Abstract
Background
Since the Global Initiative for Chronic Obstructive Lung Disease (GOLD) groups A–D were introduced, the lung function changes according to group have been evaluated rarely.
Objective
We investigated the rate of decline in annual lung function in patients categorized according to the 2014 GOLD guidelines.
Methods
Patients with COPD included in the Korean Obstructive Lung Disease (KOLD) prospective study, who underwent yearly postbronchodilator spirometry at least three times, were included. The main outcome was the annual decline in postbronchodilator forced expiratory volume in 1 second (FEV1), which was analyzed by random-slope and random-intercept mixed linear regression.
Results
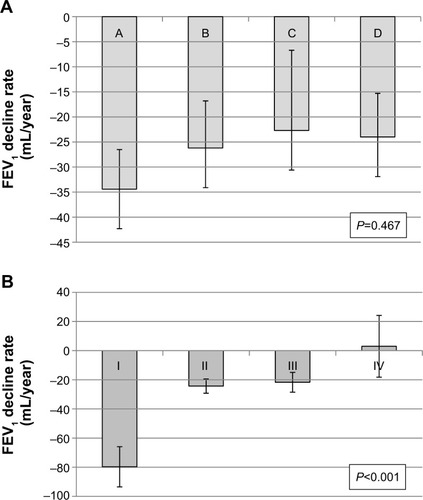
A total 175 participants were included. No significant postbronchodilator FEV1 decline was observed between the groups (−34.4±7.9 [group A]; −26.2±9.4 [group B]; −22.7±16.0 [group C]; and −24.0±8.7 mL/year [group D]) (P=0.79). The group with less symptoms (−32.3±7.2 vs −25.0±6.5 mL/year) (P=0.44) and the low risk group (−31.0±6.1 vs −23.6±7.7 mL/year) (P=0.44) at baseline showed a more rapid decline in the postbronchodilator FEV1, but the trends were not statistically significant. However, GOLD stages classified by FEV1 were significantly related to the annual lung function decline.
Conclusion
There was no significant difference in lung function decline rates according to the GOLD groups. Prior classification using postbronchodilator FEV1 predicts decline in lung function better than does the new classification.
Introduction
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) proposed a new classification system that grouped patients with COPD into four categories, groups A–D, based on symptom and risk categories.Citation1 Several studies have since evaluated clinical outcomes in COPD patients, including exacerbation and mortality, according to the GOLD groups.Citation2–Citation4 However, the decline in lung function, which is another critical therapeutic target,Citation5 has been investigated in only two studies.Citation2,Citation6 Furthermore, in these studies, the lung function decline rates were analyzed with no adjustmentCitation2 or with adjustment by using only disease severity as the sole covariate.Citation6 Finally, the impact of specific details related to the GOLD classification (ie, “more symptoms” group vs “less symptoms” group or GOLD high risk group vs low risk group), which could provide information on the use of the GOLD classification system, was not fully investigated.Citation2,Citation6 In the present study, we evaluated the annual lung function decline according to the GOLD guidelines, by using data from the Korean Obstructive Lung Disease (KOLD) cohort study, a multicenter prospective longitudinal study of patients with COPD in South Korea.Citation7
Methods
Participant eligibility and study protocol
The KOLD cohort included patients diagnosed with COPD or asthma who were recruited from pulmonary clinics at eleven referral hospitals in Korea from June 2005 to October 2012. The inclusion criteria were: (1) age ≥40 years; (2) postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <0.7; (3) current or ex-smoker with a smoking history ≥10 pack-years; and (4) greater than three annual pulmonary function tests (PFTs). Patients with “pure” asthma were excluded based on the judgement of physicians. As there was a 2-week period to wash out therapeutic drugs just before enrollment in the KOLD cohort study, it was expected that lung functions would increase in the early period of study, which is commonly observed in large clinical trials. Therefore, the 12th month from the initial enrollment was set as a baseline of the study, and analysis of lung function changes started from the baseline and continued to the last follow-up at 1-year intervals. The study was approved by the ethics committees of the Seoul National University Hospital Institutional Review Board and by the institutional review boards of the other 16 hospitals; and all patients provided written, informed consent. We adhered to the Declaration of Helsinki.
Definition of groups and stages
The recent GOLD guidelines categorize patients with COPD into groups A–D according to symptoms based on either the modified Medical Research Council (mMRC) grade or the COPD assessment test (CAT) score. Risk is based on either the FEV1 or the exacerbation history. As CAT scores were not measured in the KOLD study before 2011, we used the St George’s Respiratory Questionnaire (SGRQ) score as a surrogate for the CAT score.Citation3 According to the most recent GOLD guidelines, a symptom score equivalent to an SGRQ score ≥25 should be used as the cutoff for considering regular treatment of COPD symptoms.Citation5 Patients with an mMRC ≥2 or a SGRQ score ≥25 were placed in the more symptoms group. Those with a postbronchodilator FEV1 <50% of the predicted value or who experienced ≥ two exacerbations (hospitalization ≥ one time) in the previous year were placed in the high risk group.
GOLD stages were classified based on the percentage of predicted FEV1:Citation5 stage I was defined as predicted FEV1 ≥80%; stage II was defined as predicted FEV1 50%–79%; stage III was defined as predicted FEV1 30%–49%; and stage IV was defined as predicted FEV1 <30%.
Pulmonary function test
PFT was performed according to American Thoracic Society guidelinesCitation8 using the Vmax 22 (Sensor Medics, Yorba Linda, CA, USA) and PFDX (Medgraphics, St Paul, MN, USA). The spirometry reference values were based on the Korean equation.Citation9 Postbronchodilator spirometry values were measured 15 minutes after administering 400 μg of salbutamol. Lung volume, including the total lung capacity (TLC) and residual volume (RV), was measured using body plethysmography which were V6200 (CareFusion, San Diego, CA, USA), PFDX, or Vmax 22.Citation10 The diffusing capacity of carbon monoxide (DLCO) was measured by assessing the single-breath carbon monoxide uptake (Vmax 22 or PFDX).Citation11 The postbronchodilator FEV1 and FVC, TLC, RV, and DLCO were measured at baseline and at each annual visit.
Statistical analysis
Baseline characteristics between groups were compared using the χ2 and Fisher’s exact tests for categorical variables and the t-test and Mann–Whitney U-test for continuous variables. The main outcome was the annual rate of change in the postbronchodilator FEV1. Assuming an annual rate of decline in the FEV1 between groups A and D of 30 mL/year with a 45 mL/year standard deviation, the estimated sample size was calculated as 192 (48 patients per group) with a 5% type 1 error and 90% power.
The annual FEV1 decline rates were subjected to random-slope and random-intercept mixed linear regression analyses. Covariates in the models included time (year), time by group (or category) interaction, age, sex, height, weight, smoking status,Citation12,Citation13 and the medication possession ratio of respiratory medications. Respiratory medications included combined inhaled corticosteroids and long-acting β-agonists (ICS/LABA), and inhaled long-acting muscarinic antagonists (LAMA), as these drugs may affect the decline in lung function according to recent large random controlled trials.Citation13,Citation14 Akaike information criterion (AIC) was used to select a better statistical model. All statistical analyses were performed using Stata 13.1 (StataCorp, College Station, TX, USA). A P-value <0.05 indicated a significant difference.
Results
A total 175 patients were included, with 58 (33.1%) categorized as group A, 45 (25.7%) as group B, 15 (8.6%) as group C, and 57 (32.6%) as group D. summarizes the baseline characteristics, including the demographic, symptomatic, and spirometry values, and exacerbation history. The subjects underwent a mean 4.7±1.2 annual PFTs over the follow-up period (mean 48.2 months). A total 59.4% underwent annual PFTs more than five times, and 78.3% underwent PFT more than four times. The proportion of patients who had ever used LAMA during the follow-up period was similar among the four groups (P=0.17), meanwhile patients who had more severe symptoms or lower FEV1 were likely to use ICS/LABA (P=0.003).
Table 1 Baseline patient characteristics and long-term outcomes
The adjusted annual rates of decline in the postbronchodilator FEV1, postbronchodilator FVC, DLCO, and RV/TLC were −28.3±4.9 mL/year, −26.8±8.6 mL/year, −1.02%±0.38%, and −0.40%±0.23%, respectively (). The decline in postbronchodilator FEV1 was highest in the GOLD group A, although no significant differences were observed according to group (). The group with the fewest symptoms at baseline based on the mMRC and SGRQ criteria showed the fastest decline in postbronchodilator FEV1, although this trend was not statistically significant (P=0.44). The risk group at baseline was not associated with the decline in postbronchodilator FEV1. However, GOLD stage was significantly related with the annual FEV1 decline rates. Stage I COPD patients showed the fastest lung function decline, while stage IV patients the slowest (P<0.001). The GOLD stage was a better predictor than was the GOLD group (AIC =423.75 vs 622.08). The decline rates in postbronchodilator FVC were more prominent in groups A and C, which included patients with the least severe symptoms (P=0.13). When data were examined according to the percent change per year, the trends were similar (). The GOLD stage was also a better predictor was than the GOLD group in the annual decline rates of FEV1%. (AIC =5,384.6 vs 5,497.9) There were no significant differences in the annual rates of decline in the postbronchodilator FEV1/FVC, DLCO, and RV/TLC. The severity of decline in RV/TLC decreased from group A to group D ().
Table 2 Annual rate of decline in postbronchodilator FEV1 and FVC (mL/year)
Table 3 Annual rate of percentage decline in postbronchodilator FEV1 and FVC (%/year)
Table 4 Annual rate of decline in postbronchodilator FEV1/FVC, DLCO, and RV/TLC (%/year)
Figure 1 Annual rate of decline in postbronchodilator FEV1 adjusted by age, sex, height, weight, smoking status, and medication possession ratio of respiratory medications (ICS-LABA and LAMA).
Abbreviations: FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS-LABA, inhaled corticosteroid/long-acting β-agonist; LAMA, long-acting muscarinic antagonist.
Discussion
Our findings show that the rate of lung function decline did not differ significantly among the GOLD 2014 groups, which is similar to results in the ECLIPSE cohort study.Citation2
The new GOLD guidelines emphasize that the goals of COPD assessment are to determine the disease severity and its impact on the patient’s current health and risk of future events; to this end, four patient categories were introduced, groups A–D.Citation5 However, it remains unclear whether the GOLD category can accurately predict future risk, including mortality, exacerbations, and the rate of lung function decline. The ECLIPSE cohort study, which included 2,101 COPD patients, showed that group D had the highest mortality and group A the lowest, but surprisingly, group C showed a lower mortality rate than did group B.Citation2 Similar results were reported in a Danish study, which also observed higher mortality in group B than in group C.Citation15 In a Norwegian study (HUNT2 study), the mortality increased in order from group A to D in men with COPD, but this trend was not observed in the female patients.Citation16 A similar trend was observed for severe exacerbation requiring hospitalization and the GOLD group – the risk of severe exacerbation in groups B and C were similar in the Danish study,Citation15 ECLIPSE study,Citation2 and the COPDGene study.Citation3
The association between the rate of annual lung function decline, another important outcome, and the GOLD groups has rarely been investigated. Although a post hoc analysis of the UPLIFT trial showed group C had lower annual FEV1 decline rates compared with group B (38 vs 48 mL) (P=0.01), various covariates were not adjusted in that analysis.Citation6 Only one cohort study has examined the relationship,Citation2 and this report found that the annual FEV1 decline rates did not differ significantly between groups A, B, C, and D (−33.4, −38.0, −30.2, and −31.9 mL/year, respectively) (P=0.157). Similarly, we did not observe any significant rate change according to the GOLD groups. In the present study, the annual FEV1 decline rates in groups A, B, C, and D in the KOLD cohort were −34.4, −26.2, −22.7, and −24.0 mL/year, respectively (P=0.467).
The lack of significant change in the annual lung function decline between GOLD groups A to D may be explained in several ways. First, the exacerbation history and FEV1 may exert differing effects on the annual lung function decline. The exacerbation history and FEV1 are the two criteria used to categorize patients into the low vs high risk GOLD groups.Citation2 In the ECLIPSE study, the prior exacerbation history did not affect the annual FEV1 change (P=0.57).Citation12 Interestingly, a lower FEV1 was associated with a slower rate of lung function decline in the BODE cohortCitation17 and a pooled analysis of 15 randomized controlled trials.Citation18 These varying effects of exacerbation history and FEV1 on long-term lung function decline may eliminate any difference in the rate of declines between groups C and D (high risk group) and groups A and B (low risk group). Consistent with these earlier results, in our study, there was no statistical difference between the high risk and low risk groups.
Second, there is no evidence that more severe symptoms are associated with a faster decline in lung function. In a Hokkaido COPD study, the mMRC dyspnea scale score ≥2 did not predict rapid vs slow decline in lung function (P=0.60 [multivariate model excluding emphysema score]; P=0.75 [multivariate model excluding transfer factor coefficient of the lung for carbon monoxide]).Citation19 The ECLIPSE study found that symptoms of chronic bronchitis did not predict a faster decline in lung function.Citation12 These data suggest that there may not be any difference in the lung function decline between groups A and C (less symptoms) and groups B and D (more symptoms).
Potentially, a lower statistical power may be unable to detect any difference in the rates of lung function decline. In the UPLIFT trial, in which the primary end point was the annual FEV1 decline rate, the sample size was estimated as 758 patients per group to detect a 15 mL/year difference between groups (5% significance, 90% power, and 4-year standard deviation of 90 mL).Citation20 The KOLD study included fewer patients per group (15 to 58), which is a weakness of our study. However, the similar estimation of lung function decline rates between the groups (range from −34mL/year to −24mL/year) suggests that there may not be any significant differences among the groups. Furthermore, we still found no significant difference after adjustment of possible confounders, including ICS/LABA and LAMA. However, the initial GOLD stage was significantly related with the lung function decline rate. Compared with the model using GOLD groups, the statistical model using GOLD stages was the better one in the prediction of the annual lung function declines. Similarly, a study using the data in the UPLIFT trial reported that the GOLD stage showed a better model fitness, although the GOLD group was also significantly associated with lung function decline in the study.Citation6 Interestingly, in our study, the better the initial FEV1% was, the faster annual FEV1 decline was observed (P<0.001), as reported in previous studies.Citation17,Citation18
The strength of our study is that a sufficient number of annual spirometry examinations were performed. The subjects underwent a mean 5.4 annual spirometry tests during the study period, and ~90% of patients underwent more than four annual PFTs. In addition, we adjusted for multiple covariates, including the medication possession ratio of ICS/LABA and LAMA, which can affect lung function declineCitation13,Citation14 in the random-slope and random-intercept mixed linear regression models.
This study has several limitations. The most notable limitation is that the study may be statistically underpowered due to the relatively small number of participants, as mentioned earlier, although our sample size met the initial sample size estimation. All participants were Korean, and most were male, which may limit any generalization; however, Korean men have a higher prevalence and more severe disease than Korean women (25.8% [men] vs 9.6% [women]).Citation21 Third, we used the SGRQ score as a surrogate for the CAT score, as suggested by the new GOLD classification. However, the results of both questionnaires are strongly correlated,Citation22,Citation23 and the SGRQ score was also used to assess COPD classification in the COPDGene study.Citation3 In addition, the recent GOLD guidelines recommend that a symptom score equivalent to an SGRQ score ≥25 should be used as the cutoff for considering regular treatment of symptoms.Citation5
In conclusion, we found no significant differences in the rate of lung function decline according to the GOLD groups. Compared with the new classification model using GOLD 2014, the older classification model using solely FEV1 could predict the rate of decline in lung function better.
Acknowledgments
The authors thank Woo Jin Kim, Kwang Ha Yoo, Sei Won Lee, Jae Seung Lee, Chin Kook Rhee, and Hyun-Jeong Lee.
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. Revised 2011Global Initiative for Chronic Obstructive Lung Disease, Inc Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdfAccessed July 12, 2015
- AgustiAEdwardsLDCelliBECLIPSE InvestigatorsCharacteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohortEur Respir J201342363664623766334
- HanMKMuellerovaHCurran-EverettDGOLD 2011 disease severity classification in COPDGene: a prospective cohort studyLancet Respir Med201311435024321803
- SorianoJBAlfagemeIAlmagroPDistribution and prognostic validity of the new Global Initiative for Chronic Obstructive Lung Disease grading classificationChest2013143369470223187891
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. Updated 2014Global Initiative for Chronic Obstructive Lung Disease, Inc Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdfAccessed July 12, 2015
- GoossensLMLeimerIMetzdorfNBeckerKRutten-van MölkenMPDoes the 2013 GOLD classification improve the ability to predict lung function decline, exacerbations and mortality: a post-hoc analysis of the 4-year UPLIFT trialBMC Pulm Med20141416325326750
- ParkTSLeeJSSeoJBStudy design and outcomes of Korean Obstructive Lung Disease (KOLD) cohort studyTuberc Respir Dis (Seoul)201476416917424851130
- MillerMRHankinsonJBrusascoVATS/ERS Task ForceStandardisation of spirometryEur Respir J200526231933816055882
- ChoiJKPaekDLeeJONormal predictive values of spirometry in Korean populationTuberc Respir Dis2005583230242
- WangerJClausenJLCoatesAStandardisation of the measurement of lung volumesEur Respir J200526351152216135736
- MacintyreNCrapoROViegiGStandardisation of the single-breath determination of carbon monoxide uptake in the lungEur Respir J200526472073516204605
- VestboJEdwardsLDScanlonPDECLIPSE InvestigatorsChanges in forced expiratory volume in 1 second over time in COPDN Engl J Med2011365131184119221991892
- CelliBRThomasNEAndersonJAEffect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH studyAm J Respir Crit Care Med2008178433233818511702
- DecramerMCelliBKestenSLystigTMehraSTashkinDPUPLIFT investigatorsEffect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trialLancet200937496961171117819716598
- LangePMarottJLVestboJPrediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general populationAm J Respir Crit Care Med20121861097598122997207
- LeivsethLBrumptonBMNilsenTIMaiXMJohnsenRLanghammerAGOLD classifications and mortality in chronic obstructive pulmonary disease: the HUNT Study, NorwayThorax2013681091492123611880
- CasanovaCde TorresJPAguirre-JaímeAThe progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohortAm J Respir Crit Care Med201118491015102121836135
- TantucciCModinaDLung function decline in COPDInt J Chron Obstruct Pulmon Dis20127959922371650
- NishimuraMMakitaHNagaiKHokkaido COPD Cohort Study InvestigatorsAnnual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20121851445222016444
- DecramerMCelliBTashkinDPClinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the UPLIFT trialCOPD20041230331217136995
- KimDSKimYSJungKSKorean Academy of Tuberculosis and Respiratory DiseasesPrevalence of chronic obstructive pulmonary disease in KoreaAm J Respir Crit Care Med2005172784284715976382
- RingbaekTMartinezGLangePA comparison of the assessment of quality of life with CAT, CCQ, and SGRQ in COPD patients participating in pulmonary rehabilitationCOPD201291121522292593
- JonesPWBrusselleGDal NegroRWProperties of the COPD assessment test in a cross-sectional European studyEur Respir J2011381293521565915
