Abstract
Background
In the 2014 Global initiative for chronic Obstructive Lung Disease guidelines, bronchiectasis was for the first time defined as a comorbidity of chronic obstructive pulmonary disease (COPD), and this change has been retained in the 2015 update, which emphasizes the influence of bronchiectasis in the natural history of COPD. The present meta-analysis was aimed at summarizing the impact of bronchiectasis on patients with COPD.
Methods
Databases including Embase, PubMed, and the Cochrane Central Register of Controlled Trials were searched comprehensively to identify all relevant human clinical studies published until August 2014. Bronchiectasis was confirmed either by computed tomography or high-resolution computed tomography. One or more clinicopathological or demographical characteristics, including age, sex, smoking history, daily sputum production, exacerbations, inflammatory biomarkers, lung function, and colonization by potentially pathogenic microorganisms (PPMs), were compared between COPD patients with and without bronchiectasis.
Results
Six observational studies with 881 patients were included in the meta-analysis. The mean prevalence of bronchiectasis in patients with COPD was 54.3%, ranging from 25.6% to 69%. Coexistence of bronchiectasis and COPD occurred more often in male patients with longer smoking history. Patients with COPD and comorbid bronchiectasis had greater daily sputum production, more frequent exacerbation, poorer lung function, higher level of inflammatory biomarkers, more chronic colonization by PPMs, and higher rate of Pseudomonas aeruginosa isolation.
Conclusion
In spite of the heterogeneity between included studies and detectable publication bias, this meta-analysis demonstrated the impact of bronchiectasis in patients with COPD in all directions, indicating that coexistence of bronchiectasis should be considered a pathological phenotype of COPD, which may have a predictive value.
Introduction
With the increased use of computed tomography (CT) in the assessment of chronic obstructive pulmonary disease (COPD), the presence of coexisting bronchiectasis is being identified more frequently. Several studies have revealed a high prevalence of bronchiectasis in patients with COPD, ranging from 20% to 69%.Citation1–Citation4 In the 2014 Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines, bronchiectasis was for the first time defined as a comorbidity of COPD, and this change has been retained in the 2015 update,Citation5 which emphasizes the influence of bronchiectasis in the natural history of COPD.Citation6
There are evidences that bronchiectasis influences the clinical outcomes of COPD. Studies have shown that patients with COPD and comorbid bronchiectasis have higher risk of becoming chronic sputum producers, have more purulent sputum, have more airway or systemic inflammation, and have more exacerbations.Citation1–Citation3,Citation7–Citation10
The worse clinical outcomes in patients with COPD and comorbid bronchiectasis may be associated with chronic colonization of potentially pathogenic microorganisms (PPMs). In earlier studies, PPMs were isolated more frequently from the sputum of patients with COPD and comorbid bronchiectasis during the stable phase,Citation7,Citation9 as well as during acute exacerbation.Citation3,Citation10
However, in previous studies, controversial results on clinical outcomes, such as lung function and exacerbations, have been reported with relatively small patient populations (44–118 patients).Citation1,Citation4,Citation7,Citation9,Citation10,Citation11–Citation17 Hence, the present meta-analysis was aimed at summarizing the reports on the comorbidity of bronchiectasis and COPD and further clarifying the impact of bronchiectasis on patients with COPD.
Methods
The recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) were followed for conducting and the reporting of this review.Citation18 All analyses were performed on data of previously published studies, and thus no ethical approval and patient consent were required.
Search strategy for identification of studies
A literature search was performed to identify the clinical studies that addressed the impact of bronchiectasis in patients with COPD. Databases including Embase, PubMed, and the Cochrane Central Register of Controlled Trials were searched comprehensively to identify all relevant human clinical studies published until August 2014. The search strategy used was as follows: ([“pulmonary disease, chronic obstructive”] OR [“pulmonary” AND “disease” AND “chronic” AND “obstructive”] OR [“chronic obstructive pulmonary disease”] OR [“COPD” AND (“bronchiectasis”) AND English [language]).
Study selection
Two investigators independently obtained the full texts of potentially eligible manuscripts based on their titles and abstracts. To avoid the double counting of patients recruited in more than one study, the authors were contacted for inquiry when necessary.
Inclusion and exclusion criteria
Manuscripts were included in this meta-analysis if they met the following criteria: 1) According to the GOLD guidelines, COPD was defined as follows: postbronchodilator ratio of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) of <70% in a patient with a smoking history of >10 pack-years;Citation6 2) bronchiectasis was confirmed either by CT or high-resolution CT (HRCT), and patients with known cause of bronchiectasis were excluded; and 3) one or more clinicopathological or demographical characteristics, including age, sex, smoking history, daily sputum production, exacerbations, inflammatory biomarkers, lung function, and colonization by PPMs, were compared between COPD patients with and without bronchiectasis. Conference abstracts, case reports, editorials, and narrative reviews were excluded in this meta-analysis.
Data extraction
Two investigators independently extracted the following data from the selected studies: the first author’s name and year of publication; patients’ age, sex, smoking history, daily sputum production, and exacerbations in previous year; albumin and C-reactive protein (CRP) levels; postbronchodilator FEV1% predicted and postbronchodilator ratio of FEV1/FVC; and rate of PPM colonization and rate of Pseudomonas aeruginosa isolation. The quality of included studies was evaluated according to the Agency for Healthcare Research and Quality standard.Citation19
Statistical analysis
The meta-analysis was conducted using the open source Review Manager (RevMan) software (Version 5.3.4, available at the Web site http://ims.cochrane.org/revman/download). Irrespective of the presence of heterogeneity between studies, the random-effects model was used to combine individual effect-size estimates. The relationship between clinicopathological or demographical profiles, including sex, daily sputum production, rate of PPM colonization, and rate of P. aeruginosa isolation, and coexistence of bronchiectasis in COPD were assessed using the inverse variance method, and effect estimates were assessed as odds ratio (OR)Citation20 and 95% confidence interval (95% CI). Differences in age, smoking history, exacerbation in previous year, levels of albumin and CRP, postbronchodilator FEV1% predicted, and postbronchodilator ratio of FEV1/FVC between COPD patients with and without bronchiectasis were estimated using the inverse variance method, and contrasts were expressed in the form of weighted mean difference (WMD) and 95% CI. For those manuscripts without standard deviation (SD), an average SD was estimated using the methods described by Hozo et al.Citation21
To test the interstudy heterogeneity, the chi-square value was calculated for the assumption of homogeneity. The fixed-effects model was chosen when there was no heterogeneity, while the random-effects model was chosen when there was heterogeneity. Publication bias was assessed using funnel plots.
Results
Search results
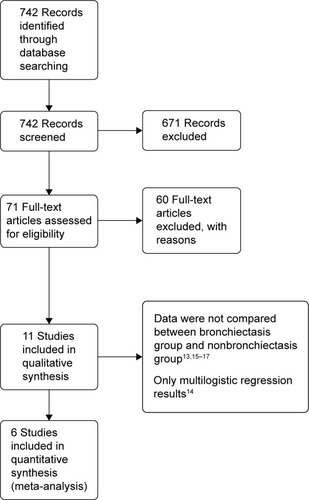
Through database search, 742 potentially relevant articles were identified. After reviewing the abstracts and full texts, 12 manuscripts about bronchiectasis in patients with COPD were found.Citation1,Citation4,Citation6,Citation11–Citation17,Citation21,Citation22 Of those, six studies met the inclusion criteria, which included patients with COPD and comorbid bronchiectasis (n=550) and patients with COPD and without comorbid bronchiectasis (n=331).Citation1,Citation4,Citation7,Citation11–Citation13 The flow diagram of the database search is represented in .
The mean prevalence of bronchiectasis in patients with COPD was 54.3% (range: 25.6%–69%). HRCT was used to diagnose bronchiectasis in all studies, except in the study by Gatheral et alCitation4 wherein a part of patients were diagnosed with bronchiectasis using CT images of 2–3 mm slice thickness (134 patients out of 406 patients, 33%) or 5–7 mm slice thickness (106 patients out of 406 patients, 26%). In their study, the comparative analysis of results of CT images with different slice thicknesses showed no underdetection of abnormalities in thicker CT sections; therefore, their analysis was performed irrespective of the used CT slice thickness. The characteristics of all included studies are summarized in and . The quality evaluation of included studies is shown in .
Table 1 Characteristics of included studies
Table 2 Radiological characteristics of COPD patients in the included studies
Table 3 Evaluation of quality of included studies
Demographic characteristics
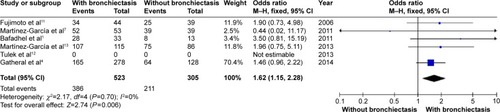
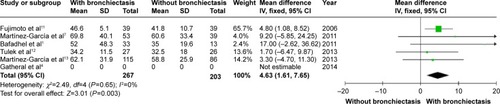
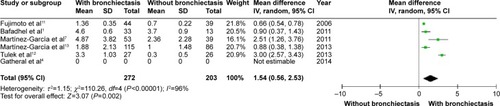
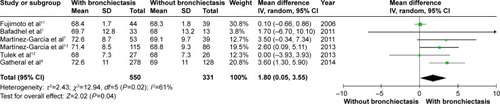
Patients with COPD and comorbid bronchiectasis were older than those without bronchiectasis (WMD: 1.8 years; 95% CI: 0.05 to 3.55; P=0.04; ); although there was a publication bias detected in the funnel plot (Figure S1). Meta-analysis of five studies providing sex information showed a higher prevalence of coexistence of bronchiectasis in male COPD patients (OR: 1.62; 95% CI: 1.15 to 2.28; P=0.006; ). Smoking history in COPD patients with bronchiectasis was also significantly longer than those without bronchiectasis (WMD: 4.63 pack-years; 95% CI: 1.61 to 7.65; P=0.003; ). Funnel plots of these two parameters showed no observable publication bias (Figures S2 and S3).
Figure 2 Forest plot of mean difference of age in COPD patients with and without bronchiectasis.
Abbreviations: CI, confidence interval; SD, standard deviation; COPD, chronic obstructive pulmonary disease; IV, inverse variance.
Clinical features
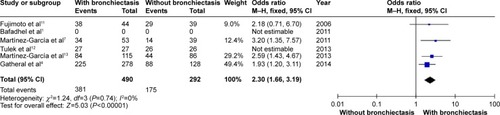
and show that patients with COPD and comorbid bronchiectasis exhibited more daily sputum production (OR: 2.30; 95% CI: 1.66 to 3.19; P<0.00001; ) and more exacerbations (WMD: 1.54 times in the previous year; 95% CI: 0.56 to 2.53; P=0.002; ) than COPD patients without bronchiectasis. Publication bias was observed in exacerbation, but not in daily sputum production (Figures S4 and S5).
Lung function
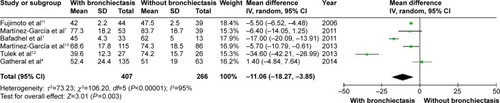
COPD patients with bronchiectasis showed a lower FEV1/FVC ratio (WMD: −8.05%; 95% CI: −10.65 to −5.45; P<0.00001; ), and airway obstruction was more severe with a lower postbronchodilator FEV1% predicted in this group of patients (WMD: −11.06; 95% CI: −18.27 to −3.85; P=0.003; ). However, funnel plots showed a significant publication bias in the FEV1/FVC ratio and postbronchodilator FEV1% predicted (Figures S6 and S7).
Figure 7 Forest plot of mean difference of postbronchodilator FEV1/FVC% in COPD patients with and without bronchiectasis.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IV, inverse variance; SD, standard deviation.
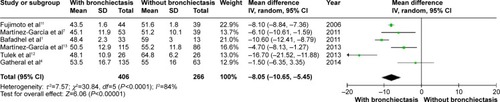
Figure 8 Forest plot of mean difference of postbronchodilator FEV1% predicted in COPD patients with and without bronchiectasis.
Inflammatory biomarkers
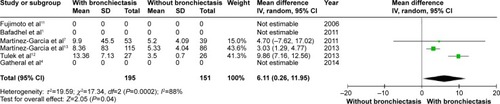
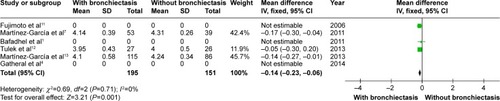
CRP level was higher in COPD patients with bronchiectasis compared with those without bronchiectasis (WMD: 6.11; 95% CI: 0.26 to 11.95; P=0.04; ). However, a significant publication bias was detectable by funnel plots (Figure S8). Albumin level was lower in COPD patients with bronchiectasis compared with those without the same (WMD: −0.14; 95% CI: −0.23 to −0.06; P=0.001; ), and no publication bias was observed (Figure S9).
Microbiological study
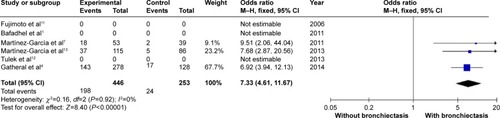
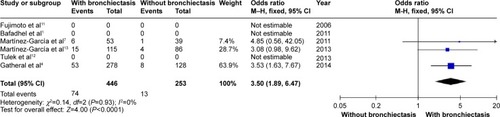
COPD patients with bronchiectasis were at increased risk of having chronic PPM colonization, compared with those without bronchiectasis (OR: 7.33; 95% CI: 4.61 to 11.67; P<0.00001; ). Moreover, P. aeruginosa was more frequently isolated in COPD patients with bronchiectasis (OR: 3.5; 95% CI: 1.89 to 6.47; P<0.0001; ). Funnel plots showed no observable publication bias for these two variables (Figures S10 and S11).
Sensitivity analysis
In the study by Gatheral et alCitation4 HRCT was not performed for each enrolled patient, which might lead to underestimation of the prevalence of bronchiectasis, although the authors had confirmed that there was no underdetection of bronchiectasis in their study. As the study by Gatheral et alCitation4 had a significant weightage in meta-analysis, a sensitivity analysis was carried out for five studies excluding the study by Gatheral et al.Citation4 The sensitivity analysis showed that the meta-analysis results on age, sex, daily sputum production, lung function, and PPM isolation did not change after excluding the study by Gatheral et al (Figures S12–S18).
Discussion
COPD is an airway disease with a variable clinical course that is not well predicted based solely on the usual objective tests such as spirometry.Citation5 Research has been conducted to identify specific COPD phenotypes. An emerging area of COPD phenotype research is the use of radiologic imaging to categorize patients into different phenotypes based on the degree of emphysema and bronchial wall thickening, a prominent imaging feature of bronchiectasis.Citation1,Citation11,Citation25–Citation27 The “emphysema hyperinflation phenotype” was relatively well established in previous studies, defining patients with COPD who present with dyspnea and intolerance to exercise as the predominating symptoms, accompanied by signs of hyperinflation.Citation28 However, the role of bronchiectasis in COPD remains unclear. In this meta-analysis, we tried to clarify the impact of coexistence of bronchiectasis in patients with COPD.
The present study as well as previous studies showed a high rate of coexistence of bronchiectasis and COPD, which could be explained by overlapped pathological mechanisms. In both bronchiectasis and COPD, neutrophils and T lymphocytes are the predominant inflammatory cells;Citation29,Citation30 the neutrophils release proteases, which cause pulmonary structure damage, whereas T lymphocytes lead to enlargement of peribronchial lymph nodes and chronic airway inflammation, resulting in airflow obstruction.Citation31,Citation32 In contrast, only 4% of COPD patients in the ECLIPSE cohort had bronchiectasis, which may be secondary to the fact that patients with other pulmonary conditions were excluded.Citation33
In the present study, it was shown that coexistence of bronchiectasis and COPD happened more often in elder male patients with longer smoking history. This finding is consistent with the consideration that tobacco exposure is one of the most important causes of chronic bronchiolitis, the latter being considered to be the fundamental pathogenic mechanism of bronchiectasis.Citation34–Citation36
The most significant results of the meta-analysis were the high OR of chronic PPM colonization and P. aeruginosa isolation (7.33 and 3.59, respectively). However, it should be noted that “chronic colonization” was replaced by “persistent infection” in the study by Gatheral et alCitation4 this replacement could lead to the increased rate of chronic PPM colonization in the present meta-analysis. However, sensitivity analysis showed no difference after excluding the study by Gatheral et al.Citation4 Previous studies have not confirmed the significant association between P. aeruginosa and bronchiectasis in patients with COPD.Citation37 With this meta-analysis of patients with COPD (n=742), the association of chronic PPM colonization, as well as P. aeruginosa isolation, with bronchiectasis in COPD patients has been definitely determined. According to the hypothesis of Cole,Citation38 it is possible that the presence of PPM and concomitant proteolytic products is responsible for triggering the mechanism that generates bronchiectasis, along with the chronic inflammation of the bronchial mucosa secondary to this phenomenon in some patients with COPD. Moreover, P. aeruginosa, one of the most important PPMs, has been isolated from 3% to 20% of patients with COPD and, more frequently, from patients with severe disease and during exacerbations.Citation39–Citation42 P. aeruginosa isolation has been associated with higher 3-year mortality, more hospital admissions in the previous year, higher BODE index scores, and more systemic steroid treatment.Citation9,Citation43 In the present meta-analysis, two of the included studies provided information about treatment during exacerbation, which indicated that patients with COPD needed more acute oral steroid treatments along with acute antibiotic treatments.Citation7,Citation13 Unfortunately, data were not enough to carry out a meta-analysis about the exacerbation treatments.
Permanent dilatation of the airways and impairment of mucociliary clearance may result in bacterial colonization in patients with bronchiectasis. Chronic airway infection, in turn, triggers an intense inflammatory response.Citation29 Higher levels of sputum interleukin (IL)-6 and IL-8 were associated with coexistence of bronchiectasis in COPD.Citation9 Due to the lack of enough studies, meta-analysis could not be performed on airway inflammatory cytokines. Thus, we tried to carry out the meta-analysis of some systemic biomarkers. The results showed a lower albumin level and a higher CRP level in patients with COPD and comorbid bronchiectasis, indicating a higher level of acute phase protein and anti-acute phase protein.
Bronchiectasis, as a result of the interaction of chronic PPM colonization and systemic inflammation, leads to distinctive clinical features, including more sputum production, frequent exacerbations, and more severe airway obstruction, as indicated in the present meta-analysis. As Martínez-García et alCitation13 mentioned in their study, the presence of bronchiectasis in patients with COPD has a greater correlation with the parameters of patients with COPD with chronic bronchitis phenotype (thicker bronchial wall, greater daily sputum production, and a high number of exacerbations) than those with the emphysematous phenotype.
All the above-mentioned factors may lead to an elevated mortality in patients with COPD and comorbid bronchiectasis. Regrettably, only a few studies described the mortality data, and they were not enough to conduct a meta- analysis. The study of Martínez-García et alCitation13 showed a higher mortality in patients with COPD and comorbid bronchiectasis (n=201), with an adjusted hazard ratio of 1.15. The study of Goeminne et alCitation15 also showed a higher mortality in patients with bronchiectasis and comorbid COPD.
Recently, Hurst et alCitation44 mentioned a new concept named COPD–bronchiectasis overlap syndrome in their review article. Considering the high prevalence of coexistence of bronchiectasis with COPD, as well as its poorer prognosis, the authors suggested that anatomical airway abnormalities of bronchiectasis in patients with COPD are best considered a phenotype of the COPD disease spectrum, and when managed in these patients, some treatments for bronchiectasis exacerbation, such as inhaled antibiotics including antipseudomonal agents, should be considered, but with a shorter course.
There are some limitations in our meta-analysis. First, there were significant biases for several outcomes, including age, exacerbation, lung function, and CRP level, as shown in funnel plots. This may be related to the publication bias and the small number of included studies. The limitation of search language and outcome bias may also play a role in these biases. Second, there were detectable interstudy heterogeneities in the meta-analysis, which were difficult to avoid as the number of included studies was too small. Among the six included studies, CT was used instead of HRCT in one of the studies to assess the patients. Although there was no underdetection of bronchiectasis, as the authors declared in their study, HRCT still showed a higher accuracy in diagnosing minor and mild bronchiectasis.Citation45 Alternatively, different diagnosis criteria between studies may influence the results as well. Third, due to lack of data, meta-analyses of quality of life, treatment, sputum characteristics, blood gas parameters, and mortality were not performed.
Conclusion
Although with some limitations, this meta-analysis highlighted the impact of bronchiectasis in patients with COPD in all directions. Coexistence of bronchiectasis should be considered a pathological phenotype of COPD, which may have a predictive value.
Acknowledgments
We thank Professor Robert A Stockley for providing full text information.
Disclosure
The authors report no conflicts of interest in this work.
References
- BafadhelMUmarIGuptaSThe role of CT scanning in multidimensional phenotyping of COPDChest2011140363464221454400
- O’BrienCGuestPJHillSLStockleyRAPhysiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary careThorax200055863564210899238
- RocheNKouassiBRabbatAYield of sputum microbiological examination in patients hospitalized for exacerbations of chronic obstructive pulmonary disease with purulent sputumRespiration2007741192516675894
- GatheralTKumarNSansomBCOPD-related bronchiectasis; independent impact on disease course and outcomesCOPD201411660561424983298
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease2014 Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jun11.pdfAccessed October 10, 2014
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease2014 Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Apr2.pdfAccessed May 11, 2015
- Martínez-GarcíaMÁSoler-CataluñaJJDonat SanzYFactors associated with bronchiectasis in patients with COPDChest201114051130113721546440
- ParrDGGuestPGReynoldsJHPrevalence and impact of bronchiectasis in alpha1-antitrypsin deficiencyAmerican Journal of Respiratory and Critical Care Medicine2007176121215122117872489
- PatelISVlahosIWilkinsonTMBronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary diseaseAmerican Journal of Respiratory and Critical Care Medicine2004170440040715130905
- Garcia-VidalCAlmagroPRomaníVPseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective studyEuropean Respiratory Journal20093451072107819386694
- FujimotoKKitaguchiYKuboKHondaTClinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomographyRespirology200611673174017052301
- TulekBKivrakASOzbekSPhenotyping of chronic obstructive pulmonary disease suing the modified Bhalla scoring system for high-resolution computed tomographyCan Respir J2013202919623616965
- Martínez-GarcíaMAde la Rosa CarrilloDSoler-CataluñaJJPrognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary diseaseAmerican Journal of Respiratory and Critical Care Medicine2013187882383123392438
- GallegoMPomaresXEspasaMPseudomonas aeruginosa isolates in severe chronic obstructive pulmonary disease: characterization and risk factorsBMC Pulmonary Medicine20141410324964956
- GoeminnePCNawrotTSRuttensDMortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysisRespiratory Medicine2014108228729624445062
- LinSHJiBCShiYMComorbid pulmonary disease and risk of community-acquired pneumonia in COPD patientsInt J Tuberc Lung Dis201317121638164424200282
- KimSSSeoJBLeeYHChronic obstructive pulmonary disease: lobe-based visual assessment of volumetric CT by using standard images – comparison with quantitative CT and pulmonary function test in the COPD gene studyRadiology2013266262663523220894
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementInt J Surg20108533634120171303
- RostomADubeCCranneyACeliac DiseaseRockville (MD)Agency for Healthcare Research and Quality (US)20049Evidence Reports/Technology Assessments, 104 Appendix D. Quality Assessment Forms. Available from: http://www.ncbi.nlm.nih.gov/books/NBK35156
- SweetingMJSuttonAJLambertPCWhat to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse dataStat Med20042391351137515116347
- HozoSPDjulbegovicBHozoIEstimating the mean and variance from the median, range, and the size of a sampleBMC medical research methodology200551315840177
- Gon calvesaJRPereirabMCDe CerqueiraaEPSevere obstructive disease: Similarities and differences between smoker and non-smoker patients with COPD and/or bronchiectasisRev Port Pneumol2013191131823017504
- GoddardPRNicholsonEMLaszloGWattIComputed tomography in pulmonary emphysemaClin Radiol1982333793877083738
- BhallaMTurciosNAponteVCystic fibrosis: scoring system with thin-section CTRadiology19911797837882027992
- HanMKBartholmaiBLiuLXClinical significance of radiologic characterizations in COPDCOPD20096645946719938970
- KinsellaMMüllerNLAbboudRTQuantitation of emphysema by computed tomography using a ‘density mask’ program and correlation with pulmonary function testsChest19909723153212298057
- NakanoYMuroSSakaiHComputed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung functionAmerican Journal of Respiratory and Critical Care Medicine20001623 Pt 11102110810988137
- MiravitllesMCalleMSoler-CataluñaJJClinical phenotypes of COPD: identification, definition and implications for guidelinesArch Bronconeumol2012483869822196477
- FuschilloSDe FeliceABalzanoGMucosal inflammation in idiopathic bronchiectasis: cellular and molecular mechanismsEur Respir J200831239640618238949
- BarnesPJChronic obstructive pulmonary diseaseN Engl J Med2000343426928010911010
- WhitwellFA study of the pathology and pathogenesis of bronchiectasisThorax19527321323912984409
- HoggJCChuFUtokaparchSThe nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med2004350262645265315215480
- AgustiACalverleyPMCelliBEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- BoucherRCRelationship of airway epithelial ion transport to chronic bronchitisProc Am Thorac Soc200411667016113415
- ReidLMReduction in bronchial subdivision in bronchiectasisThorax19505323324714776716
- HansellDMWellsAURubensMBColePJBronchiectasis: functional significance of areas of decreased attenuation at expiratory CTRadiology199419323693747972745
- NovosadSABarkerAFChronic obstructive pulmonary disease and bronchiectasisCurr Opin Pulm Med201319213313923287285
- ColePJInflammation: a two-edged sword the model of bronchiectasisEur J Respir Dis Suppl1986147615
- EnglerKMühlemannKGarzoniCColonization with Pseudomonas aeruginosa and antibiotic resistance patterns in COPD patientsSwiss Med Wkly2012142w1350922290607
- PatelIS1SeemungalTAWilksMRelationship between bacterial colonization and the frequency, character, and severity of COPD exacerbationsThorax200257975976412200518
- GroenewegenKHWoutersEFBacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1-year prospective studyRespir Med200397777077712854626
- MarinAMonsóEGarcia-NuñezMVariability and effects of bronchial colonization in patients with moderate COPDEur Respir J201035229530219643939
- AlmagroPSalvadóMGarcia-VidalCPseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary diseaseRespiration20128413643
- HurstJRElbornJSDe SoyzaACOPD-bronchiectasis overlap syndromeEur Respir J201545231031325653262
- HillATPasteurMCornfordCWelhamSBiltonDPrimary care summary of the British Thoracic Society Guideline on the management of non-cystic fibrosis bronchiectasisPrim Care Respir J201120213514021336465