Abstract
Purpose
Screening for abdominal aortic aneurysm (AAA) in “men aged over 65 years who have ever smoked” is a recommended policy. To reduce the number of screenings, it may be of value to define subgroups with a higher prevalence of AAA. Since chronic obstructive pulmonary disease (COPD) and AAA are associated with several common risk factors, this study investigates the prevalence of AAA in COPD patients.
Patients and methods
Patients with COPD were identified via the hospital information system. Inclusion criteria were: COPD stage I–IV, ability to give full consent, and age >18 years; exclusion criteria were: patient too obese for an ultrasound check, previously diagnosed AAA, prior surgery for AAA, or ethical grounds such as concomitant advanced malignant or end-stage disease. The primary endpoint of the study was an aortic diameter measured by ultrasound of ≥30 mm. Defined secondary endpoints were evaluated on the basis of medical records and interviews.
Results
Of the 1,180 identified COPD patients, 589 were included in this prospective study. In 22 patients (3.70%), the aortic diameter was ≥30 mm, representing an AAA prevalence of 6.72% among males aged >65 years. The risk of AAA increased with the following comorbidities/risk factors: male sex (odds ratio [OR] 2.98), coronary heart disease (OR 2.81), peripheral arterial occlusive disease (OR 2.47), hyperlipoproteinemia (OR 2.77), AAA in the family history (OR 3.95), and COPD stage I/II versus IV (OR 1.81).
Conclusion
The overall AAA prevalence of 3.7% in our group of COPD patients is similar to that of the general population aged >65 years. However, the frequency of AAA in male COPD patients aged >65 years is considerably higher (6.72%) and increased further still in those individuals with additional comorbidities/risk factors. Defining subgroups with a higher risk of AAA may increase the efficiency of screening.
Introduction
In the US,Citation1 the UK,Citation2,Citation3 Denmark,Citation4 and Western Australia,Citation5 recommendations for a population-based screening program for abdominal aortic aneurysms (AAA) exist, as the general benefits of this approach have been proven.Citation6–Citation10 To date, such programs have been realized in the US, England, Sweden, Scotland, Northern Ireland, and Norway.Citation11 However, it seems useful to reduce the number of patients to be screened by identifying groups at a higher risk of developing AAA. In a previous study, we found an elevated AAA prevalence of 8.6% in patients who had been treated for arterial disease previously.Citation12 Whether other diseases are also related to AAA remains unclear. The US Preventive Services Task Force (USPSTF) defined the criteria for the identification of persons who should be screened. The main group to screen includes “men aged over 65 years who ever smoked”.Citation13,Citation14 It seems meaningful to look for additional conditions that may be associated with a higher prevalence of AAA. While a higher prevalence of cardiovascular disease in chronic obstructive pulmonary disease (COPD) patients has been shown,Citation15 the figures for AAA in COPD patients are unclear. Therefore, the objective of the present study is to estimate the proportion of hospitalized patients with COPD who are also afflicted by AAA.
Patients and methods
We performed a prospective cross-sectional study in our hospital. All pulmonary department inpatients with a COPD diagnosis were identified via the hospital information system. The study was approved by the ethics committee of the Charité – Universitätsmedizin Berlin (reg nr EA4/142/13). All aspects of the study were in accordance with the Declaration of Helsinki (in its current, revised form). A study nurse assessed the identified patients for inclusion and exclusion criteria. Inclusion criteria were: COPD stage I–IV,Citation16 minimum age of 18 years, and ability to give full consent. Patients too obese for an ultrasound check on technical grounds, those with previously diagnosed AAA, and patients who had already undergone surgery for AAA were excluded, as were some patients on ethical grounds (see “Results”).
If patients met the inclusion criteria, the study nurse contacted the responsible physician for further information about the patient and his/her circumstances, in order to avoid contacting patients in a situation not appropriate for discussing a study (eg, concomitant advanced malignant or end-stage disease).
Once the treating medical team had established that the patient was in an appropriate situation and had agreed to the patient being contacted, the patient was informed about the study. After obtaining the patient’s oral and written consent, the following factors were assessed in a standardized interview: smoking status, hypertension, coronary heart disease (CHD), myocardial infarction, peripheral arterial occlusive disease (PAOD), stroke, diabetes, hyperlipoproteinemia (HLP), pulmonary embolism, thrombosis, hyperuricemia, malignancy, abdominal symptoms, and the family history on AAA. Respiratory and other data were extracted from the patient’s record.
Ultrasound screening for AAA was performed by a study nurse (trained on AortaScan®AMI 9700; Verathon, Bothell, WA, USA). AAA was defined as an aortic diameter ≥30 mm. Where an aortic diameter ≥30 mm was found or in cases of ambiguous findings, an angiologist was involved.
Patient characteristics are presented as frequencies and percentages for categorical data, and as means with standard deviations (SDs) or medians with ranges for continuous data. For the analysis, COPD stages I and II were combined. Associations between AAA and suspected risk factors were evaluated by univariate logistic regression; results are presented as odds ratios (ORs) with 95% confidence intervals (CIs) and P-values, which are considered explorative (without adjusting for multiple testing). Analyses were performed in SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
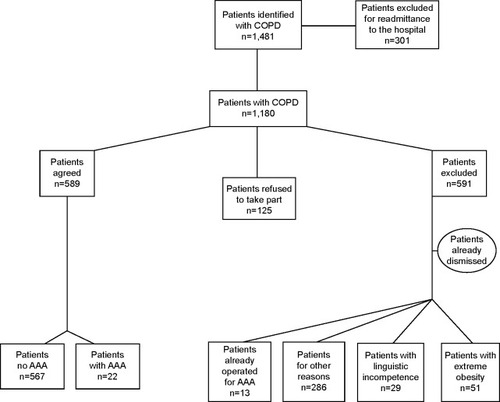
In a 24-month period (February 2011–February 2013), 1,481 patients with COPD were identified via the clinical information system. Of these, 301 patients were excluded because of readmission to the hospital. A total of 1,180 patients thus qualified for the study, of whom 589 agreed to participate and 591 were excluded. Patients were excluded either because they had already received surgery for AAA (13 patients); or on ethical grounds, such as concomitant advanced malignant or end-stage disease (286 patients); or because they did not agree to participate in the study (125 patients). For linguistic reasons, 29 patients were not able to comply with the requirements for giving informed consent and 87 patients had left the hospital prior to being contacted. In 51 patients, obesity rendered an ultrasound check technically impossible ().
Figure 1 Study population flowchart.
The study participants were Caucasian, had a mean age of approximately 70 years (only eight patients below 50 years), a mean body mass index (BMI) of 25.4 kg/m2, and 54.2% were males. The average tobacco consumption was 50.2 pack-years. C-reactive protein (CRP) was elevated on average (26.8 mg/L; normal value <5 mg/L). More than half (56%) of the patients were COPD stage IV, with mean forced expiratory volume in 1 second (FEV1) values of FEV1 0.9 L and FEV1 predicted (pred) 36.8%; maximum vital capacity (VCmax) values of VCmax 2.0l and VCmax pred 57.8%, and FEV1/VCmax 43.2% ().
Table 1 Basic characteristics and pulmonary function
shows the frequency of comorbidities and potential confounding factors in these COPD patients. Patients were predominately current or former smokers (n=546, 92.9%) and had additional comorbidities, such as hypertension (n=369, 63.1%), CHD (n=139, 23.7%), myocardial infarction (n=78, 13.3%), PAOD (n=65, 11.1%), and stroke (n=44, 7.5%). At least one kind of arterial disease was suffered by 210 patients (35.7%). Overall, 109 patients (18.6%) had one arterial disease, 87 patients (14.8%) had two, 13 patients (2.2%) had three, and one patient (0.2%) suffered from four arterial diseases.
Table 2 Comorbidities and potential contributory factors (n=589)
In terms of other comorbidities, 141 patients (24%) had diabetes, 59 (10%) had HLP, and 127 patients (22%) had malignancies, of which 91 (72%) were in the lower respiratory tract (mainly lung cancer). Abdominal symptoms were experienced by 43 patients (7.3%), and 16 (2.7%) knew of AAA in their families ().
Among the 589 patients, 22 (3.7%) had an AAA (aortic diameter ≥30 mm; ). Of these 22 patients, 17 (77.0%) were males, 20 (91.0%) were current or former smokers, 14 (63.0%) had hypertension, ten (42.0%) suffered from CHD, four (18.0%) had already had a myocardial infarction, five (23.0%) suffered from PAOD, three (13.6%) had experienced a stroke, four (18.0%) had diabetes, five (23.0%) had HLP, and eight (36.4%) had a malignancy. AAA diameters are summarized in ; in most cases, the diameter was between 30 mm and 45 mm. The prevalence of AAA was 5.3% among males and only 1.9% among females. The prevalence rates of AAA among patients aged >65 years were 6.72% and 2.54% for males and females, respectively.
Table 3 Comorbidities of patients with AAA (≥30 mm; n=22)
Table 4 Diameter of identified AAA (≥30 mm)
Of all patients with malignancies, 6.3% were afflicted by AAA. In the case of AAA in the family history, 9.0% of patients had an AAA themselves. Individuals with HLP had an AAA in 8.5% of cases, but only 2.8% of patients with diabetes had an AAA. Of patients with PAOD, 5.3% were identified as carrying an AAA, as were 6.1% of CHD patients, 2.8% of patients with a previous myocardial infarction, and 6.8% of patients with a previous stroke. Of the patients complaining of abdominal symptoms, only 2.5% had an AAA. The proportion of patients with thrombosis, pulmonary embolism, hypertension, or current or former tobacco consumption with AAA in this subgroup ranged between 3.4% and 3.8%, and thus did not differ from the overall median values. COPD in stage I or II was associated with AAA in 4.5% of cases, COPD in stage III in 4.2%, and COPD in stage IV was associated with AAA in 2.5% of patients.
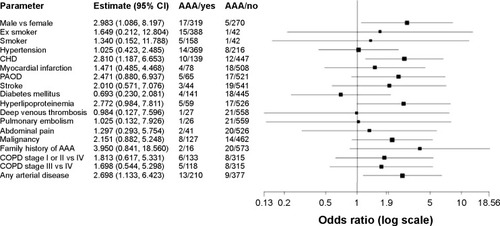
In addition, we looked for further patient data and parameters in the patient’s history that might possibly be associated with a higher prevalence of AAA. Besides age (OR 1.08 per year, P-value 0.004, 95% CI 1.03–1.14) and male sex (OR 3.0, P-value 0.034, 95% CI 1.1–8.2), which were associated with a higher prevalence of AAA; further significant associations were found between CHD (OR 2.8, P-value 0.019, 95% CI 1.2–6.7) and the presence of any arterial disease (OR 2.7, P-value 0.025, 95% CI 1.13–6.42) and AAA. The presence of PAOD alone (OR 2.47, P-value 0.086, 95% CI 0.88–6.94) or HLP (OR 2.8, P-value 0.054, 95% CI 0.98–7.81) also showed an increased risk; however, these effects did not reach statistical significance. Factors tested but not significantly associated with AAA were: BMI (OR 1.02, P-value 0.468, 95% CI 0.96–1.09), cigarette pack-years (OR 1.00, P-value 0.580, 95% CI 0.99–1.01), and CRP (OR 1.00, P-value 0.166, 95% CI 1.00–1.01; ).
Figure 2 Comorbidities and risk factors in COPD.
Abbreviations: CHD, coronary heart disease; PAOD, peripheral arterial occlusive disease; AAA, abdominal aortic aneurysm; COPD, chronic obstructive pulmonary disease; CI, confidence interval; vs, versus.
Discussion
This prospective study of 589 COPD patients showed a prevalence of 3.7% for AAA defined as an aortic diameter ≥30 mm. This prevalence of AAA among COPD patients is similar to that seen in screening studies of the general population, ie, those not limited to COPD patients (eg, the multicenter aneurysm screening study [MASS] 4.9%,Citation3 Lindholt et al 4.0%Citation3,Citation4). If the calculation is based on the same criterion as in the quoted general screening studies – ie, men aged >65 years – the prevalence of 6.72% is considerably higher and very close to the rate of 6.5% recently cited by Divo et al in an overview on COPD comorbidities.Citation17,Citation18 There fore, an amendment to the US recommendations for AAA screening would generate a higher prevalence of AAA if COPD patients were to be defined as a subgroup among “men aged >65 years who have ever smoked” who are particularly worth screening. In addition, some other subgroups of COPD patients with an even higher prevalence of AAA were identified (CHD, HLP, malignoma, positive family history for AAA). This is in accordance with the literature, where various studies showing, eg, a CHD-related prevalence of AAA ranging from 9.0%–14.4% were found.Citation19–Citation22 A highly significant risk factor was sex. To be a male with COPD means having a three-fold higher risk of developing an AAA. In contrast to the situation in males, a Cochrane Review for screening in women found that 3–5 years after screening, women had an OR 1.99 (95% CI 0.36–10.88; versus men OR 0.60, 95% CI 0.47–0.78) as compared to an unscreened population. This means that more women died in the screened population, thus the frequency of ruptured AAA could not be reduced by screening in women.Citation23 The same result was found by Scott et al.Citation24 The results of a recent Swedish study suggest screening subgroups, such as women older than 70 years who have ever smoked.Citation25 It thus seems more relevant to develop a screening program that includes more risk factors than just age, sex, and one major disease like COPD. The combination of the above-mentioned risk factors might help to develop screening strategies that substantially reduce the number of necessary screenings.
In addition to the 22 patients in our study in whom AAA was detected, 13 (3.73%) of the 591 excluded patients had received prior surgery for AAA, suggesting that within the group of excluded patients, AAAs are also present. Of the 22 patients with an aortic diameter ≥30 mm, six (27.0%) had passed the limit of an AAA diameter of 50 mm at which therapeutic options have to be considered. An urgent indication for considering therapy was detected in four patients.
The observed comorbidities are typical for a population with a high prevalence of smokers or ex-smokers with a relatively high average of 50.2 pack-years. The nicotine load was so high in this population that no difference could be observed for AAA patients. Overall, 65 patients (11.0%) were already afflicted by PAOD, which shows the importance of also screening COPD patients for this disease, as well as for other vascular diseases like CHD (23.7%). These anamnestic hints agree with reports in the literature about a higher prevalence of AAA in patients with CHDCitation19–Citation22 or noncardiac arterial disease.Citation12,Citation26
The Lung Health Study has shown a close relationship between COPD and an increased risk of cardiovascular disease and death.Citation27 This was confirmed by a recently published investigation in which 65% of men and 55% of women with COPD were afflicted by cardiovascular disease. In the latter study, cardiovascular diseases (all grouped together, ie, not separated into subgroups) were not a factor contributing to the reduction in COPD patients’ health-related quality of life.Citation28 Inflammation seems to play a major common role in COPD and atherosclerosis.Citation29 A study on the association of aortic aneurysms and reduced lung function with risk factors such as cigarette smoking, cardiovascular disease, and increased inflammatory and hemostatic activity found that individuals with AAA had a higher frequency of impaired lung function and a greater incidence of COPD compared to controls without an aneurysm.Citation30 In this study, the markers of activated inflammation and hemostasis had the most important effect. The main factors for the identification of an activation of inflammation and hemostasis were plasmin–antiplasmin complexes and D-dimers. Although impaired lung function – determined by FEV1, FEV1/forced vital capacity (FVC), and peak expiratory flow rate (PEFR) – could be demonstrated in aneurysm patients compared to a control group with PAOD, cigarette smoking and cardiovascular disease in COPD patients were not as strongly related to aneurysm formation as these markers of inflammation. The authors hypothesized that activation of inflammation and hemostasis in response to injury may be an important explanation for the association between aneurysm formation and reduced respiratory function. The higher incidence of COPD in aneurysm patients was shown in a Dutch case–control study, where the FEV1/FVC ratio was reduced in an AAA group versus PAOD patients. This was apparent in current, former, and never smokers; the largest difference was found for the latter group. The authors concluded that the pronounced airway obstruction cannot be explained by cigarette smoking alone. They suggest that other factors in addition to smoking are responsible for the association between COPD and AAA.Citation31 Their analysis of the factors underlying development of COPD and AAA is in accordance with the results of an earlier study, where the largest difference in the FEV1/FVC ratio was also found in the group of never smokers.Citation32 This association could not be assessed in the present study, due to the lack of a sufficiently large control group of never smokers.
Smoking is not only a risk factor for AAA, but also for CHD. However, the relationship between impaired lung function and AAA is tighter than the relationship between decreased lung function and CHD.Citation31,Citation32 The question is, whether both pathologies are part of a process that Fabbri and Rabe call “chronic systemic inflammatory syndrome”,Citation33 a generalized inflammatory process which they assume is responsible for various clinical entities. This inflammation could cause COPD in the lung and AAA in the vascular system. Inflammatory factors such as IL-6, IL1-β, TNF-α, MMP-9, MCP-1, and high-sensitivity CRP are elevated in COPD patients,Citation29 these factors are known to be elevated in AAA patients as well. Reports that statins, with their anti-inflammatory activity, might have a beneficial role in COPD treatment also fit into this pattern.Citation34,Citation35 Statins are known to suppress inflammatory activity in the wall of AAA, although it has not yet been proven that AAA growth can be retarded by statin medication.Citation36–Citation38
In our cohort, an activated level of inflammation with a mean CRP of 26.81 mg/L was found, which would be consistent with the hypothesis of general inflammation, but is certainly not proof of it. We did not find a statistically significant relationship between CRP level and an increased risk of AAA.
Our findings cannot contribute to solving the problem of whether there is a generalized state of inflammation that initiates different clinical diseases or whether one of these diseases leads to an inflammation that subsequently causes other clinical manifestations. Only the third option, ie, that these comorbidities exist independently of each other, seems to be excluded by both our findings and other reports in the literature revealing strong epidemiologic evidence for reduced FEV1 being a marker for cardiovascular mortality, independent of smoking history.Citation39
One limitation of the present study is that out of 1,180 screened COPD patients, only 589 could be included. Therefore, selection bias might be present and may have resulted in a deviation from the “true” prevalence of AAA in this population. However, in half of the excluded patients, the grounds for their noninclusion were ethical. Because these patients are unlikely to be screened in a real-life setting, this patient selection should not lower the practical importance of our findings. A further limitation could be the inclusion of hospital inpatients only, who might have more severe comorbidities than outpatients with COPD. The current study group contained twice as many COPD patients with diabetes, almost four-times as many patients with CHD, and approximately 12-times as many individuals with malignancies as COPD patients observed by general practitioners.Citation15
Conclusion
The prevalence of AAA in a group of hospitalized COPD patients is similar to that of the general population aged over 65 years. However, identifying risk factors such as male sex and CHD may significantly increase the efficiency of screening for AAA. Other factors, such as HLP, PAOD, malignancy, and/or a positive family history for AAA also increased the risk for AAA, but failed to reach statistical significance.
Disclosure
The authors report no conflicts of interest in this study.
References
- U.S. Preventive Services Task ForceScreening for abdominal aortic aneurysm: recommendation statementAnn Intern Med2005142319820215684208
- ScottRATisiPVAshtonHAAllenDRAbdominal aortic aneurysm rupture rates: a 7-year follow-up of the entire abdominal aortic aneurysm population detected by screeningJ Vasc Surg19982811241289685138
- Multicentre Aneurysm Screening Study GroupMulticentre aneurysm screening study (MASS): cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trialBMJ200232573731135114212433761
- LindholtJSJuulSFastingHHennebergEWScreening for abdominal aortic aneurysms: single centre randomised controlled trialBMJ2005330749475015757960
- NormanPEJamrozikKLawrence-BrownMMPopulation based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysmBMJ200432974771259126515545293
- DabareDLoTTMcCormackDJKungVWWhat is the role of screening in the management of abdominal aortic aneurysms?Interact Cardiovasc Thorac Surg201214439940522268069
- BöcklerDLangWDebusESRandomisierte studien mit EBM-level 1 beweisen es: ein screening programm für abdominelle aortenaneurysmen ist sinnvoll! [Randomised studies with EBM level 1 prove it. A screening programme for abdominal aortic aneurysms makes sense]Gefässchirurgie200914350361
- EcksteinHHBocklerDFlessenkamperISchmitz-RixenTDebusSLangWUltrasonographic screening for the detection of abdominal aortic aneurysmsDtsch Arztebl Int20091064165766319946430
- FerketBSGrootenboerNColkesenEBSystematic review of guidelines on abdominal aortic aneurysm screeningJ Vasc Surg20125551296130421324630
- MollFLPowellJTFraedrichGEuropean Society for Vascular SurgeryManagement of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgeryEur J Vasc Endovasc Surg201141suppl 1S1S5821215940
- StatherPWSidloffDARhemaIAChokeEBownMJSayersRDA review of current reporting of abdominal aortic aneurysm mortality and prevalence in the literatureEur J Vasc Endovasc Surg201447324024224368205
- FlessenkamperIKendziaAStalkeJMultizentrisches Screening eines arteriell vorerkrankten patientenkollektivs in hinblick auf die prävalenz infrarenaler aortenaneurysmen. BARE – Berliner aeurysma raten evaluation. [Multicenter aortic aneurysm screening trial in an arterial sick cohort. BARE – Berlin aneurysm rate evaluation]Gefaesschirurgie2009145376383
- FlemingCWhitlockEPBeilTLLederleFAScreening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. preventive services task forceAnn Intern Med2005142320321115684209
- Guirguis-BlakeJMBeilTLSengerCAWhitlockEPUltrasonography screening for abdominal aortic aneurysms: a systematic evidence review for the U.S. preventive services task forceAnn Intern Med2014160532132924473919
- CazzolaMBettoncelliGSessaECricelliCBiscioneGPrevalence of comorbidities in patients with chronic obstructive pulmonary diseaseRespiration201080211211920134148
- BakkePSRonmarkEEaganTEuropean Respiratory Society Task ForceRecommendations for epidemiological studies on COPDEur Respir J20113861261127722130763
- SchnellKWeissCOLeeTThe prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999–2008BMC Pulm Med2012122622695054
- DivoMJMartinezCHManninoDMAgeing and the epidemiology of multimorbidityEur Respir J20144441055106825142482
- Dall’OlmoCIppolitoAMcIlduffJKinningWFortinGEPics I study: evaluation of possible abdominal aortic aneurysms (in patients who have undergone previous CABG)Vasc Dis Manag20074116
- DurieuxRvan DammeHLabropoulosNHigh prevalence of abdominal aortic aneurysm in patients with three-vessel coronary artery diseaseEur J Vasc Endovasc Surg201447327327824456737
- LongABuiHTBarbeCPrevalence of abdominal aortic aneurysm and large infrarenal aorta in patients with acute coronary syndrome and proven coronary stenosis: a prospective monocenter studyAnn Vasc Surg201024560260820371161
- MonneyPHayozDTinguelyFHigh prevalence of unsuspected abdominal aortic aneurysms in patients hospitalised for surgical coronary revascularisationEur J Cardiothorac Surg2004251656814690734
- CosfordPALengGCScreening for abdominal aortic aneurysmCochrane Database Syst Rev20072CD00294517443519
- ScottRABridgewaterSGAshtonHARandomized clinical trial of screening for abdominal aortic aneurysm in womenBr J Surg200289328328511872050
- SvensjoSBjorckMWanhainenACurrent prevalence of abdominal aortic aneurysm in 70-year-old womenBr J Surg2013100336737223192439
- KentKCZwolakRMEgorovaNNAnalysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individualsJ Vasc Surg201052353954820630687
- AnthonisenNRConnettJEEnrightPLManfredaJHospitalizations and mortality in the lung health studyAm J Respir Crit Care Med2002166333333912153966
- SundhJJohanssonGLarssonKComorbidity and health-related quality of life in patients with severe chronic obstructive pulmonary disease attending Swedish secondary care unitsInt J Chron Obstruct Pulmon Dis20151017318325653516
- HanMKMcLaughlinVVCrinerGJMartinezFJPulmonary diseases and the heartCirculation2007116252992300518086941
- FowkesFGAnandanCLLeeAJReduced lung function in patients with abdominal aortic aneurysm is associated with activation of inflammation and hemostasis, not smoking or cardiovascular diseaseJ Vasc Surg200643347448016520158
- MeijerCAKokjeVBvan TongerenRBAn association between chronic obstructive pulmonary disease and abdominal aortic aneurysm beyond smoking: results from a case-control studyEur J Vasc Endovasc Surg201244215315722705161
- SakamakiFOyaHNagayaNKyotaniSSatohTNakanishiNHigher prevalence of obstructive airway disease in patients with thoracic or abdominal aortic aneurysmJ Vasc Surg2002361354012096254
- FabbriLMRabeKFFrom COPD to chronic systemic inflammatory syndrome?Lancet2007370958979779917765529
- JandaSParkKFitzGeraldJMEtminanMSwistonJStatins in COPD: a systematic reviewChest2009136373474319376844
- SoysethVBrekkePHSmithPOmlandTStatin use is associated with reduced mortality in COPDEur Respir J200729227928317050558
- AssarANMedical treatment of small abdominal aortic aneurysmJ Cardiovasc Surg (Torino)2012534517525
- RughaniGRobertsonLClarkeMMedical treatment for small abdominal aortic aneurysmsCochrane Database Syst Rev20129CD00953622972146
- van der MeijEKoningGGVriensPWA clinical evaluation of statin pleiotropy: statins selectively and dose-dependently reduce vascular inflammationPLoS One201381e5388223349755
- SinDDWuLManSFThe relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literatureChest200512761952195915947307
