (3 patients); 25% (14 patients) in GOLD 3; and 22% (8 patients) in GOLD 4. Severe PH (mPAP ≥35 mmHg) was identified in four patients (2.8%). Arterial partial oxygen pressure was the outcome most strongly associated with PH (r=−0.29, P<0.001). EIPH was observed in 60 patients (71%) and had a similar prevalence in both GOLD 2 and 3, and was present in all GOLD 4 patients. Patients with PH had lower cardiac index during exercise than patients without PH (5.0±1.2 versus 6.7±1.4 L/min/m2, respectively; P=0.001).
Conclusion
PH has a similar prevalence in COPD patients with severe and very-severe airflow limitation, being associated with the presence of arterial hypoxemia. In contrast, EIPH is highly prevalent, even in moderate COPD, and might contribute to limiting exercise tolerance.
Introduction
Pulmonary hypertension (PH) is a relevant complication in the natural history of patients with chronic obstructive pulmonary disease (COPD), since its presence is associated with shorter survival, increased risk of exacerbations, and greater use of health care resources.Citation1 The actual prevalence of PH in COPD remains unsettled and varies widely according to the targeted population, the definition applied, and the diagnostic approach used to identify PH.Citation2 Right-heart catheterization is the gold standard to diagnose PH.Citation3 In COPD, most hemodynamic studies have been performed in patients with advanced disease in whom PH is expected to occur more frequently, with a prevalence of PH ranging from 23%–91%.Citation4–Citation12 Information on the prevalence of PH in patients with milder disease is scarce. Based on the former Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric classification,Citation13 Hilde et alCitation14 reported a prevalence of 5%, 27%, and 53% in patients with GOLD stages 2, 3, and 4, respectively.
Patients with COPD may develop PH during exercise, a condition associated with an increased risk for subsequently developing PH at rest.Citation12 Current understanding of the hemodynamic response of the pulmonary circulation to exercise in patients with COPD and its impact over exercise intolerance is limited. Studies have shown a cardiovascular limitation to exercise in patients with severe PH which seems to be distinct from the ventilatory exhaustion usually observed in COPD.Citation15,Citation16
In order to expand our understanding of the hemodynamic profile of PH across the spirometric degrees of COPD, we analyzed data from 139 COPD patients assessed by right-heart catheterization at rest. A subset of these patients (n=85) had hemodynamic measurements during exercise. The main objective of the present study was to assess the prevalence of PH in this cohort of patients according to the GOLD spirometric grades. Secondary aims were to describe the hemodynamic characteristics at rest and during exercise, and to analyze the potential relationships between pulmonary hemodynamics and lung function tests.
Methods
This was a retrospective study, which included data from seven previously reported studies.Citation17–Citation23 All studies were approved by our institutional ethics committee and the procedures were performed after obtaining written informed consent. All involved patients were clinically stable (ie, at least 3 months from the last COPD exacerbation), without cardiac failure or other coexisting chronic respiratory disease, and received regular treatment for COPD, including long-term oxygen if it was deemed necessary. No patient was treated with PH targeted therapy, namely prostanoids, endothelin-receptor antagonists, or phosphodiesterase-5 inhibitors. All were current (n=53) or former (n=86) smokers (≥10 pack-year). Patients were classified according to the GOLD spirometric grade system.Citation24
PH was defined by a mean pulmonary artery pressure (mPAP) ≥25 mmHg and a pulmonary capillary wedge pressure (PCWP) <15 mmHg.2 Severe PH was considered when mPAP was ≥35 mmHg or ≥25 mmHg along with a cardiac index (CI) <2.0 L/min/m2, according to the most updated clinical classification of PH in chronic lung diseases.Citation3 An abnormal increase of mPAP during exercise was considered when the change in mPAP ([ΔmPAP] in mmHg) was >3- times greater than the change in cardiac output ([CO] in L/min) (ΔCO).Citation14,Citation25 As age may influence exercise mPAP, we also analyzed the mPAP values during exercise according to those reported by Kovacs et alCitation26 who established the upper limit of normal as 30 mmHg for subjects <50 years and as 46 mmHg for subjects ≥50 years.
Measurements
Lung function
Forced spirometry, static lung volumes, and single-breath carbon monoxide diffusing capacity (DLco) were measured according to the American Thoracic Society/European Respiratory Society recommendations,Citation27 using our own predicted equations.Citation28–Citation29 Arterial blood gas samples were drawn for measuring partial pressure of arterial oxygen (PaO2), partial pressure of arterial carbon dioxide (PaCO2), and pH at rest and during exercise.
Right-heart catheterization
A triple-lumen Swan–Ganz catheter (Edwards Laboratories, Santa Ana, CA, USA) was placed into the pulmonary artery under pressure wave monitoring (M1166A; Hewlett-Packard, Boeblingen, Germany). Transducers were zeroed at the level of right atrium. Right atrial pressure (RAP), pulmonary artery pressure (PAP), and PCWP were measured at the end of expiration. CO was measured by thermodilution and expressed as the mean of three measurements. CI, pulmonary vascular resistance (PVR), and total pulmonary resistance were calculated using standard formulae.Citation30
Exercise measurements
Prior to catheterization, the highest workload that patients could tolerate was determined by an incremental exercise test on a cycle ergometer in 85 subjects. The workload was increased using a ramp of 5 Watts every minute until the maximal load limited by symptoms was achieved. Hemodynamic and gas exchange measurements were performed in the semirecumbent position at a workload equivalent to 60% of the maximal workload tolerated in the previous incremental test.
Statistical analysis
Data are expressed as mean ± standard deviation unless otherwise stated. One-way analysis of variance with Bonferroni correction was used to compare more than two groups. Relationships between variables were assessed using Pearson’s or Spearman’s correlation coefficients depending on data distribution. Changes in hemodynamic data from rest to exercise were compared using paired t-tests. The Mann–Whitney U-test was used to assess CI during exercise in patients with and without PH. Estimations of risk for PH were made using odds ratios and their 95% confidence intervals from univariate logistic regression models for PaO2, DLco, PaCO2, inspiratory capacity, or forced expiratory volume in 1 second (FEV1). In order to assess a multivariate model, we used a forward stepwise procedure with a criterion of 0.05 to select variables for the final model. All data were analyzed with SPSS version 18 and P-values <0.05 were considerate as statistically significant.
Results
The means (± standard deviation) of demographic and lung function measurements for all participants are given in as a function of the current GOLD spirometric classification.Citation24 One hundred and thirty-nine patients (134 men, 5 women) were included. Mean age was 63±8 years. The mean of post-bronchodilator FEV1 was 41%±16% of predicted; PaO2 was 69±12 mmHg; PaCO2 was 40±6; and alveolar–arterial oxygen partial pressure difference (AaPO2) was 32±10 mmHg. Thirty-seven patients were under long-term oxygen therapy.
Table 1 Anthropometric and functional characteristics of 139 patients grouped according to GOLD spirometric grades
Hemodynamic characteristics at rest
shows the hemodynamic measurements at rest. The mean mPAP of the whole cohort was 20±8 mmHg. PH was present in 25 patients (18% of cases of the whole cohort). Most of them had severe or very-severe airflow limitation, although PH prevalence was similar in GOLD 3 and 4.
Table 2 Pulmonary hemodynamics at rest of 139 patients grouped according to the GOLD spirometric grades
Mean PVR was normal in GOLD 2 and 3, although with a wide dispersion, and elevated in GOLD 4. RAP, PCWP, and CI were on average within normal range in all GOLD grades. The CI correlated with FEV1 (r=0.38, P<0.001). Severe PH was noticed in four patients (2.8% of the whole cohort). All of them were male. Their lung function and hemodynamic characteristics are listed in .
Table 3 Subject characteristics in four COPD patients with severe pulmonary hypertension
Hemodynamic characteristics during exercise and changes in blood gases
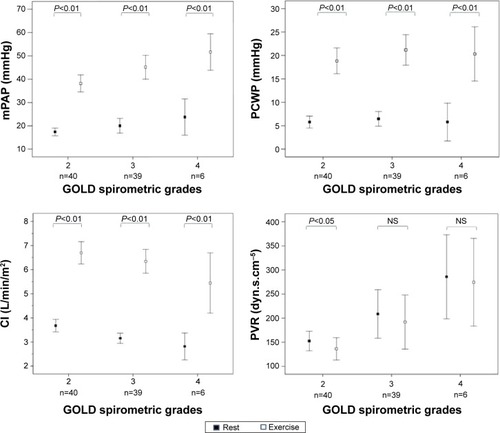
Both mPAP and PCWP increased significantly during exercise in all GOLD grades (; ). An abnormal increase of mPAP during exercise, defined by a ΔmPAP/ΔCO ratio >3, was observed in 60 patients (71%): 28 patients (70%) were in GOLD 2, 26 (67%) in GOLD 3, and the remaining six patients in GOLD 4. During exercise, patients with PH, compared with those without PH, had lower CI (5.0±1.2 and 6.7±1.4 L/min/m2, respectively; P=0.001) (). Patients with associated PH had, as compared with patients without PH, lower PaO2 and higher PaCO2 both at rest and during exercise ().
Table 4 Pulmonary hemodynamics during submaximal exercise in patients grouped to the GOLD spirometric grades (n=85)
Table 5 Arterial blood gas profile at rest and during exercise in 85 patients with or without pulmonary hypertension
Figure 1 Changes from rest to peak exercise in the hemodynamic variables in 85 patients who underwent exercise testing, grouped according GOLD stage.
Note: P-value differences from rest to exercise (paired t-tests).
Abbreviations: CI, cardiac index; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; NS, not significant.
Variables associated with PH
In the univariate analysis, the following outcomes were associated with the presence of PH at rest: PaO2; DLco (% predicted); PaCO2; inspiratory capacity (% predicted); and FEV1 (% predicted). In the stepwise multiple regression model, PaO2 was the only covariate associated with the presence of PH ().
Table 6 Univariate and multivariate logistic regression analysis of variables associated with pulmonary hypertension
Discussion
The current study, which includes pulmonary hemodynamic measurements at rest and during exercise in a stable population of COPD across the whole spirometric spectrum of severity, identified several findings. First, the overall prevalence of PH in our cohort was relatively low (18%). Second, patients in GOLD 3 and 4 had similar a prevalence of PH. Third, a significant increase of both mPAP and PCWP during exercise was noticed in all patients with a high proportion of an “exercise hypertensive response” in the entire cohort. And fourth, CI during exercise was affected by the presence of PH.
Data about the prevalence of PH in COPD, particularly in patients with moderate-to-severe COPD, is limited. The prevalence found in our study was lower than in a recent study by Hilde et alCitation14 who reported a prevalence of PH of 27% in stable patients in GOLD 2–4. Interestingly, the prevalence of PH observed in the present study in patients with GOLD 2 and 3 was similar to that reported by Hilde et al.Citation14 In contrast, the prevalence of PH in patients with GOLD 4 was lower in our study (22%) than in Hilde’s study (53%). This discrepancy may be due to the fact that the current GOLD spirometric classification, which we used, does not consider arterial blood gas measurements for the definition of GOLD 4.Citation24 Accordingly, in patients with severe-to-very-severe COPD, the degree of airflow limitation is not related to the presence of PH. What makes a difference regarding PH in those patients is the presence of concomitant abnormal pulmonary gas exchange. In fact, if we classify patients in our series according to the previous GOLD classification, the prevalence of PH in GOLD 4 stage is 33%, in line with that reported by Hilde et al.Citation14 That said, we cannot overlook that the current A-B-C-D GOLD classification, based on a multidimensional assessment of airflow limitation, symptoms, and risk of exacerbations,Citation24 might more accurately identify differences in PH prevalence between categories. Nevertheless, the retrospective nature of our study precludes such a combined assessment.
As previously reported, the degree of PH in this cohort was of mild-to-moderate magnitude with a well preserved CO, and mPAP correlated with airflow obstruction severity.Citation1,Citation31 In the whole cohort, both resting PCWP and RAP were within normal limits. The prevalence of patients with severe PH (2.8%) in our study is comparable with other reported series.Citation6,Citation7,Citation9 Likewise, patients with severe PH showed less severe airflow limitation, more severe arterial hypoxemia, and lower DLco than patients with moderate PH. It is important to identify these patients with predominant vascular involvement since they represent a clinically distinct phenotype within the COPD population that carries poor prognosis.Citation6,Citation9,Citation32
The exercise-induced increase in mPAP was interpreted according to the increase in CO as proposed by recent reports.Citation14,Citation25 The latter proposal is based on the concept that during exercise, the pulmonary microcirculation is progressively recruited under physiological conditions, resulting in the maintenance of relatively low arterial pressure despite increasing flow.Citation33 The abnormal increase in mPAP during exercise in COPD patients has been documented in different studies.Citation12,Citation14,Citation16,Citation34 In spite of the fact that there is no current definition of exercise-induced PH, we observed that 71% of the patients developed a “hypertensive response”, defined on the basis of pressure/flow relationships. Such an abnormal vascular response was noticed even in moderate grades of COPD severity, whereas it was present in all GOLD 4 patients. The clinical significance of the increased PAP during exercise remains to be established. Conceivably, it could limit exercise capacity. In fact, an increased mPAP has been associated with poor results in the 6 minutes walking test.Citation5,Citation10 Furthermore, a PAP threshold >30 mmHg during exercise is a strong predictor for the development of PH in the long-term.Citation12 We also found that PVR does not decrease during exercise in GOLD grades 3 and 4, in contrast with what occurs in healthy subjects.Citation35 This fall in PVR during exercise is explained by the distensibility of resistive pulmonary vessels. Reduced pulmonary artery compliance observed in COPD might explain this response.Citation14 Furthermore, CI during exercise was lower in patients with associated PH. Although ventilatory impairment is the main factor limiting exercise tolerance in COPD, it cannot be disregarded that hemodynamic alterations may also contribute to exercise intolerance. Indeed, Boerrigter et alCitation15 reported a distinctive exercise pattern in COPD patients with severe PH (mPAP >40 mmHg). Although in our study the limited number of patients with severe PH precluded an analysis in this subset of patients, the finding of lower CI values during exercise in patients with PH concurs with the findings by Boerrigter et al.Citation15
Pulmonary gas exchange impairment was associated with the presence of PH. However, the fact that patients without arterial hypoxemia also had PH in our cohort underlines the concept that the genesis of PH is complex and multifactorial.Citation36 The weak correlation in COPD between PH and lung function measurements has been largely known and fits with our results.Citation7,Citation11,Citation34 This makes it difficult in daily clinical practice to use predictive models to identify patients with pretest probability of presenting PH. Skjørten et alCitation37 suggested that PaO2 values at rest and peak exercise below 71 mmHg and 64 mmHg, respectively, indicate the need for further evaluation of coexisting PH. In our study, we failed to find an adequate model with functional variables that could help to predict the presence of PH.
Our investigation has some limitations. First, there is the possibility that selection bias might have influenced the significance of our findings due to its retrospective, single-center, and observational design. However, because the purpose of the study was to describe PH and hemodynamic characteristics in COPD patients with different grades of airflow limitation, we believe that the population addresses the needs of the research question. Secondly, since there were no patients with mild airflow limitation (GOLD grade 1) in the study, our findings may not be extensively applicable to this population. Thirdly, the number of women included was small, such that our observations may not be generalized to all individuals with COPD. This sex bias is due to the lower prevalence of COPD in women than in men in our country and the underdiagnoses of COPD in women.Citation38,Citation39 Finally, we do not exclude that previous oxygen therapy or vasodilator treatment in these patients could influence the hemodynamic measurements.
Conclusion
In summary, the present study that analyzed a representative cohort of COPD patients with varying degrees of airflow limitation severity by means of right-heart catheterization shows that PH at rest is uncommon in patients with moderate airflow limitation but has a similar prevalence to patients with severe and very-severe airflow obstruction from other studies, highlighting that airflow limitation is a poor predictor of PH occurrence. In advanced COPD, the coexistence of pulmonary gas exchange impairment is of great influence on the development of PH. In contrast, an abnormal vascular response to exercise was observed in the majority of patients, even in those with mild airflow limitation, highlighting the notion that pulmonary vascular derangement is an early event in the natural history of COPD. Progression of these abnormalities may lead to the development of PH that restrains the increase of CO during exercise, which might contribute to limiting exercise tolerance.
Acknowledgments
The authors would like to thank to Dr Ignasi Garcia-Olivé and Dr Diego Rodriguez for their assistance in the statistical analysis. This paper was supported by grants from the Societat Catalana de Pneumologia, Sociedad Española de Neumología y Cirugía Torácica and Instituto de Salud Carlos III (EC07/90049).
Disclosure
The authors report no conflicts of interest in this work.
References
- BarberàJAPeinadoVISantosSPulmonary hypertension in chronic obstructive pulmonary diseaseEur Respir J200321589290512765440
- HoeperMMBarberàJAChannickRNDiagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertensionJ Am Coll Cardiol2009541 SupplS85S9619555862
- SeegerWAdirYBarberàJAPulmonary hypertension in chronic lung diseasesJ Am Coll Cardiol20136225 SupplD109D11624355635
- Oswald-MammosserMApprillMBachezPEhrhartMWeitzenblumEPulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous typeRespiration1991585–63043101792422
- SimsMWMargolisDJLocalioARPanettieriRAKawutSMChristieJDImpact of pulmonary artery pressure on exercise function in severe COPDChest2009136241241919318664
- ThabutGDauriatGSternJBPulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantationChest200512751531153615888824
- ScharfSMIqbalMKellerCHemodynamic characterization of patients with severe emphysemaAm J Respir Crit Care Med2002166331432212153963
- DoiMNakanoKHiramotoTKohnoNSignificance of pulmonary artery pressure in emphysema patients with mild-to-moderate hypoxemiaRespir Med200397891592012924518
- ChaouatABugnetASKadaouiNSevere pulmonary hypertension and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005172218919415831842
- CutticaMJKalhanRShlobinOACategorization and impact of pulmonary hypertension in patients with advanced COPDRespir Med2010104121877188220547449
- AndersenKHIversenMKjaergaardJPrevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary diseaseJ Heart Lung Transplant201231437338022226804
- KesslerRFallerMWeitzenblumE“Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung diseaseAm J Respir Crit Care Med2001164221922411463591
- RabeKFHurdSAnzuetoAGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- HildeJMSkjørtenIHansteenVHaemodynamic responses to exercise in patients with COPDEur Respir J20134151031104122903957
- BoerrigterBGBogaardHJTripPVentilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertensionChest201214251166117422556320
- PynnaertCLamotteMNaeijeRAerobic exercise capacity in COPD patients with and without pulmonary hypertensionRespir Med2010104112112619577458
- BarberàJARogerNRocaJRoviraIHigenbottamTWRodriguez-RoisinRWorsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary diseaseLancet199634789994364408618485
- RogerNBarberàJARocaJRoviraIGómezFPRodriguez-RoisinRNitric oxide inhalation during exercise in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19971563 Pt 18008069309996
- AgustíAGBarberáJARocaJWagnerPDGuitartRRodriguez-RoisínRHypoxic pulmonary vasoconstriction and gas exchange during exercise in chronic obstructive pulmonary diseaseChest19909722682752298050
- RibasJDíazOBarberàJAInvasive exercise testing in the evaluation of patients at high-risk for lung resectionEur Respir J1998126142914359877504
- RibasJJiménezMJBarberàJAGas exchange and pulmonary hemodynamics during lung resection in patients at increased risk: relationship with preoperative exercise testingChest2001120385285911555520
- PeinadoVIGómezFPBarberàJAPulmonary vascular abnormalities in chronic obstructive pulmonary disease undergoing lung transplantJ Heart Lung Transplant201332121262126924263025
- BlancoIGimenoEMunozPAHemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertensionAm J Respir Crit Care Med2010181327027819875684
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- SaggarRLewisGDSystromDMChampionHCNaeijeRSaggarRPulmonary vascular response to exercise: a haemodynamic observationEur Respir J201239223123422298608
- KovacsGBergholdAScheidlSOlschewskiHPulmonary arterial pressure during rest and exercise in healthy subjects: a systematic reviewEur Respir J200934488889419324955
- MillerMRCrapoRHankinsonJGeneral considerations for lung function testingEur Respir J200526115316115994402
- RocaJSanchisJAgusti-VidalASpirometric reference values for a Mediterranean populationBull Eur Physiopathol Respir19862232172243730638
- RocaJRodriguez-RoisinRCoboEBurgosFPerezJClausenJLSingle-breath carbon monoxide diffusing capacity prediction equations from a Mediterranean populationAm Rev Respir Dis19901414 Pt 1102610322327636
- NelsonLDRutherfordEJPrinciples of hemodynamic monitoringPinskyMRDhainautJFPathophysiologic Foundations of Critical CareBaltimore, MDWilliams and Wilkins1993322
- BarberàJAThe pulmonary vasculature of COPDRennardSIRodríguez-RoisinRHuchonGRocheNClinical management of chronic obstructive lung disease2nd edNew YorkInforma Healthcare2008189205
- HurdmanJCondliffeRElliotCAPulmonary hypertension in COPD: results from the ASPIRE registryEur Respir J20134161292130123018917
- LauEMManesACelermajerDSGalièNEarly detection of pulmonary vascular disease in pulmonary arterial hypertension: time to move forwardEur Heart J201132202489289821616950
- ChristensenCCRygMSEdvardsenASkjønsbergOHRelationship between exercise desaturation and pulmonary haemodynamics in COPD patientsEur Respir J200424458058615459136
- KovacsGOlschewskiABergholdAOlschewskiHPulmonary vascular resistances during exercise in normal subjects: a systematic reviewEur Respir J201239231932821885394
- PeinadoVIPizarroSBarberàJAPulmonary vascular involvement in COPDChest2008134480881418842913
- SkjørtenIHildeJMMelsomMNHansteenVSteineKHumerfeltSPulmonary artery pressure and PaO2 in chronic obstructive pulmonary diseaseRespir Med201310781271127923768734
- MiravitllesMSorianoJBGarcia-RioRPrevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activitiesThorax2009641086386819553233
- AncocheaJMiravitllesMGarcía-RíoFUnderdiagnoses of chronic obstructive pulmonary disease in women: quantification of the problem, determinants and proposals for actionArch Bronconeumol2013496223229 English, Spanish23317767