Abstract
COPD is a common cause of disability, morbidity and mortality worldwide and a major global health problem with enormous direct and indirect health care costs. Different reasons can be advanced to explain it, but among them the possibility that the recommended diagnostic and therapeutic approaches to COPD were less effective than they could be, should be also considered. The pharmacological baseline treatment of stable COPD has been widely based on the severity of airflow obstruction and recently, of chronic symptoms and on the annual number of previous exacerbations. These recommendations do not take into account the underlying prevalent disease that should be treated and the future risk. Our suggestion is that the therapy must be firstly tailored on the prevalent disease leading to COPD, independently from the degree of FEV1 reduction and chronic dyspnea and only after that, according to the severity of the disorder (and age of patient), to establish the level of the treatment in order to freeze, when possible, and not to follow the underlying pathological process, running after it. Moreover, given the relevance of exacerbations in the natural history of COPD, greater effort should be placed on recognition of their prevalent type in frequent exacerbators and to prevent them using more tailored and specific treatment.
Introduction
COPD is a common cause of disability, morbidity, and mortality worldwide and a major global health problem with enormous direct and indirect health care costs.Citation1,Citation2 The worst notice, however, is that the mortality rate (number of deaths per 100,000 persons) for COPD in the last 50 years (at least in USA) has progressively increased in both men and women older than 60 years in every 5-year period.Citation3 Moreover, among the 20 leading causes of deaths in USA, the only one that has substantially increased the age-standardized relative rank of death in the last 20 years is COPD.Citation4 In Europe, the situation seems better, and in many European countries, the mortality rate (number of deaths for 100,000 persons) for COPD is decreasing, particularly in men.Citation5 However, for 100,000 deaths, the percentage of mortality due to COPD is increasing, suggesting that among chronic noncommunicable diseases COPD is less effectively treated.Citation5
Therefore, according to this trend, it is estimated that COPD will become the third worldwide cause of mortality in 2030.Citation6 Of course, there are many causes for this, but among them the possibility that the recommended diagnostic and therapeutic approaches to COPD were less effective than what they could be should be seriously considered.
Definition
Let us start with definition which is a fundamental point. The most disseminated definition says that COPD is a disease.Citation1 In fact, D in the COPD acronym stands for disease. It must be acknowledged, however, that COPD is recognized in the definition as an obstructive functional defect associated with a chronic immune inflammatory process, occurring in the lungs of susceptible individuals with known risk factors, in the absence of other causes. Thus, strictly speaking, D in the COPD acronym should stand for disorder. Since the 1960s, based on pathological findings, it has been clear that similar functional disorder could arise from different diseases, essentially two, one originating from small airways and the other from lung parenchyma.Citation7
Today, we know that chronic bronchiolitis, which is a progressive, proliferative, and fibrosing pathology of the membranous and terminal bronchioli due to persistent inflammatory, immune, and remodeling process, is by far the most common disease leading to COPD.Citation8 In contrast, panlobular emphysema, which is a destructive and hyporegenerative pathology of the alveolar walls with some autoimmune features, is a rare disease that may originate among individuals with severe alpha-1-antitrypsin deficiency and heavy smokers and also leads to COPD.Citation9,Citation10
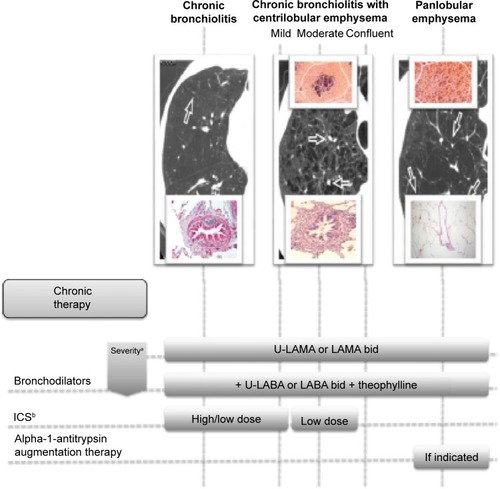
Moreover, chronic bronchiolitis can be frequently associated with centrilobular emphysema, when the inflammatory process involves the respiratory bronchioli with subsequent destruction of the secondary lobule, from the center to periphery. This may configure the presence of mild (<5% of the lung volume with low attenuation area [LAA] <−950 HU, showing round- or oval-shaped LAA with well-defined border), moderate (>5% of the lung volume with LAA <−950 HU, showing polygonal- or irregular-shaped LAA with ill-defined border), or confluent (showing irregular-shaped LAA with ill-defined border coalescent with each other) centrilobular emphysema.Citation11 Actually, centrilobular emphysema does not occur without chronic bronchiolitis, and there is growing evidence that injury of terminal bronchioli would precede the enlargement of centrilobular spaces.Citation12
In a cross-sectional high-resolution computed tomography analysis, it is often impossible to distinguish between panlobular emphysema and confluent centrilobular emphysema, the only distinctive clue being the prevalence of destructive lesions in the lower lobes in the former.
At the stage of severe-to-very severe airflow obstruction, fibrosing chronic bronchiolitis with (same degree of) centrilobular emphysema is the pathological condition most frequently encountered. Although not definitely proved, isolated fibrosing chronic bronchiolitis should be the most common form of COPD at earlier stages of airflow obstruction.
In all circumstances, however, a progressive reduction in maximal expiratory and inspiratory flow rates may develop because of an increase in airway resistance and/or a decrease in pulmonary static elastance. Diminution of the elastic recoil pressure can augment the expiratory flow resistance by distortion and collapse of the small airways because of reduction in tethering forces. This phenomenon is amplified by the loss of airway–parenchyma interdependence due to progressive disappearance of alveolar attachments.
Diagnostic and severity assessment
With the above-mentioned background, in a patient with COPD, the priority should be to define the prevalent underlying disease. Generally, clinical, radiological (chest X-ray), and adequate functional assessments are sufficient to achieve this ( and ), but sometimes this task requires a more extensive work-up including high-resolution computed tomography lung scan.
Table 1 Functional aspects of patients with prevalent chronic bronchiolitis
Table 2 Functional aspects of patients with prevalent emphysema
Is it useful? Many lines of evidence suggest yes. Based on different underlying disease, namely prevalent chronic bronchiolitis versus prevalent pulmonary emphysema, in patients with COPD the effect of anti-inflammatory treatments (inhaled corticosteroids and/or phosphodiesterase 4 inhibitors, for instance) on top of long-acting bronchodilators is different in terms of improvement in function, symptoms, quality of life,Citation13,Citation14 and prevention of exacerbations.Citation15–Citation17 In addition, the functional decline is differently affected by treatment,Citation18,Citation19 the all-cause and respiratory mortality is different in long-term follow-up,Citation20 and finally, the clinical phenotypes and some comorbidities tend to cluster differently.Citation21,Citation22
After that it is mandatory to stage the COPD severity, which cannot rely only on forced expiratory volume in 1 second (FEV1) and, as recently suggested, on symptoms, requiring instead a multidimensional approach not only to have a prognostic evaluation but also to establish the level of initial treatment and timing of monitoring. Presently, the updated BODE (body mass index, airflow obstruction, dyspnea and exercise capacity) index appears the most suitable tool for such purpose,Citation23 taking into account also the distribution of the symptoms during the day and the gas exchange abnormalities. Prospectively, determination of some plasma biomarkers, such as C-protein and fibrinogen, might be useful.Citation24 All of this information must be weighted for the age of the patient.
Subsequently, an annual history of COPD exacerbations, as much as is possible to detail, must be performed because a high frequency of COPD exacerbations is an undisputable marker of disease severity that needs to be aggressively controlled. It is of limited utility to simply count the previous COPD exacerbations, and every effort should be made to recognize the prevalent type, and so choose the best available strategy to prevent them.Citation25
The various comorbidities should finally be investigated and graduated by severity in order to treat adequately those known to have a major impact on mortality in patients with COPD.Citation26
Therapeutic approach
The pharmacological baseline treatment of stable COPD has been widely based on the severity of airflow obstruction and recently also of chronic symptoms and on the number of exacerbations in the previous year.Citation1
With the interesting but somehow confusing exception of the Spanish guidelines,Citation27 these recommendations do not take into account the underlying prevalent disease that should be treated and the future risk.
Moreover, the exacerbations are just enumerated with no attempt to define their etiology, always suggesting the stereotyped use of inhaled corticosteroids (ICS) on top of bronchodilators when they are two or more in the previous year (or one severe, requiring hospitalization).Citation1
In contrast, the therapy must be first tailored on the prevalent disease underlying COPD, independent from the degree of FEV1 reduction and chronic dyspnea score, and only after that, according to the severity of the disorder (and age of the patient), to establish the level of the treatment in order to freeze, when possible, and not to follow the underlying pathological process, running after it ().
Figure 1 The baseline pharmacological approach to COPD according to the prevalent underlying disease.
Abbreviations: bid, twice daily; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroids; FENO, fractional exhaled nitric oxide; LABA, long-acting beta-adrenoceptor agonist; LAMA, long-acting muscarinic antagonist; U-LABA, ultralong-acting beta-adrenoceptor agonist; U-LAMA, ultralong-acting muscarinic antagonist.
We do not need expensive randomized controlled trials (RCTs) anymore where thousands of patients with COPD having different underlying diseases and having different severity of airflow obstruction are enrolled all together, trying to understand the effect of a same treatment. On the contrary, more valuable information about any given targeted intervention might be collected, studying small numbers of well-selected patients with COPD, with same underlying disease, similar clinical phenotypes, same degree of airflow obstruction and/or BODE index, and similar age range, for an adequate span of time.
It can be speculated, but it must be proved with well-designed prospective studies that this approach will be more effective in terms of lung function decline and patient-related outcomes, particularly if applied in the initial phases of COPD, implying an early diagnosis of chronic airflow obstruction and a better characterization of the underlying disease.
Perhaps, we would learn that more aggressive treatments have to be implemented in the earlier stages of COPD, instead of using them in the more advanced ones as recommended today, to obtain the best possible outcomes. This has been suggested by the post hoc subgroups analysis in the previous interventional large studies on COPD where improvement in symptoms, exacerbation frequency, FEV1 annual decline, and all-cause mortality was demonstrated only for patients with COPD stages II and III (Global Initiative for Chronic Obstructive Lung Disease).Citation28,Citation29
The goal should be to have patients dying with COPD (when allowed by the underlying disease, essentially chronic bronchiolitis) and not because of COPD.
Exacerbations
The prevention of COPD exacerbations is a point of paramount importance in the management of COPD that needs a completely different approach because it cannot be addressed simply with the baseline pharmacological treatment. We know a lot about COPD exacerbations, even if their diagnosis, essentially based on worsening of chronic symptoms reported as relevant by the patients, is presently still based on the exclusion of other diseases. To suffer from two or more COPD exacerbations or from one severe COPD exacerbation leading to hospitalization in the previous year is without doubt a marker of COPD severity, independent from the underlying disease, degree of airflow obstruction, and entity of symptoms or BODE index, even if lower FEV1 is associated with higher risk of frequent and more severe exacerbations. Although the probability of having a new COPD exacerbation is greater in those patients with COPD who previously experienced COPD exacerbations (so-called frequent exacerbators),Citation30 such status, with few exceptions,Citation31–Citation33 cannot be identified as a distinct phenotype, rather as a condition requiring more strict social and medical attention. In fact, it is quite easy to shift from a frequent exacerbator to a nonfrequent exacerbator and vice versa,Citation34 sometimes without an obvious reason, but often clearly because of a more adequate and comprehensive treatment.Citation35
Given the prognostic importance of COPD exacerbations,Citation36 however, we cannot be limited to simply counting exacerbations; we must learn how to consistently recognize the prevalent type in a single patient. Such an approach is crucial to prevent more effectively the COPD exacerbations using more tailored and specific therapies ().
Figure 2 The different preventive approach to acute exacerbation in COPD.
Abbreviations: AECOPD, acute exacerbations of chronic obstructive pulmonary disease; vit, vitamin; ICS, inhaled corticosteroids; C-PAP, continuous positive airway pressure.
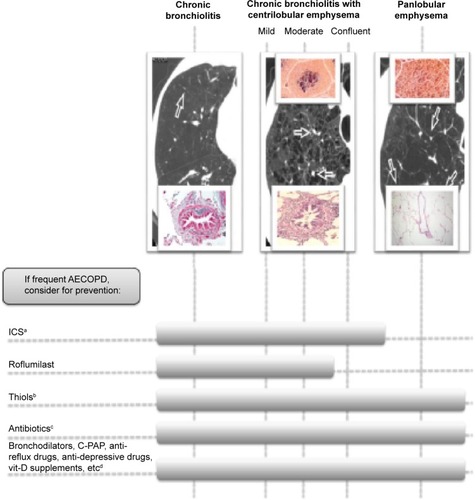
Some plasma, blood, or sputum biomarkers have been shown to be associated with high sensitivity and specificity to a prevalent clinical type of COPD exacerbation: eosinophilic, infectious either virus or bacteria associated, or pauci-inflammatory, due to several possible causes that have to be identified.Citation37 More interestingly, these specific biomarkers tend to be detectable also in stable conditions, at least in eosinophilia- and bacteria-associated exacerbations, allowing an easier identification of the most likely future pattern of COPD exacerbations.Citation37
Thus, when possible, each prevalent type of COPD exacerbation in a given patient who is a frequent exacerbator should be appropriately prevented by specific treatments that cannot be invariably the use of high-dose ICS, as presently recommended.Citation1,Citation27
Long-acting bronchodilators and mainly ultralong-acting bronchodilators (both beta-2 agonist and antimuscarinic drugs) are able to prevent up to 30% of COPD exacerbations when given aloneCitation38 and possibly more when given in combination.Citation39
ICS are very useful to prevent the eosinophilic exacerbations,Citation15–Citation17 but it is difficult to see how ICS can prevent infectious exacerbations, or even noneosinophilic and noninfectious exacerbations, apart from strengthening the action of bronchodilators such as long-acting beta-2 agonists (LABA) in some circumstances.Citation38
In patients with COPD having bronchiectasis and chronic bronchitis, COPD exacerbations are often infectious and bacterial in nature and must be prevented with antibiotic prophylaxis, at least in the winter season. There is strong evidence that macrolides may significantly reduce COPD exacerbations,Citation40–Citation42 suggesting that this kind of exacerbation can be prevented in some clinical phenotypes of patients with COPD having high risk of bacterial colonization or chronic infections.
Presently, only adequate immunization against influenza virus can be offered for viral exacerbations in patients with COPD, and we urgently need the same effective tools for rhinovirus infection, the most common cause of virus-associated COPD exacerbation.
All other types of COPD exacerbations (noninfectious and noneosinophilic) deserve accurate work-up and specific preventative treatment should be applied when the cause is recognized (). Many of these exacerbations, in fact, can be largely avoided with targeted approaches.Citation38,Citation43–Citation45 For instance, in the presence of patients with COPD having presumable high oxidative stress (those who continue smoking, those with chronic bronchitis, and those with high exposure to environment pollution), an adequate antioxidant support might significantly reduce COPD exacerbations.Citation46,Citation47
Finally, a strict adherence to baseline chronic therapy should be assured throughout a strong relationship between patients with COPD and their physicians and caregivers because this aspect is crucial to obtain improved control of COPD exacerbations and their consequences.Citation48
Conclusion
In summary, in patients with COPD, first treat as soon as possible the underlying disease, aiming to freeze the disease and halt progression, ready to implement treatment (essentially with different bronchodilators, including theophylline) to control symptoms and improve lung function, exercise tolerance, and quality of life, if necessary. Second, look at the exacerbations of COPD, if they are frequent (more than one in a year), define their prevalent phenotype (eosinophilic, bacterial, or due to other causes) and treat to prevent accordingly.
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Pulmonary DiseaseGlobal strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary DiseaseGlobal Initiative for Chronic Obstructive Pulmonary Disease: 2014 Available from: http//www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jun11.pdfAccessed December 15, 2014
- MennPHeinrichJHuberRMKORA Study GroupDirect medical costs of COPD – an excess cost approach based on two population-based studiesRespir Med201210654054822100535
- ThunMJCarterBDFeskanichD50-year trends in smoking-related mortality in the United SatesN Engl J Med201336835136423343064
- MurrayCJLPhilDLopezADMeasuring the global burden of diseaseN Engl J Med201336944845723902484
- ERS White BookThe Burden of Lung DiseaseSheffieldERS White Book2013 Chapt 1:1–15 and COPD Chapt 13:1–12
- World Health OrganizationBurden of COPDGenevaWorld Health Organization2014
- BurrowsBFletcherCMHeardBEJonesNLWootliffJSThe emphysematous and bronchial types of chronic airways obstruction. A clinicopathological study of patients in London and ChicagoLancet196618308354159957
- HoggJCChuFUtokaparchSThe nature of small-airway obstruction in chronic obstructive pulmoinary diseaseN Engl J Med20043502645265315215480
- ErikssonSPulmonary emphysema and alpha-1-antitrypsin deficiencyActa Med Scand196417519720514124635
- FinkelsteinRFraserRSGhezzoHCosioMAlveolar inflammation and its relation to emphysema in smokersAm J Respir Crit Care Med1995152166616727582312
- KimWDEidelmanDHIzquierdoJLGhezzoHSaettaMPCosioMCentrilobular and panlobular emphysema in smokers. Two distinct morphologic and functional entitiesAm Rev Respir Dis1991144138513901741553
- McDonoughJEYuanRSuzukiMSmall-airway obstruction and emphysema in chronic obstructive pulmonary diseaseN Engl J Med20113651567157522029978
- BrightlingCEMcKennaSHargadonBSputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary diseaseThorax20056019319815741434
- LeeJ-HLeeYKKimE-KKimT-HResponse to inhaled long-acting beta-agonist and corticosteroid according to COPD subtypeRespir Med201010454255119926461
- SivaRGreenRHBrightlingCEEosinophilic airway inflammation and exacerbations of COPD: a randomized controlled trialEur Respir J20072990691317301099
- Di SantostefanoRLLiHRubinDBStempelDAWhich patients with chronic obstructive pulmonary disease benefit from the addition of an inhaled corticosteroid to their bronchodilator? A cluster analysisBMJ Open2013343e001838
- LieskerJJWBathoornEPostmaDSVonkJMTimensWKerstjensAMSputum inflammation predicts exacerbations after cessation of inhaled corticosteroids in COPDRespir Med20111051853186021802933
- LapperreTSSnoeck-StrobandJBGosmanMMGroningen Leiden Universities Corticosteroids in Obstructive Lung Disease Study GroupEffect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary diseaseAnn Intern Med200915151752719841453
- NishimuraMMakitaHNagaiKHokkaido COPD Cohort Study InvestigatorsAnnual Change in pulmonary function and clinical phenotype in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2012185445222016444
- JohannessenASkorgeTDBottaiMMortality by level of emphysema and airway wall thicknessAm J Respir Crit Care Med201318760260823328525
- HershCPMakeBJLynchDACOPDGene and ECLIPSE InvestigatorsNon-emphysematous chronic obstructive pulmonary disease is associated with diabetes mellitusBMC Pulm Med20141416425341556
- HanMKKazerooniEALynchDACOPDGene InvestigatorsChronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypesRadiology201126127428221788524
- PuhanMAGarcia-AymerichJFreyMExpansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADOindexLancet200937470471119716962
- AgustíAEdwardsLDRennardSIEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsPersistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotypePLoS One201275e3748322624038
- HurstJRExacerbation phenotyping in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201118462562621920921
- DivoMCoteCde TorresJPBODE Collaborative GroupComorbidities and risk of mortality in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201218615516122561964
- MiravittlesMSoler-CatalunaJJCalleMSpanish guideline for COPD (gesEPOC). Update 2014Arch Bronconeumol201448suppl 1258
- JenkinsCRJonesPWCalverleyPMEfficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomized placebo-controlled TORCH studyRespir Res200910596819566934
- DecramerMCelliBKestenSUPLIFT InvestigatorsEffect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomized controlled trialLancet20093741171117819716598
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obtructive pulomary diseaseNew Engl J Med2010631128113820843247
- LinCLSiuLKLinJCMannose-binding lectine gene polymorphism contributes to the recurrence of exacerbation in patients with COPDChest2011139435120688922
- ForemanMGDeMeoDLHershCPPolymorphic variation in surfactant protein B is associated with COPD exacerbationsEur Respir J20083293894418550614
- MalliaPMessageSDGielenVExperimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbationAm J Respir Crirt Care Med2011183734742
- DonaldsonGCMüllerovaHLocantoreNFactors associated with change in exacerbation frequency in COPDRespir Res201314798823899210
- WedzichaJARabeKFMartinezFJEfficacy of roflumilast in the COPD frequent exacerbator phenotypeChest20131431302131123117188
- Soler-CatalunaJJMartìnez-GarcìaMARomàn SanchezPSalcedoENavarroMOchadoRSevere acute exacerbation and mortality in patients with chronic obstructive pulmonary diseaseThorax20056092593116055622
- BafadhelMMcKennaSTerrySAcute exacerbations of chronic obstructive pulmonary disease. Identification of biollogic clusters and their biomarkersAm J Respir Crirt Care Med2011184662671
- WedzichaJADecramerMSeemungalARThe role of bronchodilators in the prevention of exacerbation of COPDEur Respir J2012401545155422835613
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopironium and tiotropium (SPARK): a randomized, double-blind, parallel-group studyLancet Respir Med2013119920924429126
- SeemungalTARWilkinsonTMAHurstJRPereraWRSapsfordRJWedzichaJALong-term erytromycin therapy is associated with dcreased chronic obstructive pulmonary disease exacerbationsAm J Respir Crti Care Med200817811391147
- AlbertRKConnettJBaileyWCCOPD Clinical Research NetworkAzithromicin for prevention of exacerbations of COPDNew Engl J Med201136568969821864166
- UzunSDjaminRSKluytmansJAAzithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomized, double-blind, placebo-controlled trialLancet Respir Med2014236136824746000
- WashkoGRFanVSRamseySDNational Emphysema Treatment Trial Research GroupThe effect of lung volume reduction surgery on chronic obstructive pulmonary disease exacerbationsAm J Respir Crirt Care Med2008177164169
- MarinJMSorianoJBCarrizoSJBoldovaACelliBROutcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. The overlap sindromeAm J Respir Crirt Care Med2010182325331
- LehouckAMathieuCCarremansCHigh does of Vitamin D to reduce exacerbations in chronic obstructive pulmonary diseaseAnn Intern Med201215610511422250141
- ZhengJPKangJHuangSGEffect of carbocistein on acute exacerbation of chronic obstructive pulmonary disease (PEACE study): a randomized placebo-controlled studyLancet20083712013201818555912
- ZhengJPWenFQBaiCXOn behalf of the PANTHEON study groupTwice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trialLancet Respir Med2014218719424621680
- VestboJAndersonJACalverleyPMAdherence to inhaled therapy, mortality and hospital admission in COPDThorax20096493994319703830
