Abstract
Rationale
Pigment epithelium-derived factor (PEDF) is a 50 kD small secreting glycoprotein that participates in multiple physiological and pathological processes. Recent studies have reported that PEDF plays an important role in inflammatory responses in several diseases. However, the role of PEDF in the pathogenesis of chronic obstructive pulmonary disease (COPD) remains unclear.
Objective
The aim of the present study is to explore the potential relationship between PEDF and COPD.
Methods
We used differential proteomics – stable isotope labeling with amino acids in cell culture – to investigate protein expression profile changes in cigarette smoke extract-treated pulmonary cells and found that the neurotrophic and antiangiogenic protein PEDF was abnormally expressed. Furthermore, Western blotting was used to detect the expression of PEDF in the lung tissue of rats that were exposed to cigarette smoke. Eighty subjects between the ages of 40–90 years, including 20 healthy nonsmokers, ten smoking volunteers, and 50 COPD patients, were recruited from September 2012 until August 2013 in Sichuan Province, People’s Republic of China. We measured the plasma PEDF concentration and classic proinflammatory cytokines by multiplex enzyme-linked immunosorbent assay. In addition, we performed a spirometry examination to diagnose COPD patients and we also analyzed the correlation between PEDF and lung function.
Results
First, we found that the expression of PEDF in cigarette smoke extract-treated cells increased 16.2-fold when compared with the control group. Next, we confirmed that 4 weeks’ exposure to cigarette smoke can upregulate PEDF levels in rat lung tissues. We also discovered that plasma PEDF in COPD patients was significantly increased when compared with either healthy nonsmoking or smoking subjects. Furthermore, circulating PEDF was correlated with inflammatory cytokine and blood neutrophil numbers, but it was reversely associated with a decline in forced expiratory volume in 1 second percent predicted.
Conclusion
Our findings provide a novel link between PEDF and COPD. Elevated PEDF levels may be involved in promoting the development of COPD by performing proinflamma-tory functions.
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease characterized by progressive airflow limitations that are poorly reversible and closely associated with aberrant inflammatory responses to noxious particles.Citation1 Recent research has found in both pulmonary and systemic circulation in COPD patients the release of proinflammatory cytokines, which are considered to play significant roles in the pathogenesis of COPD.Citation2,Citation3
Pigment epithelium-derived factor (PEDF) is a 50 kD small secreting glycoprotein that belongs to a noninhibitory serpin family group. PEDF was first identified as a potent angiostatic cytokine that originated from human retinal pigment epithelium.Citation4 Several other observations showed that PEDF is also expressed in many tissues, such as adipose and lung tissues.Citation5,Citation6 PEDF participates in multiple physiological and pathological processes.Citation7,Citation8 It has been reported that PEDF is a potent endogenous molecule that induces tumor cell apoptosis via the Fas–nuclear factor-kappa B (NF-kB) and caspase families.Citation9,Citation10 Furthermore, increased PEDF has been observed in the peripheral blood of patients with obesity or atherosclerosis.Citation11,Citation12 Finally, a previous study has demonstrated PEDF-induced inflammatory signaling in muscle and fat cells.Citation13 However, whether PEDF is involved in the pathogenesis of COPD is unknown.
The aim of the present research was to explore the potential role of PEDF in COPD. In the present study, we measured PEDF expression in both cigarette smoke extract (CSE)-stimulated epithelial cells and lung tissues of rats exposed to cigarette smoke (CS). We also detected the plasma concentration of PEDF and classic proinflammatory cyto-kines in nonsmoker (NS) controls, healthy smokers (HS), and stable COPD patients. In addition, we performed a spirometry examination and analyzed the correlations between PEDF and lung function in the study subjects. Our findings provided a novel link between PEDF and COPD, suggesting that PEDF is involved in the pathogenesis of COPD.
Methods
Cell culture
Human lung cells (NCI-H292) were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured at 37°C in a 5% CO2 environment in Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen; Thermo Fisher Scientific) and 100 U/mL of penicillin and streptomycin antibiotics (penicillin–streptomycin, Invitrogen; Thermo Fisher Scientific). CSE in RPMI medium was freshly prepared for each experiment, as previously described.Citation14
Animals
Male specific pathogen-free grade Sprague Dawley rats (weighing 180–220 g) were obtained from the Laboratory Animal Center of Sichuan University (Chengdu, People’s Republic of China). Animals had free access to regular food and tap water, and they were maintained on a 12-hour light/12-hour dark cycle at a room temperature of 23°C±2°C. All rat experiments were performed according to the Laboratory Animal Care Guidelines of West China School of Medicine, Sichuan University.
Patient population
Eighty subjects aged from 40 to 90 years were recruited from September 2012 until August 2013 in Sichuan Province, People’s Republic of China. We collected questionnaires and performed physical examinations to obtain the subjects’ background information. We also performed diagnostic tests, including pulmonary function tests, using a Spirotel® spirometer purchased from Mir Medical International Research Srl (Rome, Italy) and chest X-ray to screen COPD patients. Twenty nonsmoking volunteers with normal spirometry results and without a history of cigarette smoking were included as the healthy NS group. Ten smoking volunteers with normal spirometry results and a tobacco smoking history were considered as the HS group. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classifications, subjects with postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <70% were enrolled as the COPD patient group. All subjects taking glucocorticoids (inhaled and oral) and bronchodilators in the previous 3 months were excluded. In addition, participants with respiratory disorders (bronchiectasis, lung cancer, tuberculosis, and chronic bronchitis) or diseases known to be associated with an increase in circulating PEDF (retinopathy) were not enrolled. Our study was approved by the Ethics Committees at the West China School of Medicine, Sichuan University. All subjects provided their signed informed consent.
Measurements of circulating PEDF and blood parameters
We drew the venous peripheral blood of subjects, and the samples were then centrifuged for 10 minutes at 400 g at 4°C. The plasma was collected and stored at −80°C until measurement. Concentrations of PEDF, C-reactive protein (CRP), interleukin (IL)-8, IL-6, and tumor necrosis factor (TNF)-α were determined using Milliplex MAP circulating enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s protocol (Merck KGaA, Darmstadt, Germany).
Blood samples were detected by an SF-3000 blood counter system, and white blood cells were categorized. Blood glucose levels and cholesterol levels were measured via a biochemistry method.
Stable isotope labeling with amino acids in cell culture
Briefly, H292 cells were labeled with a stable isotope when confluence reached approximately 70%. The cells were then starved with FBS and free RPMI medium and incubated with or without CSE for 24 hours. Afterwards, the cells were harvested and the differences in expressing proteins were detected using mass spectrometry-based quantitative proteomics.
Exposure of rats to CS
Cigarettes were purchased from the Cigarette Factory and contained 1.0 mg of nicotine and 14 mg of tar per cigarette (Tianxiaxiu, Chengdu, People’s Republic of China). Animals were handled and exposed to CS, as previously described.Citation15 Briefly, wild-type rats were subsequently exposed to CS (five cigarettes) for 30 minutes twice a day, 6 days per week, and for up to 4 weeks using a mechanical smoking chamber. Sham rats were exposed to ambient air as control. After 4 weeks of CS exposure, the rats were sacrificed and lung tissues were collected for Western blotting.
Western blotting analysis
The lung tissues of the sham and CS rat groups were homogenized with radioimmunoprecipitation assay (RIPA) lysis buffer supplemented with a protease inhibitor cocktail and phenylmethylsulfonyl fluoride (PMSF) (Biocolor BioScience Technology Company, Shanghai, People’s Republic of China). The lysates were centrifuged at 12,000 rpm for 30 minutes at 4°C, and the supernatants were subsequently collected. Concentrations of the total protein were measured by the BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). The procedure was performed as previously described.Citation16 Equal amounts of protein were immunoblotted with anti-PEDF primary antibody. The density of the bands was quantified using ImageJ software and normalized against anti-β-actin an as a control.
Statistical analysis
Values were presented as the mean ± standard error, unless otherwise indicated. All statistical analyses were conducted using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The normal distribution of these data has been confirmed. For multiple comparisons, one-way analysis of variance and least significant difference were used to analyze the experimental data. Pearson’s correlation analysis was used to investigate the correlations between the PEDF levels and inflammatory cytokine or lung function parameters. The association between patient characteristics and PEDF levels was evaluated by multivariate linear regression analysis. P#0.05 was considered statistically significant.
Results
Elevated PEDF levels in CSE-treated pulmonary epithelial cells and in the lung homogenate of rats exposed to CS
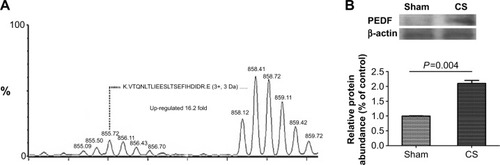
To investigate the candidate proteins involved in the pathogenesis of COPD, we stimulated the pulmonary epithelial cells with CSE. As described in the Methods section, we cultured the human pulmonary epithelium NCI-H292 cell line with/without CSE treatment for 24 hours. Afterwards, the protein expression changes of the samples were identified using a mass spectrometer. We found that CSE dramatically induced PEDF secretion as much as 16.2-fold more than in the untreated cells (). To further confirm this observation, we also collected the lung tissues of rats that were exposed to CS for 4 weeks, and we detected PEDF expression in lung homogenate by Western blotting. It was found that the PEDF protein consistently increased more than twofold in the CS-treated mice when compared with the mice in the sham group (). Considering that cigarette smoking is the most common cause of COPD, our data indicate that PEDF may be involved in sustained inflammation, which is induced by cigarette smoking in COPD.
Figure 1 Cigarette smoke (CS)-induced pigment epithelium-derived factor (PEDF) expression both in vitro and in vivo.
Clinical characteristics of COPD patients
To examine whether PEDF is a potential mediator in COPD pathogenesis, we measured the circulating PEDF levels in recruited healthy participants with or without a history of smoking and also in stable COPD patients. The general clinical characteristics of the healthy volunteers with or without a history of smoking, as well as the COPD patients, are shown in . Subjects in the NS, HS, and COPD groups were age matched, and there were no differences in body mass index, blood pressure, and metabolism parameters. The HS group had more male subjects than did the NS group, but not more than the COPD group. COPD patients had a lower PaO2 level when compared with the NS subjects. In addition, HS had a higher, though not significant, mean cumulative tobacco consumption of 33.00±5.37 packs/year, as compared with the COPD patients, who had a mean tobacco consumption of 21.48±2.46 packs/year.
Table 1 Characteristics of healthy volunteers and COPD patients in stable condition
Circulating PEDF is upregulated in COPD patients
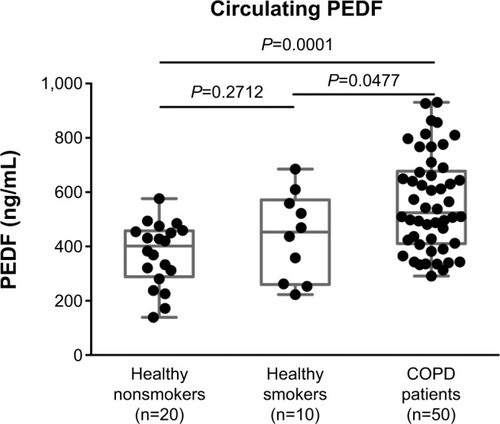
We then measured the PEDF levels using multiplex ELISA. We found that PEDF levels had increased significantly in the plasma of COPD patients in comparison with both the NS and HS groups (551.16±23.66 versus 378.80±27.92 ng/ml; and 551.16±23.66 versus 439.80±51.78 ng/ml, respectively; ). There were no differences found among the healthy volunteers ().
Figure 2 Levels of pigment epithelium-derived factor (PEDF) in the plasma from the healthy nonsmokers group, healthy smokers group, and chronic obstructive pulmonary disease (COPD) group. Circulating PEDF concentrations were measured using multiplex enzyme-linked immunosorbent assay. COPD patients had a significantly elevated PEDF level when compared with those of the healthy nonsmoker and smoking subjects (P=0.0001 and P=0.0477, respectively). There was no difference between the healthy nonsmokers and smokers (P=0.2712). The expression of PEDF is presented as the median (interquartile range) and compared by one-way analysis of variance. A value of P<0.05 was considered to be statistically significant.
PEDF may play a role in mediating the proinflammatory response in COPD
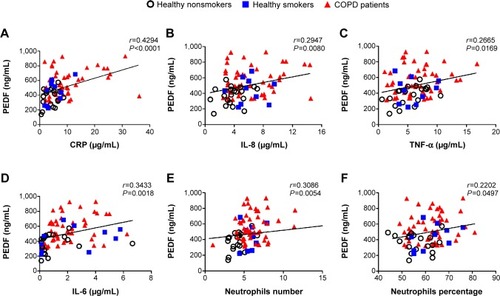
Next, we investigated the relationship between PEDF and COPD inflammatory mediators. In our study, selected inflammation biomarkers, including CRP, IL-8, TNF-α, and IL-6 levels in peripheral blood, were distinguished among the COPD patients, the NS group, and the HS group. In the univariate analysis, there were positive correlations between PEDF and CRP (r=0.4294; P<0.0001), IL-8 (r=0.2947; P<0.008), TNF-α (r=0.2665; P<0.0169), and IL-6 (r=0.3433; P<0.0018). Since neutrophils were reported to be responsible for lung destruction and chronic inflammatory processes, we then analyzed the correlation between PEDF expression and neutrophil count via whole blood analysis. As shown in –F, PEDF levels were positively correlated with neutrophil count and percentage (r=0.3086, P<0.0054; and r=0.2202, P=0.0497, respectively). We also performed a univariate analysis on the PEDF levels in association with the subjects’ age, sex, and smoking pack-years, which were not related (data not shown).
Figure 3 Circulating pigment epithelium-derived factor (PEDF) levels were correlated with established chronic obstructive pulmonary disease (COPD) biomarkers. PEDF concentration was positively correlated with (A) serum C-reactive protein (CRP) (r=0.4294; P<0.0001); (B) interleukin (IL)-8 (r=0.2947; P=0.0080); (C) tumor necrosis factor (TNF)-α (r=0.2665; P=0.0169); and (D) IL-6 (r=0.3433; P=0.018). (E, F) The absolute value of PEDF was also correlated with either neutrophil number or neutrophil percentage (r=0.3086, P=0.0054; r=0.2202, P=0.0497, respectively). The solid line denotes the line of best fit, and Pearson’s correlation coefficient is presented as an r-value.
PEDF is reversely correlated with lung function
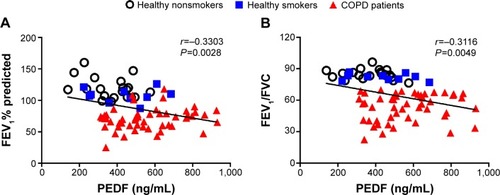
shows the spirometry results of the healthy volunteers and COPD patients. Inverse correlations between PEDF and FEV1 percent predicted (r=−0.3303, P=0.0028; ) and FEV1/FVC (r=−0.3116, P=0.0049; ) were observed.
Table 2 Spirometry results of healthy volunteers and COPD patients in stable condition
Figure 4 Correlation analysis between PEDF levels and pulmonary function.
Abbreviations: PEDF, pigment epithelium-derived factor; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease.
Multivariate linear regression analysis
Finally, we evaluated the interaction of the aforementioned parameters on changes in PEDF levels across all subjects using multivariate linear regression analysis. As shown in , CRP and IL-6 were the independent parameters associated with PEDF.
Table 3 Multivariable linear regression analysis for circulating PEDF in volunteers and COPD patients
Discussion
In the present study, we found that CSE can induce levels of PEDF that are 16 times greater than those in the control groups in cultured epithelial cells. Correspondingly, PEDF expression in rat lungs was upregulated twofold by exposure to CS. These results captured our attention, and we proposed that PEDF may participate in the development of COPD. We then compared the circulating PEDF concentrations in COPD patients in a stable condition with those of their counterpart controls. We first demonstrated that plasma PEDF levels in COPD patients were significantly higher than those in either the healthy nonsmoking or smoking subjects. Furthermore, PEDF was correlated with the presence of classic inflammatory biomarkers in systemic COPD. We also found correlations between PEDF levels and lung function decline. Our results indicated the potential relationship between increased PEDF levels and the inflammation involved in COPD, which implies that PEDF may participate in the pathogenesis of COPD.
The identification of PEDF as a proinflammatory factor is reported in several recent studies.Citation5,Citation13,Citation17 Chavan et alCitation5 observed that the culture medium of 3T3-L1 adipocytes significantly activated macrophages to produce TNF. PEDF was identified as the exact molecule involved in adipocyte-secreted proteins, which regulate macrophages via the PEDF receptor, ATGL/iPLA2. In accordance with the positive correlation found between PEDF and proinflammatory cytokines in our study, it was reported that exogenous PEDF activated the expression of TNF, IL-1, and IL-6 in a dose-dependent manner. Notably, PEDF performed a potent function in regulating multiple inflammatory signaling. The treatment of recombinant PEDF directly induced p38 mitogen-activated protein kinase (p38 MAPK) and extracellular signal regulated kinase (ERK)1 and ERK2.Citation13 In COPD, p38 MAPK and ERK1/2 were revealed to be activated in lung structure and inflammatory cells, strengthening inflammatory responses and weakening steroid responses.Citation18,Citation19 Moreover, NF-kB plays an essential role in COPD to induce the enhancement of multiple inflammatory genes.Citation20 In human vascular smooth muscle cells, PEDF can acutely activate the NF-kB signaling pathway, as well as initiate mTOR and AKT signaling.Citation13 Therefore, the elevation of PEDF levels in COPD patients may play a role in the pathogenesis of COPD by mediating multiple inflammatory signaling processes.
Apoptosis is newly observed in COPD patients and contributes to a loss of pulmonary parenchyma.Citation21,Citation22 The proapoptotic property of cellular PEDF is comprehensively discussed. Recent studies have reported that PEDF participated in regulating classic apoptosis pathways, including the PEDF–Fas/FasL and PEDF–NF–kB–caspase 8 pathways.Citation9,Citation23 Moreover, our unpublished data showed that PEDF was closely correlated with levels of mitochondrial deoxyribo-nucleic acid, which can be released into the extracellular space from apoptotic cells. Suzuki et alCitation24 found decreased levels of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) I in the bronchoalveolar lavage fluid, induced sputum, and serum of COPD patients. The overexpression of PEDF also induced cell death by inhibiting the phosphorylation of VEGF and VEGFR I, leading to the caspase-3 cascade.Citation25,Citation26 These interesting findings showed the possibility that PEDF-induced apoptosis may also contribute to the loss of pulmonary parenchyma in COPD.
We first identified that PEDF was increased in the circulation of COPD patients. The findings of our preliminary research showed the possibility that PEDF could contribute to chronic inflammatory responses in the development of COPD. Although there was an increase in the levels in COPD patients as a group, PEDF does not seem to be significantly related to COPD severity. These results might be influenced by several factors. First, whether the destruction of lung parenchyma contributes to the expression of PEDF levels is unclear. Second, the elevation of systemic mediator expression is generally considered to be the outcome of a “spill-out” of PEDF from the lungs of COPD patients; however, complications may also increase PEDF concentrations in circulation. To confirm this hypothesis, we need to conduct more research on the PEDF levels in the bronchoalveolar lavage fluid of COPD patients and obtain pathological evidence from lung tissues. Another explanation for these results is that we did not have a high enough number of severe COPD patients, and this may have had some influence on the relationship between PEDF levels and COPD severity. Therefore, it is necessary to recruit more stage III and stage IV COPD patients in subsequent studies. A lack of information regarding the molecular mechanisms that regulate PEDF in COPD patients also limits our understanding of the role of this multifunctional protein, which also needs further study.
In conclusion, we first identified that PEDF was induced in either CSE-treated pulmonary epithelial cells or rat lung tissues exposed to CS. Accordingly, we also found that elevated PEDF levels in circulation may be related to the inflammatory response in COPD, and that this can be correlated with the decline in lung function in these patients. Our findings reveal the potential correlation that PEDF may participate in the inflammation involved in COPD.
Author contributions
FW and XL designed experiments. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We are grateful to Professor Wei (State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, West China Medical School, Sichuan University) for support in this work. The authors acknowledge Dr F Guo for comments on the manuscript. Funding: this study was supported by grants 81470236, 31171103, and 8123001 from the National Natural Science Foundation of China to Dr F Q Wen. Patient consent: our patient consent form was approved by the Ethics Committees at West China Hospital, Sichuan University, People’s Republic of China. Consents were signed by the patients. Ethics approval: the Ethics Committees at West China Hospital, West China Medical School, Sichuan University, People’s Republic of China.
Disclosure
The authors have no conflicts of interest to report.
References
- ManninoDMBuistASGlobal burden of COPD: risk factors, prevalence, and future trendsLancet2007370958976577317765526
- CasadevallCCoronellCRamirez-SarmientoALUpregulation of pro-inflammatory cytokines in the intercostal muscles of COPD patientsEur Respir J200730470170717626109
- SinDDManSFWhy are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary diseaseCirculation2003107111514151912654609
- Tombran-TinkJChaderGGJohnsonLVPEDF: a pigment epithelium-derived factor with potent neuronal differentiative activityExp Eye Res19915334114141936177
- ChavanSSHudsonLKLiJHIdentification of pigment epithelium-derived factor as an adipocyte-derived inflammatory factorMol Med201281161116822714715
- CosgroveGPBrownKKSchiemannWPPigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesisAm J Respir Crit Care Med2004170324225115117744
- BecerraSPNotarioVThe effects of PEDF on cancer biology: mechanisms of action and therapeutic potentialNat Rev Cancer201313425827123486238
- CutlerPAkuffoELBodnarWMProteomic identification and early validation of complement 1 inhibitor and pigment epithelium-derived factor: two novel biomarkers of Alzheimer’s disease in human plasmaProteomics Clin Appl20082446747721136851
- HirschJJohnsonCLNeliusTKennedyRRieseWFilleurSPEDF inhibits IL8 production in prostate cancer cells through PEDF receptor/phospholipase A2 and regulation of NFkappaB and PPARgammaCytokine201155220221021570865
- HeSSShiHSYinTAAV-mediated gene transfer of human pigment epithelium-derived factor inhibits Lewis lung carcinoma growth in miceOncol Rep20122741142114822218393
- TaharaNYamagishiSTaharaASerum level of pigment epithelium-derived factor is a marker of atherosclerosis in humansAtherosclerosis2011219131131521733518
- WangPSmitEBrouwersMCPlasma pigment epithelium-derived factor is positively associated with obesity in Caucasian subjects, in particular with the visceral fat depotEur J Endocrinol2008159671371818787046
- FamullaSLamersDHartwigSPigment epithelium-derived factor (PEDF) is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cellsInt J Obes (Lond)201135676277220938440
- HanSXHeGMWangTLosartan attenuates chronic cigarette smoke exposure-induced pulmonary arterial hypertension in rats: possible involvement of angiotensin-converting enzyme-2Toxicol Appl Pharmacol2010245110010720178811
- ChenLSunBBWangTCigarette smoke enhances {beta}-defensin 2 expression in rat airways via nuclear factor-{kappa}B activationEur Respir J201036363864520150208
- YangTLuoFShenYQuercetin attenuates airway inflammation and mucus production induced by cigarette smoke in ratsInt Immunopharmacol2012131738122465384
- KenchegowdaSHeJBazanHEInvolvement of pigment epithelium-derived factor, docosahexaenoic acid and neuroprotectin D1 in corneal inflammation and nerve integrity after refractive surgeryProstaglandins Leukot Essent Fatty Acids2013881273122579364
- PetecchiaLSabatiniFVaresioLBronchial airway epithelial cell damage following exposure to cigarette smoke includes disassembly of tight junction components mediated by the extracellular signal-regulated kinase 1/2 pathwayChest200913561502151219447922
- RendaTBaraldoSPelaiaGIncreased activation of p38 MAPK in COPDEur Respir J2008311626917959643
- RahmanIAdcockIMOxidative stress and redox regulation of lung inflammation in COPDEur Respir J200628121924216816350
- DemedtsIKDemoorTBrackeKRJoosGFBrusselleGGRole of apoptosis in the pathogenesis of COPD and pulmonary emphysemaRespir Res200675316571143
- ComerDMKidneyJCEnnisMElbornJSAirway epithelial cell apoptosis and inflammation in COPD, smokers and nonsmokersEur Respir J20134151058106722878876
- VolpertOVZaichukTZhouWInducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factorNat Med20028434935711927940
- SuzukiMBetsuyakuTNagaiKDecreased airway expression of vascular endothelial growth factor in cigarette smoke-induced emphysema in mice and COPD patientsInhal Toxicol200820334935918300052
- ZhangYHanJYangXPigment epithelium-derived factor inhibits angiogenesis and growth of gastric carcinoma by down-regulation of VEGFOncol Rep201126368168621617872
- CaiJJiangWGGrantMBBoultonMPigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1J Biol Chem200628163604361316339148
