Abstract
Background
COPD exacerbations requiring intensive care unit (ICU) admission have a major impact on morbidity and mortality. Only 10%–25% of COPD exacerbations are eosinophilic.
Aim
To assess whether eosinophilic COPD exacerbations have better outcomes than non-eosinophilic COPD exacerbations in the ICU.
Methods
This retrospective observational cohort study was conducted in a thoracic, surgery-level III respiratory ICU of a tertiary teaching hospital for chest diseases from 2013 to 2014. Subjects previously diagnosed with COPD and who were admitted to the ICU with acute respiratory failure were included. Data were collected electronically from the hospital database. Subjects’ characteristics, complete blood count parameters, neutrophil to lymphocyte ratio (NLR), delta NLR (admission minus discharge), C-reactive protein (CRP) on admission to and discharge from ICU, length of ICU stay, and mortality were recorded. COPD subjects were grouped according to eosinophil levels (>2% or ≤2%) (group 1, eosinophilic; group 2, non-eosinophilic). These groups were compared with the recorded data.
Results
Over the study period, 647 eligible COPD subjects were enrolled (62 [40.3% female] in group 1 and 585 [33.5% female] in group 2). Group 2 had significantly higher C-reactive protein, neutrophils, NLR, delta NLR, and hemoglobin, but a lower lymphocyte, monocyte, and platelet count than group 1, on admission to and discharge from the ICU. Median (interquartile range) length of ICU stay and mortality in the ICU in groups 1 and 2 were 4 days (2–7 days) vs 6 days (3–9 days) (P<0.002), and 12.9% vs 24.9% (P<0.034), respectively.
Conclusion
COPD exacerbations with acute respiratory failure requiring ICU admission had a better outcome with a peripheral eosinophil level >2%. NLR and peripheral eosinophilia may be helpful indicators for steroid and antibiotic management.
Introduction
COPD exacerbations have a major impact on morbidity and mortality when intensive care unit (ICU) admission is required.Citation1,Citation2 Acute respiratory failure (ARF) due to COPD exacerbation is, as a first choice, primarily managed by noninvasive mechanical ventilation (NIMV), and the initiation of NIMV is determined by the severity of ARF.Citation3–Citation5 In addition to the application of NIMV, an optimized medical regime should be initiated for successful management.Citation3
The optimal medical treatment for COPD exacerbations in the ICU is still controversial. The majority of COPD exacerbations have an infectious origin.Citation6 Some studies have investigated the decision to use steroids or antibiotics based on the COPD exacerbation.Citation7–Citation11 Sputum and peripheral eosinophilia (2%–3% eosinophils) have been found in only 10%–45% of COPD exacerbations.Citation12–Citation16 The presence of either sputum or peripheral eosinophilia can help physicians to decide on steroid or antibiotic management.Citation11–Citation16 Peripheral eosinophilia is thought to be a result of the inflammatory process of COPD exacerbation.Citation12 In addition to the presence of eosinophilia, other inflammatory markers such as neutrophil to lymphocyte ratio (NLR),Citation17 platelet (PLT) to mean platelet volume (MPV),Citation18 and PLT/MPV ratio are not well defined in COPD subjects with ARF requiring ICU admission.
There are limited data on peripheral eosinophilia, NLR, PLT/MPV, and outcomes of COPD exacerbation that lead to ARF and require ICU admission. We hypothesized that the presence of peripheral eosinophilia may result in a better outcome compared with non-eosinophilic exacerbations.
Methods
This retrospective observational cohort study was conducted in a thoracic, surgery level III respiratory ICU of a tertiary teaching hospital for chest diseases from 2013 to 2014. The Sureyyapasa Chest Disease and Surgery Research Hospital Local Ethics Committee approved the study and was in accordance with the Declaration of Helsinki. Due to the retrospective nature of the study design, informed consent was not obtained. During the study period, the same intensivist specialist team worked in the ICU, which they staffed 24 hours per day.
Subjects
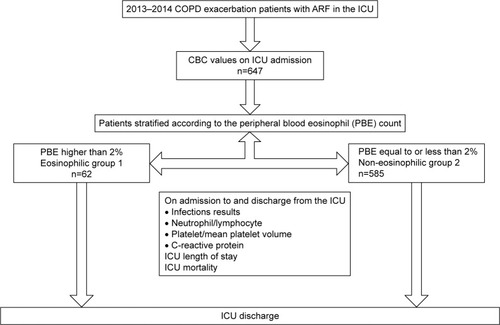
Subjects previously diagnosed with COPD (ICD coding as J 44) and admitted to the ICU with ARF were included and grouped according to their peripheral eosinophil count on the day of admission. Subject inclusion is summarized in a flowchart (). The COPD diagnosis was established by a physician who evaluated airflow obstruction on spirometry, forced expiratory volume in 1 second (FEV1) of 70% predicted or less, and an FEV1 and forced vital capacity ratio of 70% or less.Citation3 Spirometry test data could not be recorded from the subjects’ charts. The smoking history of patients was recorded.
Definitions
Hypoxic ARF was defined as the ratio of partial arterial oxygen pressure to inspired fractionated oxygen (PaO2/FiO2) <300 and partial arterial carbon dioxide pressure (PaCO2) <45 mmHg. Hypercapnic/hypoxemic ARF was defined as PaCO2 >45 mmHg and PaO2/FiO2 <300. Hypercapnic ARF was PaCO2 >45 mmHg and PaO2/FiO2 >300.Citation5,Citation19
The COPD exacerbation due to an infectious origin was defined by the presence of all three Anthonisen criteria, as follows: worsening of dyspnea, increased volume of pulmonary secretions (endotracheal, sputum), and increased purulence of respiratory secretions.Citation3,Citation20
Peripheral eosinophilia, defined by an eosinophil count higher than 2%, was accepted as eosinophilic COPD exacerbation, and if the peripheral blood eosinophil count was equal to or less than 2%, it was defined as a non-eosinophilic COPD exacerbation.Citation13 The cutoff of 2% peripheral blood eosinophils has been shown to have a sensitivity of 90% and specificity of 60% for identifying a sputum eosinophilia of greater than 3% at the time of exacerbation.Citation13
The NLR was calculated as an indicator of the systemic inflammatory response. PLT to MPV: MPV correlates with PLT activity.
Delta values were used to show the differences between values at ICU admission and at the time of ICU discharge.
Data
Data were collected electronically from the hospital database. Subject’s characteristics, comorbid diseases, APACHE II scores,Citation21 complete blood count (CBC) parameters, NLR, delta NLR (admission minus discharge), C-reactive protein (CRP), biochemistry and arterial blood gas (ABG) values on admission to and discharge from the ICU, length of ICU stay (LOS days), and mortality were recorded. COPD subjects were divided into two groups according to their peripheral eosinophil count (group 1, eosinophilic [>2%]; group 2, non-eosinophilic [≤2%]) (). The groups were compared with the recorded data.
Mechanical ventilation
Initially, NIMV was applied to all COPD subjects with hypercapnic respiratory failure unless absolutely contraindicated.Citation19 Contraindications of NIMV were defined as 1) absolute, respiratory arrest and inability to fit mask and 2) relative, medically unstable (hypotensive shock, uncontrolled cardiac ischemia or arrhythmia, uncontrolled copious upper gastrointestinal bleeding), agitation, uncooperativeness, inability to protect airway, impaired swallowing, excessive secretions not managed by secretion clearance techniques, multiple (two or more) organ failure, and recent upper airway or upper gastrointestinal surgery.Citation5,Citation22 NIMV was provided in pressure assist-control mode with ICU mechanical ventilators via a double-tube circuit with a full-face mask. Pressure support was initially set at 8–10 cm H2O and gradually increased to a maximum of 30 cm H2O until the exhaled tidal volume was 5–7 mL/kg and guided by subject tolerance. Positive end-expiratory pressure was set at 5 cm H2O and raised or lowered to treat hypoxemia or enhance subject comfort, respectively. The reasons for NIMV failure in hypercapnic subjects were no pH improvement, no change or a rise in breathing frequency after 1–2 hours, and lack of cooperation.
Invasive mechanical ventilation (IMV) was applied in the presence of absolute or relative contraindications for NIMV, as mentioned earlier. It was applied by assist-control ventilation in pressure control mode. Inspiratory pressure was set for a target tidal volume of 5–7 mL/kg ideal body weight (Airway plateau pressure <30 cm H2O) under a sedation protocol. The Richmond agitation sedation scale was used for infusion and assessment of the daily need for sedation.Citation23 When subjects met the previously described criteria for weaning, the pressure support ventilation mode was used and gradually decreased (1–2 cm H2O every 1–2 hours), and when the T-piece trial duration was 30 minutes, they were extubated.Citation24
Bronchodilator and anti-inflammatory treatment in the ICU
While the subjects were under NIMV or IMV, a short-acting β2 agonist (salbutamol, 100 μg per puff) and ipratropium bromide (100 μg/20 μg per puff) was given every 2–4 hours (four to ten puffs) via a metered dose inhaler chamber (Aerovent, Altech®, Altera Firm, Izmir, Turkey). A nebular form of salbutamol (2.5 mg/2.5 mL per nebule) was given every 15 minutes to 4 hours, or ipratropium bromide/salbutamol (0.5 mg/3.01 mg/2.5 mL per nebule) was given every 2–4 hours for subjects breathing without mechanical ventilation support. Long-acting β2 agonists were not used in COPD subjects with ARF in the ICU. Intravenous methylprednisolone (40–60 mg) was given one–two times daily as an anti-inflammatory for those subjects unable to take oral medications, or those (eligible) subjects with impaired gastrointestinal absorption. The steroid dose was tapered gradually and discontinued over 7 to 10 days. Methylxanthines (theophylline and aminophylline) were not given. All subjects received oxygen for COPD, as well as medication to treat the underlying cause of ICU admission or ARF and comorbidities.Citation1,Citation25,Citation26
Statistical analysis
A descriptive analysis was used to investigate subject demographics and ICU data. Groups were compared using the Mann–Whitney U-tests for nonparametric continuous variables or the Student’s t-tests for parametric continuous variables. The chi-square test was employed for dichotomous variables. The median with interquartile range (IQR) was employed for nonparametric continuous variables, and mean ± standard deviation was used for parametric continuous variables. Count and percentage were used when applicable. Logistic regression analysis was performed to define mortality risk factors in the study population. Age, sex, body mass index, IMV, NIMV applications, peripheral eosinophilia, long-term oxygen or NIMV use, APACHE II score on admission to the ICU, septic shock, resistant pathogen, a CRP greater than 50 mg/dl, and an NLR ≥16 were included in the model. A P-value <0.05 was accepted as statistically significant.
Results
During the study period, 9.6% of the enrolled subjects had eosinophilic COPD (group 1, peripheral blood eosinophil count >2%), and the remainder had non-eosinophilic COPD (group 2, peripheral blood eosinophil count ≤2%). The subject demographics, smoking history, and ICU data are summarized in . Both groups were aged more than 65 years, and the majority was male with a similar body mass index within the normal range. Where the patient was prior to ICU admission and the length of pre-ICU hospital stay are also summarized in . Three hundred and eighteen (49.1%) patients were admitted from the general ward or other ICU, and the median length of stay was 4 days (IQR, 1–7 days). Half of cases in both groups had received long-term oxygen therapy and one-third of the subjects in group 2 had home NIMV. Comorbidities were similar in both groups, except arrhythmia. The non-eosinophilic COPD subjects were significantly more severe (greater APACHE II score), more acidotic and hypercapnic (). Half of the subjects in both groups had cultures analyzed and the non-eosinophilic group had a significantly higher rate of resistant pathogen and septic shock (). The study groups were further sub-grouped according to the presence of arrhythmia and the use of home ventilation. There were no significant differences in the recorded data among these subgroups. However, PaO2/FiO2 ratios of patients with arrhythmia were slightly more hypoxemic in both the eosinophilic and non-eosinophilic groups (PaO2/FiO2 ratio: 130 vs 152, respectively).
Table 1 Demographics and ICU values of study groups
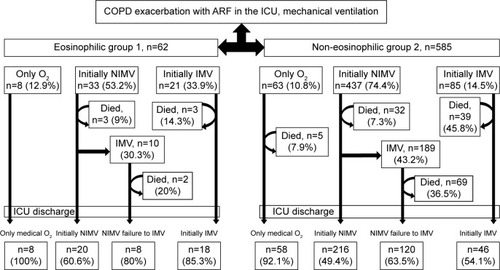
The mechanical ventilation approach and ICU mortality are summarized for the eosinophilic and non-eosinophilic COPD subjects groups in . The eosinophilic group required a higher rate of initial IMV (33.9%), and these patients had a significantly higher rate of arrhythmia (P<0.02) and deeper hypoxemia (). The mortality of those patients was 14.3%. Although the non-eosinophilic COPD patients needed 14.5% of initial IMV, the mortality rate was much higher at 45.8%. NIMV failure rate (death and intubation) was higher in the non-eosinophilic group than the eosinophilic COPD patients (50.6% vs 39.4%) (). In the present study, the non-eosinophilic group had a significantly longer ICU stay and a higher mortality.
Figure 2 Respiratory treatment approach and outcome for study groups.
CBC values on admission to and discharge from the ICU for both groups are shown in . CBC values on admission and discharge for each patient group are compared separately in . Nearly all parameters of CBC values, except mean corpuscular volume (MCV), were significantly different in both groups. The non-eosinophilic group had significantly higher neutrophils, erythrocytes, and MPV, and a lower lymphocyte and PLT count at both admission and discharge.
Table 2 Complete blood count values of study groups on admission to and discharge from the ICU
Table 3 Comparison of complete blood count values on admission and discharge for each group
shows the inflammatory biomarkers of both groups on ICU admission and on ICU discharge. CRP values (on admission to and discharge from ICU) were similar in both groups. However, delta NLR was significantly lower in the eosinophilic COPD group compared with the non-eosinophilic group. PLT to MPV was higher in the eosinophilic group, both on admission to and discharge from the ICU. Delta PLT to MPV was similar in both groups (). Steroid and antibiotic use are summarized in .
Table 4 On the day of admission to and discharge from the ICU for CRP, neutrophil to lymphocyte ratio and platelet to MPV ratio and delta values of study groups
A logistic regression analysis was performed to identify mortality risk factors. We included age, sex, body mass index, IMV, NIMV applications, peripheral eosinophilia, long-term oxygen or NIMV use, APACHE II score on admission to the ICU, presence of arrhythmia, septic shock, resistant pathogen, a CRP greater than 50 mg/mL, and an NLR ≥16. The presence of septic shock, arrhythmia, age more than 80 years, resistant pathogen infection, the need for IMV, an NLR ≥16, and a CRP greater than 50 mg/mL were all found to be risk factors for mortality. The prior use of home NIMV was found to significantly reduce the risk of mortality in the ICU ().
Table 5 Logistic regression analysis of intensive care mortality risk factors of COPD patients with acute respiratory failure
Discussion
We found that only 10% of COPD patients with ARF had peripheral blood eosinophilia. The non-eosinophilic COPD exacerbation group had a higher APACHE II score and worse arterial blood gas on admission. The non-eosinophilic group used NIMV more often initially and had a higher NIMV failure rate, a longer ICU stay, a significantly higher rate of septic shock and resistant pathogen infection, and an increased ICU mortality despite similar steroid and antibiotic usage. Patients with eosinophilic COPD exacerbation had a lower rate of NLR than those with non-eosinophilic COPD exacerbations in the ICU. In contrast to the non-eosinophilic group, the eosinophilic group showed no significant change in NLR during the treatment period. Patients with eosinophilic exacerbations had a higher rate of PLT to MPV compared with the non-eosinophilic group, both on admission to, and discharge from the ICU.
COPD exacerbation requiring ICU admission and peripheral blood biomarkers: CRP, NLR, and PLT to MPV
Studies have shown that the primary etiology of COPD exacerbations is due to infection, either viral or bacterial, or both, and these are associated with high economic costs and an accelerated lung function decline.Citation3,Citation25 The type of inflammation in subjects with COPD exacerbations is neutrophilic, but eosinophilic inflammation is also observed in 10% to 45% of COPD exacerbations.Citation11–Citation16 Bafadhel et al showed that the peripheral eosinophil count is a valid biomarker of COPD exacerbation and they also showed that the 2% threshold value is a sensitive marker for the presence of an eosinophilic attack of COPD that can be corticosteroid responsive.Citation13–Citation16 The prevalence of eosinophilic inflammation in COPD subjects was assessed by Singh et al and samples from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints cohort were included; they found that 37% of the COPD subjects had blood eosinophil counts persistently ≤2%.Citation27 In the present study, only 10% of the subjects with a COPD exacerbation requiring ICU admission had a peripheral blood eosinophil count greater than 2%. The prevalence of eosinophilic inflammation in COPD subjects requiring ICU is, thus far, not well defined.
CRP is the most well-known inflammatory biomarker. A diagnosis of COPD exacerbation can be more sensitive if the COPD patient has a CRP >8 mg/mL together with increasing dyspnea, sputum, volume, or purulence.Citation28 However, this study pointed out that CRP alone is not sufficient and not sensitive and specific enough to confirm an exacerbation. Raised CRP levels also do not reflect the clinical severity of the attack.Citation28 Daniels et al showed that if CRP values are greater than 50 mg/mL, the subjects had a better response to antibiotic treatment.Citation7 Although the present study was not designed to compare the effect of CRP levels, the majority of patients had CRP levels greater than 50 mg/mL. Almost all the patients were treated with antibiotics, either pre-ICU or in the ICU. The decision to use antibiotics was based on a standard general clinical practice approach, without controlled NLR or peripheral eosinophilia. However, if the decision to treat with antibiotics was made according to novel infectious and inflammatory markers such as NLR, MPV, and PLT/MPV, would the outcome of patients with COPD have been better? This may be the subject for future well-designed studies.
Despite steroid and antibiotic treatment, and mechanical ventilation (NIMV or IMV), COPD exacerbations still present a major issue and contribute significantly toward morbidity and mortality in this cohort. In the present study, all eosinophilic and non-eosinophilic COPD patients used steroids and antibiotics, either pre-ICU hospital stay, or in the ICU showed an overall mortality of 25%. The study design did not include an analysis of steroid use and inflammatory response, and as such, the detailed history of steroid usage (at home, and in the emergency department and ward), doses, and duration were not recorded. There are several novel monoclonal antibodies and small molecule antagonists that have been evaluated in clinical trials for the management of COPD exacerbation with promising results. These agents can be particularly successful with appropriate COPD subtype selection.Citation29 Recently, Brightling et al published a randomized placebo-controlled phase IIa study that evaluated benralizumab, an interleukin-5 receptor alpha monoclonal antibody, which depletes blood and sputum eosinophils in patients with COPD exacerbation.Citation30 They assessed the ability of benralizumab to reduce the annualized rate of acute exacerbations of COPD. Although they were unable to show a decline of the COPD annualized rate of acute exacerbations, their subgroup analysis did show that patients with baseline blood eosinophil concentrations of 200 cells per μL had a greater improvement of acute exacerbation of COPD and FEV1 (statistically nonsignificant).
Identifying biomarkers of COPD exacerbations could be helpful in defining the concept of different “exacerbation phenotypes”. The more common exacerbation is bacterial (non-eosinophilic). Recently, Günay et al studied the NLR and MPV in COPD subjects during the stable period and during acute exacerbation and they found that COPD subjects had higher NLR and CRP and lower MPV than healthy subjects (MPV and NLR: healthy subjects, 10.1 and 1.71; stable COPD subjects, 8.80 and 2.59; and exacerbation of COPD, 8.0 and 4.28, respectively).Citation17 Our study showed similar results for MPV; however, NLR was nearly three times higher in the non-eosinophilic COPD exacerbation subjects than in the COPD exacerbation subjects in Günay et al’s study.Citation17 NLR values are increased according to the severity of COPD attack. Thus, NLR may be helpful when deciding on a treatment management plan according to the type of COPD exacerbation. In the present study, the NLR was nearly three times higher in the non-eosinophilic group than the eosinophilic group, and this may help to indicate the severity of infectious and inflammatory COPD exacerbation.
ICU outcomes, length of stay, and mortality
A previously published study investigating the outcomes of ARF subjects with COPD requiring ICU admission from 2008 to 2012 (n=1,013) showed a 16.9% mortality rate.Citation31 Mortality in 698 subjects with COPD exacerbations of infectious origin was 21.6%; however, COPD subjects with pneumonia (n=241) had a higher rate of mortality (39%). Factors indicating a poor prognosis for COPD subjects in the ICU are the need for IMV, presence of pneumonia, cachexia, coronary artery diseases, arrhythmia, hypertension, and higher APACHE II scores.Citation31 Mortality risk factors in the present study were found to be similar: the need for IMV, the presence of arrhythmia as a comorbidity, the presence of septic shock and a resistant pathogen infection, CRP values ≥50 mg/mL, and an NLR ≥16 on admission to the ICU. A meta-analysis that included 979 COPD subjects from 14 studies found that COPD subjects had an average of 35% intubation for IMV and a mean mortality rate of 45%.Citation32 In the present study, IMV was found to be a risk factor for mortality in ICU patients with ARF and the overall IMV application rate was 47.3%; however, mortality was less than their meta-analysis (37%). The IMV application rate was higher in the eosinophilic group in our study, although they had a better pH and PaCO2 and lower APACHE II score on admission. This may be explained by the fact that they had higher rate of arrhythmia and hypoxemia. Steer et al claimed that the “DECAF score” (the Dyspnoea, Eosinopenia, Consolidation, Acidaemia and Atrial Fibrillation score) that uses indices can accurately predict in-hospital mortality in patients hospitalized with acute exacerbations of COPD.Citation33 In the present study, we did not use the DECAF score; however, the non-eosinophilic group had a higher rate of mortality. The study also showed that the NIMV failure rate and overall mortality were two times higher in the non-eosinophilic group. In contrast, peripheral eosinophilia has been shown to be associated with an increase in all-cause mortality in subjects with airway diseases compared with healthy subjects.Citation34,Citation35 However, comparing healthy subjects with eosinophilic COPD subjects in terms of mortality is not an equal comparison. Further studies are needed to evaluate the hospital and long-term mortality in COPD subjects with eosinophilic and non-eosinophilic exacerbations.
Limitations
There were some limitations to this study. First, it was a retrospective and single-center study with insufficient data; however, we believe that it provides valuable clinical information (due to the large sample size) for assessing ICU outcomes of COPD subjects with eosinophilic and non-eosinophilic exacerbations. Second, COPD severity and spirometry results were not recorded because of the absence of electronically available data; some asthmatic patients may have been misclassified as spirometry with reversibility tests or any tests to distinguish COPD from asthma were not done, in ICU or after discharge. However, patients with asthma, or patients with suspicious COPD, were excluded. Although this may weaken our results, the majority of subjects had severe COPD, as determined by the use of long-term oxygen therapy and NIV at home. We can claim that nearly all the COPD patients were in a severe stage of the disease. Our results cannot be generalized for all stages of patients with COPD. Third, due to the retrospective nature of the study, the management of COPD could not be controlled and as such the treatment choices may seem insufficient in some cases. However, the ICU team included all pulmonologists and intensivists, and they followed the same treatment protocol and worked 24/7 in the ICU.
The strength of this study lies in the results showing the eosinophilic COPD exacerbation rate in a large sample size study in a disease-specific respiratory ICU and conducted by a pulmonologist–intensivist. These could increase the reliability of our results.
Conclusion
COPD exacerbations requiring ICU admission showed peripheral blood eosinophilia in nearly 10%; this was a lower rate of eosinophilia than other COPD exacerbations admitted to the hospital. Non-eosinophilic COPD exacerbations can be more severe, have a higher APACHE II score, and have sepsis-related complications. As such, non-eosinophilic COPD exacerbations have a higher rate of NIMV failure, a longer LOS and a higher mortality rate.
COPD exacerbation management, especially steroid use, is not standardized in the ICU. Besides Anthonisen criteria, a higher NLR and the identification of a peripheral non-eosinophilic state can be helpful for making a decision on antibiotic vs steroid use for the management of COPD exacerbation in the ICU.
Acknowledgments
The authors thank Dr Sharon Forsyth for editing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- KhilnaniGCBangaASharmaSKPredictors of mortality of patients with acute respiratory failure secondary to chronic obstructive pulmonary disease admitted to an intensive care unit: a one year studyBMC Pulm Med200441215566574
- AlaithanAMMemonJIRehmaniRSQureshiAASalamAChronic obstructive pulmonary disease: hospital and intensive care unit outcomes in the Kingdom of Saudi ArabiaInt J Chron Obstruct Pulmon Dis2012781982323269866
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for Diagnosis, Management, and Prevention of COPDGlobal Initiative for Chronix Obstructive Lung Disease updated 2012 Available from: http://www.goldcopd.org
- ElliottMWConfalonieriMNavaSWhere to perform noninvasive ventilation?Eur Respir J2002191159116612108872
- AmbrosinoNVaghegginiGNon-invasive ventilation in exacerbations of COPDInt J Chron Obstruct Pulmon Dis2007247147618268921
- PapiABellettatoCMBraccioniFInfections and airway inflammation in chronic obstructive pulmonary disease severe exacerbationsAm J Respir Crit Care Med2006173101114112116484677
- DanielsJMSnijdersDde GraaffCSVlaspolderFJansenHMBoersmaWGAntibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201018115015719875685
- WaltersJAGibsonPGWood-BakerRHannayMWaltersEHSystemic corticosteroids for acute exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev20091CD00128819160195
- Christ-CrainMJaccard-StolzDBingisserREffect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trialLancet200436360060714987884
- SaettaMDi StefanoAMaestrelliPAirway eosinophilia in chronic bronchitis during exacerbationsAm J Respir Crit Care Med1994150164616527952628
- BrightlingCEMonteiroWWardRSputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trialLancet20003561480148511081531
- KitaguchiYKomatsuYFujimotoKHanaokaMKuboKSputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthmaInt J Chron Obstruct Pulmon Dis2012728328922589579
- BafadhelMMcKennaSTerrySAcute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkersAm J Respir Crit Care Med2011184666267121680942
- BafadhelMMcCormickMSahaSProfiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary diseaseRespiration2012831364421912093
- BafadhelMMcKennaSTerrySBlood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trialAm J Respir Crit Care Med20121861485522447964
- BafadhelMDaviesLCalverleyPMAaronSDBrightlingCEPavordIDBlood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysisEur Respir J201444378979124925917
- GünayESarınç UlaşlıSAkarONeutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective studyInflammation201437237483024078279
- WangRTLiJYCaoZGLiYMean platelet volume is decreased during an acute exacerbation of chronic obstructive pulmonary diseaseRespirology20131881244124823786593
- NavaSHillNNon-invasive ventilation in acute respiratory failureLancet2009374968525025919616722
- AnthonisenNRManfredaJWarrenCPHershfieldESHardingGKNelsonNAAntibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Inter Med19871066196204
- KnausWADraperEAWagnerDPZimmermanJEAPACHE II: a severity of disease classification systemCrit Care Med1985138188293928249
- AmbrosinoNVaghegginiGNoninvasive positive pressure ventilation in the acute care setting: where are we?Eur Respir J200831487488618378782
- SesslerCNGosnellMSGrapMJThe Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patientsAm J Respir Crit Care Med2002166101338134412421743
- BolesJMBionJConnorsAWeaning from mechanical ventilationEur Respir J20072951033105617470624
- SnowVLascherSMottur-PilsonCJoint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal MedicineEvidence base for management of acute exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med2001134759559911281744
- NavaSKarakurtSRampullaCBraschiAFanfullaFSalbutamol delivery during non-invasive mechanical ventilation in patients with chronic obstructive pulmonary disease: a randomized, controlled studyIntensive Care Med200127101627163511685304
- SinghDKolsumUBrightlingCELocantoreNAgustiATal-SingerRECLIPSE investigatorsEosinophilic inflammation in COPD: prevalence and clinical characteristicsEur Respir J20144461697170025323230
- HospersJJRijckenBSchoutenJPPostmaDSWeissSTEosinophilia and positive skin tests predict cardiovascular mortality in a general population sample followed for 30 yearsAm J Epidemiol199915048249110472948
- DasguptaANeighbourHNairPTargeted therapy of bronchitis in obstructive airway diseasesPharmacol Ther2013140321322223845862
- BrightlingCEBleeckerERPanettieriRAJrBenralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a studyLancet Respir Med201421189190125208464
- OngelEAKarakurtZSalturkCHow do COPD comorbidities affect ICU outcomes?Int J Chron Obstruct Pulmon Dis201491187119625378919
- QuonBSGanWQSinDDContemporary management of acute exacerbations of COPD: a systematic review and metaanalysisChest2008133375676618321904
- SteerJGibsonJBourkeSCThe DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary diseaseThorax2012671197097622895999
- HospersJJSchoutenJPWeissSTRijckenBPostmaDSAsthma attacks with eosinophilia predict mortality from chronic obstructive pulmonary disease in a general population sampleAm J Respir Crit Care Med19991601869187410588599
- HospersJJRijckenBSchoutenJPPostmaDSWeissSTEosinophilia and positive skin tests predict cardiovascular mortality in a general population sample followed for 30 yearsAm J Epidemiol199915048249110472948