Abstract
Background
Few data are available about the effects of respiratory muscle training with normocapnic hyperpnea (NH) in COPD. The aim is to evaluate the effects of 4 weeks of NH (Spirotiger®) on ventilatory pattern, exercise capacity, and quality of life (QoL) in COPD patients.
Methods
Twenty-six COPD patients (three females), ages 49–82 years, were included in this study. Spirometry and maximal inspiratory pressure, St George Respiratory Questionnaire, 6-minute walk test, and symptom-limited endurance exercise test (endurance test to the limit of tolerance [tLim]) at 75%–80% of peak work rate up to a Borg Score of 8–9/10 were performed before and after NH. Patients were equipped with ambulatory inductive plethysmography (LifeShirt®) to evaluate ventilatory pattern and thoracoabdominal coordination (phase angle [PhA]) during tLim. After four supervised sessions, subjects trained at home for 4 weeks – 10 minutes twice a day at 50% of maximal voluntary ventilation. The workload was adjusted during the training period to maintain a Borg Score of 5–6/10.
Results
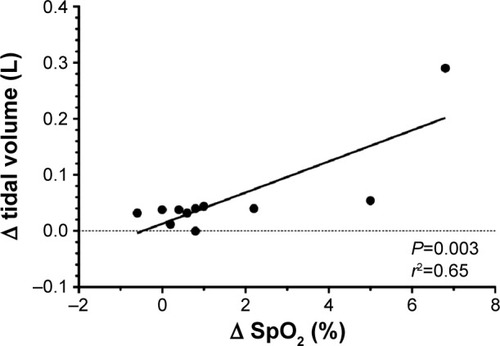
Twenty subjects completed the study. After NH, maximal inspiratory pressure significantly increased (81.5±31.6 vs 91.8±30.6 cmH2O, P<0.01); exercise endurance time (+150 seconds, P=0.04), 6-minute walk test (+30 meters, P=0.03), and QoL (−8, P<0.01) all increased. During tLim, the ventilatory pattern changed significantly (lower ventilation, lower respiratory rate, higher tidal volume); oxygen desaturation, PhA, and dyspnea Borg Score were lower for the same work intensity (P<0.01, P=0.02, and P<0.01, respectively; one-way ANOVA). The improvement in tidal volume and oxygen saturation after NH were significantly related (R2=0.65, P<0.01).
Conclusion
As expected, NH improves inspiratory muscle performance, exercise capacity, and QoL. New results are significant change in ventilatory pattern, which improves oxygen saturation, and an improvement in thoracoabdominal coordination (lower PhA). These two facts could explain the reduced dyspnea during the endurance test. All these results together may play a role in improving exercise capacity after NH training.
Introduction
Dyspnea is the primary symptom that limits exercise in many patients with COPD.Citation1,Citation2 In COPD, exercise limitation is clearly multifactorial; one of the main contributorsCitation1 is ventilatory limitation due to increased airway resistance and pulmonary hyperinflation, both increasing the work of breathing.Citation3–Citation5 In addition, COPD patients exhibit abnormal thoracoabdominal motion during exercise and this is another cause of exercise limitation.Citation6–Citation9 In particular, Chien et alCitation9 showed that asynchronous thoracoabdominal motion occurred early during exercise in patients with mild COPD, contributing to a decreased 6-minute walk distance. Respiratory muscle weakness can also play a role in dyspnea perception and exercise limitation. Indeed, inspiratory muscle weakness is observed in COPD patientsCitation10,Citation11 and contributes to dyspnea perception.Citation1,Citation12,Citation13
For this reason, there has been an interest in applying a specific training to the inspiratory muscles in COPD patients. Different forms of respiratory muscle training (RMT) have been used, and from recent meta-analysesCitation14,Citation15 it is clear that RMT increases inspiratory muscle performance and functional exercise capacity (ie, 6-minute walk distance, endurance exercise capacity, and maximal oxygen consumptionCitation14,Citation15), and decreases dyspnea, but the clinical importance of these improvements remains unclear. Most studies investigated the effects of RMT with resistive or threshold loading,Citation14 while a few studies evaluatedCitation16–Citation18 the effects of RMT with normocapnic hyperpnea (NH) even though Koppers et alCitation16 reported that NH could be the most appropriate technique for improving respiratory muscle endurance capacity, which is required during endurance exercises.
None of these studiesCitation16–Citation18 investigated the effects of NH on thoracoabdominal coordination and oxygen saturation (SpO2) during exercise in COPD patients, whereas only one studyCitation16 investigated the effects of NH on the ventilatory pattern, showing lower minute ventilation and respiratory rate and larger tidal volume during exercise.
The aim of this study was to evaluate the effects of 4 weeks of NH (using the Spirotiger® device) on thoracoabdominal coordination, SpO2, ventilatory pattern, exercise capacity, and quality of life (QoL) in COPD patients.
Methods
Subjects were consecutively recruited from the outpatient clinic of the Department of Respiratory Disease, Ferrara University Hospital, Italy.
Inclusion criteria were as follows: 1) COPD as defined according to international guidelines;Citation19 2) forced expiratory volume in 1 second (FEV1) between 30% and 80% predicted,Citation20 after bronchodilatation; and 3) stable clinical condition for at least 6 weeks. Exclusion criteria were as follows: 1) cardiac comorbidity (ie, recent myocardial infarction, myocardial ischemia, and cardiac arrhythmia); 2) inability to use the device; and 3) inclusion in any pulmonary rehabilitation program.
The Ethics Committee of the University of Ferrara approved the study (protocol number 090591). Informed consent was obtained from each subject.
Study design
On the first day of the study, patients performed a respiratory function test (forced flow–volume curve) and their inspiratory muscle strength (maximal inspiratory pressure [MIP]) was measured. After 30 minutes of rest, a 6-minute walk test (6MWT) was performed.Citation21 The St George Respiratory Questionnaire (SGRQ) for measuring health-related QoL was completed.Citation22
On the second day, an incremental walking test was performed, followed, after 30 minutes of rest, by an endurance test to the limit of tolerance (tLim) (ie, until subjects were unable to continue due to symptoms). Daily physical activity was monitored using the SenseWear Armband® (BodyMedia, Pittsburgh, PA, USA) validated for estimating energy expenditure in COPD patients.Citation23,Citation24
After 4 weeks of respiratory muscle endurance training (NH), the subjects repeated all the tests in a standardized manner and sequence.
The testing and the training procedure are detailed in the sections Exercise testing and Respiratory muscle endurance training.
Respiratory function
The forced flow–volume curve was performed (JAEGER® MasterScreen Body; San Diego, CA, USA) to measure forced vital capacity and FEV1. In accordance with the guidelines of the American Thoracic Society/European Respiratory Society,Citation25 subjects repeated the maneuver at least three times and the two largest values of forced vital capacity and FEV1 had to be within 0.15 L of each other. Reference normal values were taken from the European Community for Steel and Coal.Citation20
After respiratory function, MIP was measured; starting from residual volume, the subject was asked to inspire as deeply as possible, maintaining the maximal pressure for at least 1.5 seconds. The pressure was measured with a manometer (CARDINAL Health microRPM®; San Diego, CA, USA). Subjects repeated the maneuver a minimum of five times and reproducibility had to be within 5%–10% in accordance with the guidelines.Citation26 The highest value was considered for comparison before and after training.
Exercise testing
The 6MWT was performed in a corridor 24 m long. The subjects were instructed to cover as much distance as possible in 6 minutes and standardized verbal encouragement was given.Citation21 The maximum distance walked was recorded.
The maximal incremental exercise test was performed on a treadmill (Runner Galaxy MTC, Cavezzo, Italy). During the test, the work rate was increased every 10 meters by 0.2 km/h.Citation27 Arterial blood pressure was measured every minute. Heart rate and SpO2 were continuously monitored. At the end of the test, maximal working capacity (Speedmax) was recorded.
A constant-load endurance exercise test was performed on a treadmill at a work rate of 80%–85% of the individual’s Speedmax and continued to the tLim.Citation28 Subjects were not encouraged during the test. Perception of dyspnea and muscular fatigue were measured using the Borg ScaleCitation29 at regular intervals. The tLim was terminated when the subjects indicated they were unable to continue due to intolerable dyspnea and/or muscular fatigue (Borg Score 8–9/10), or were unable to keep up with the speed of the treadmill; the duration of the test was recorded as walking endurance time.
During tLim, patients were equipped with ambulatory inductive plethysmography (LifeShirt® System, San Diego, CA, USA).Citation30 The LifeShirt® features rib cage and abdominal inductance bands embedded within an elasticized vest, connected to a battery-operated handheld computer. The handheld computer continuously records data. Before each test, a calibration of the system was performed. After the testing session, the data were downloaded and analyzed with a dedicated software (Vivologic 3.0, Vivonoetics, San Diego, CA, USA) for calculating respiratory variables: ventilation, tidal volume, and respiratory rate. The phase angle (PhA) was obtained as a measure of asynchrony between rib cage and abdominal movements (thoracoabdominal asynchrony). A pulse oximeter and an electrocardiograph were integrated into the LifeShirt system.
Respiratory muscle endurance training
The training protocol lasted 4 weeks and was performed by means of Spirotiger® (MVM, Linate, MI, Italy), which consists of a handheld unit with a pouch and a base station. A two-way piston valve connected to a rebreathing bag permits a constant isocapnic end-tidal CO2 fractionCitation31 to be maintained.
Before starting the protocol, subjects underwent four supervised training sessions to learn the technique and to define the target training ventilation. In accordance with previous studies,Citation16–Citation18 the size of the bag was adjusted to 50%–60% of the subject’s vital capacity and the breathing frequency chosen was such that ventilation corresponded to 50%–60% of maximal voluntary ventilation (calculated as 35 times FEV1). The tidal volume was adjusted by changing the size of the bag and breathing frequency was changed accordingly to maintain target training ventilation (ie 50%–60% of maximal voluntary ventilation). The subjects trained 20 minutes daily (1 minute of exercise and 1 minute of rest, ten times, twice a day), 7 days a week, for 5 weeks. While performing NH, the patients wore a nose clip to ensure breathing occurred exclusively through the training device.
During the training period, a diary documented the compliance with home-based training: number, duration of the sessions, and rating of perceived respiratory exertion (Borg Score) were reported.
Statistical analysis
Data are reported as mean ± standard deviation. The Student’s t-test was used to compare baseline characteristics and training changes. The one-way ANOVA test with analysis of the covariate was used to evaluate the effects of training on ventilation, ventilatory pattern, and PhA; the P-values were adjusted according to the Bonferroni correction. The parametric Pearson correlation coefficient was used to describe the relationships between variables. Statistical significance was accepted at P<0.05. All the analyses were performed using GraphPad Prism 40.
Results
Twenty-six patients were included in the study. Baseline characteristics of the subjects are shown in . The mean age of the study population was 69±8 years. According to Armband® data, the physical activity status of the subjects was between low and moderate (). Medication was not changed during the study period.
Table 1 Baseline characteristics (26 subjects)
Twenty patients completed the study. Six patients did not complete the study: two because of poor tolerance to the device, three because of poor compliance, and one because of excessive fatigue.
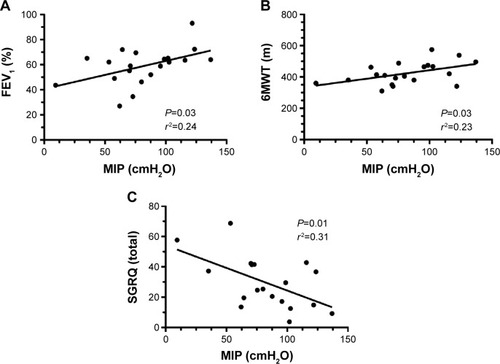
Intriguingly, at baseline (T0), a positive correlation is found between MIP and FEV1 as a percentage of predicted values () and between MIP and the 6MWT (); an inverse correlation is shown between MIP and the total SGRQ score ().
Figure 1 Correlation between MIP and FEV1 (A), 6MWT (B), and SGRQ (C) before the training.
NH training
Analysis of the diaries showed that all patients trained 20 minutes daily, every day at a workload corresponding to 51% of maximal voluntary ventilation with a mean Borg Score of 6/10.
Inspiratory muscle performance
shows the effects of 4 weeks of home-based NH on respiratory function and muscle strength. After training (T1), MIP significantly increased (+10.3 cmH2O; Student’s t-test, P=0.0001). A strong correlation was found between the value at T0 and at T1 (R2=0.90, P<0.00001). The probability plot distribution confirms normal distribution of the data.
Table 2 Pretraining and posttraining values (20 subjects)
Exercise performance and ventilatory pattern
The effects of NH on exercise performance are shown in .
Regarding the 6MWT, the distance covered increased by 30 m (Student’s t-test, P=0.03).
The duration of tLim significantly increased by 2 minutes and 30 seconds (Student’s t-test, P=0.04); during the test, average speed was 4.8±0.9 km/h, corresponding to 79% of Speedmax (6.1±1.2 km/h).
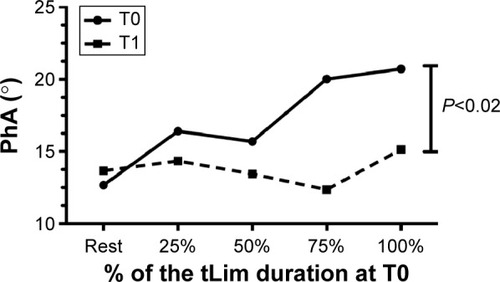
Regarding ventilatory data, a significant increase in tidal volume at rest was shown (T0: 0.29±0.74 mL and T1: 0.32±0.81 mL; Student’s t-test, P=0.02). During tLim, after the training, significantly lower ventilation and respiratory rate were found (one-way ANOVA, P<0.05; for ventilation, respiratory rate, and tidal volume, respectively). Furthermore, a lower increase in PhA was found during exercise (one-way ANOVA, P=0.02; ).
Figure 2 The ventilation (A), respiratory rate (B), and tidal volume trend (C) during tLim before T0 and after T1 training.
Notes: ANOVA test between T0 and T1. Mean value at rest, at 25%, 50%, 75%, and 100% of the tLim duration at T0. *P<0.05 between T0 and T1.
Abbreviations: tLim, endurance test to the limit of tolerance; T0, baseline; T1, after 4 weeks of respiratory muscle training; ANOVA, analysis of variance; min, minute.
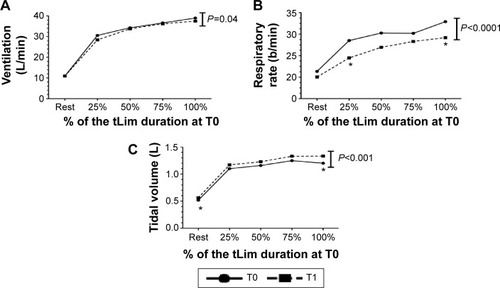
Figure 3 The PhA trend during tLim before and after training.
Abbreviations: PhA, phase angle; tLim, endurance test to the limit of tolerance; T0, baseline; T1, after 4 weeks of respiratory muscle training.
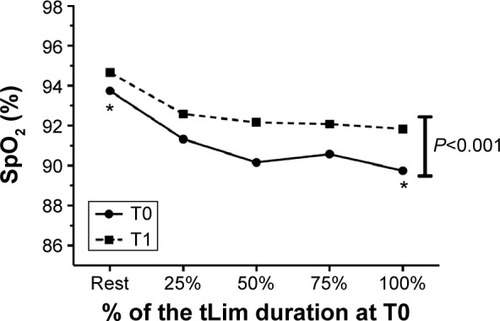
Regarding SpO2, an improvement was found at rest (Student’s t-test, P=0.02; ). The SpO2 monitoring during tLim showed significantly higher values (one-way ANOVA, P<0.01; ).
Figure 4 The SpO2 trend during tLim before and after training.
Abbreviations: SpO2, oxygen saturation; tLim, endurance test to the limit of tolerance; T0, baseline; T1, after 4 weeks of respiratory muscle training; ANOVA, analysis of variance.
After training, a correlation was found during tLim between the changes in tidal volume and SpO2 ().
Figure 5 Correlation between the changes in tidal volume and SpO2, before and after training, during tLim.
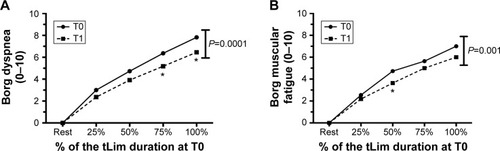
All these findings were associated with lower dyspnea and muscular fatigue Borg Score (one-way ANOVA, P<0.01; , respectively).
Figure 6 The Borg dyspnea (A) and muscular fatigue (B) trend during tLim before and after training.
Abbreviations: tLim, endurance test to the limit of tolerance; T0, baseline; T1, after 4 weeks of respiratory muscle training; ANOVA, analysis of variance.
Discussion
The aim of this study was to evaluate the effects of 4 weeks of inspiratory muscle training with NH on ventilatory pattern, exercise capacity, and QoL in COPD patients. The main results are the changes in ventilatory pattern and the improved thoracoabdominal coordination during exercise after training.
Regarding the ventilatory pattern, after training at the same work intensity, we found lower ventilation, a decrease in respiratory rate, and an increase in tidal volume. The slower and deeper ventilation allows improved alveolar ventilation, which in turn has an effect on gas exchange. The subjects exhibit improved oxygenation, which is related to the increase in tidal volume. Improved oxygenation is already evident at rest when the ventilatory pattern is characterized by a higher tidal volume at the same ventilation. Several studiesCitation32–Citation34 have already shown that deeper and slower breathing (so-called yoga breathing) improves SpO2 in both healthy subjects exposed to high altitudeCitation32 and subjects suffering from chronic diseases such as heart failureCitation33 and COPD.Citation34
To our knowledge, only KoppersCitation16 has described the ventilatory pattern changes after NH, but only during exercise. In his study,Citation16 he also highlights that deeper and slower ventilation reduces the work of breathing, delaying respiratory muscle fatigue, and dyspnea perception. Moreover, improved thoracoabdominal coordination plays a key role in dyspnea perception, even though few data are available in literature. Indeed, only two studiesCitation9,Citation35 have investigated this asynchrony in COPD subjects. In particular, ChienCitation9 has shown that thoracoabdominal asynchrony reduces exercise capacity in moderate and severe COPD. The mechanism, however, remains unclear.Citation36,Citation37 Our results are in line with this result; in fact, in our study, patients with moderate and severe COPD show an increased exercise capacity together with better thoracoabdominal coordination. Both the change in ventilatory pattern and the improved thoracoabdominal coordination can explain the reduction in dyspnea perception.
Besides the innovative results related to ventilation, we also found a significant increase in inspiratory muscle strength after NH. This is a quite controversial point. In fact, it is clear from meta-analysesCitation14,Citation15 that NH increases inspiratory muscle endurance and only one studyCitation18 showed that it improved respiratory muscle strength. However, the increase in respiratory muscle strength, and not just endurance, is clinically relevant to COPD patients.Citation38,Citation39 In fact, Terzano et alCitation38 and Tudorache et alCitation39 have demonstrated that airway obstruction is closely associated with lower respiratory muscle pressures. In our study, we also found, at baseline, a correlation between MIP and the severity of bronchial obstruction. Furthermore, Tudorache et alCitation39 found a correlation between MIP and the 6MWT distance, as in our study. However, to the best of our knowledge, there is no study in literature demonstrating a significant inverse correlation between MIP and QoL.
Regarding exercise capacity, the positive effects of NH in COPDCitation16–Citation18 are already known. In this study, we have found a higher duration of the endurance test (+43%) and an increase in walking distance during the 6MWT (+30 m), which are the minimal important difference.Citation28,Citation40,Citation41
Another interesting point is the evidence that the level of physical activity of the subjects did not change during the study period. The use of the multi-sensor Armband® allowed us to verify that the energy expenditure and the number of daily steps did not change significantly after NH. Therefore, no significant physical activity changes could have influenced our results.Citation42
This study has some limitations. The main limitation is the absence of a sham group. So far, there is no instrument available that allows NH without any workload, which should be the gold standard for control group training. Among all papers discussing NH, only threeCitation17,Citation18,Citation43 studied a control group training with an incentive spirometer with minimal resistance and a low respiratory rate; however, this device does not completely satisfy our purposes.
Another limitation is the high number of dropouts (23%). A careful analysis of the characteristics of these subjects could have helped us to better identify the subjects that could benefit from this training. However, we did not find any significant differences between the two groups as regards age, respiratory function, exercise capacity, anthropometric characteristics, and QoL. All subjects but one dropped out because of poor compliance and poor adaptation to the device. Therefore, it seems that motivation is the main factor for completing the training.
Conclusion
NH is an effective treatment for COPD to improve respiratory muscle strength, ventilatory pattern, and thoracoabdominal coordination, which have a positive effect on SpO2. As a consequence, dyspnea perception decreases, exercise capacity increases, and QoL improves. These preliminary results seem to show that NH can be used as a nonpharmacological therapy to complement exercise, or as an alternative aid for COPD patients to improve exercise capacity and QoL.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The text was revised by a professional native English person (Help S.r.l., Ferrara, Italy).
Disclosure
This study had no financial support. The authors report no conflicts of interest in this work.
References
- O’DonnelDELavenezianaPDyspnea and activity limitation in COPD: mechanical factorsCOPD20084225236
- GosselinkRTroostersTDecramerMPeripheral muscle weakness contributes to exercise limitation in COPDAm J Respir Crit Care Med19961539769808630582
- NiciLMechanisms and measures of exercise intolerance in chronic obstructive pulmonary diseaseClin Chest Med20002169370411194780
- SinderbyCSpahijaJBeckJDiaphragm activation during exercise in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20011631637164111401887
- O’DonnellDEBertleyJCChauLKWebbKAQualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanismsAm J Respir Crit Care Med19971551091159001298
- AlvesGSBrittoRRCamposFCVilacaABMoraesKSParreiraVFBreathing pattern and thoracoabdominal motion during exercise in chronic obstructive pulmonary diseaseBraz J Med Biol Res2008411194595019099148
- SharpJTGoldbergNBDruzWSFishmanHCDanonJThoraco-abdominal motion in chronic obstructive pulmonary diseaseAm Rev Respir Dis197711514756138371
- DelgadoHRBraunSRSkatrudJBReddanWGPegelowDFChest wall and abdominal motion during exercise in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis198212622002057103243
- ChienJYRuanSYHuangYCYuCJYangPCAsynchronous thoraco-abdominal motion contributes to decreased 6-minute walk test in patients with COPDRespir Care201358232032622782088
- DecramerMDemedtsMRochetteFBilletLMaximal transrespiratory pressures in obstructive lung diseaseBull Eur Physiopathol Respir1980164794907407438
- PolkeyMIKyroussisDHamnegardCHMillsGHGreenMMoxhamJDiaphragm strength in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1996154131013178912741
- KillianKJJonesNLRespiratory muscles and dyspneaClin Chest Med198892372483292125
- HamiltonNKillianKJSummersEJonesNLMuscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disordersAm J Respir Crit Care Med1995152202120318520771
- GosselinkRDe VosJVan Den HeuvelSPSegersJDecramerMKwakkelGImpact of inspiratory muscle training in patients with COPD: what is the evidence?Eur Respir J201137241642521282809
- GeddesELO’BrienKReidWDBrooksDCroweJInspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic reviewRespir Med2008102121715172918708282
- KoppersRJVosPJBootCRFolgeringHTExercise performance improves in patients with COPD due to respiratory muscle endurance trainingChest2006129488689216608934
- SchererTASpenglerCMOwassapianDImhofEBoutellierURespiratory muscle endurance training in chronic obstructive pulmonary disease: impact on exercise capacity, dyspnea, and quality of lifeAm J Respir Crit Care Med200016251709171411069801
- MadorMJDenizOAggarwalAShafferMKufelTJSpenglerCMEffect of respiratory muscle endurance training in patients with COPD undergoing pulmonary rehabilitationChest200512831216122416162709
- Global Strategy for the Diagnosis, Management, and Prevention of COPD Global Initiative for Chronic Obstructive Pulmonary disease (GOLD)2011 Available from: http://www.goldcopd.comAccessed May 12, 2015
- European Community for Steel and CoalStandardized lung function testingEur Respir J1993162527
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function LaboratoriesATS statement: guidelines for the six-minute walk testAm J Respir Crit Care Med200216611111712091180
- JonesPWQuirkFHBaveystockCMThe St George’s respiratory questionnaireRespir Med199185suppl B25311759018
- PatelSABenzoRPSlivkaWASciurbaFCActivity monitoring and energy expenditure in COPD patients: a validation studyCOPD2007410711217530503
- Van RemoortelHRasteYLouvarisZPROactive consortiumValidaty of six activity monitors in chronic obstructive disease: a comparison with indirect calorimetryPLoS One201276e3919822745715
- MillerMRHankinsonJBrusascoVStandardization of spirometryEur Respir J20052631933816055882
- American Thoracic Society/European Respiratory SocietyATS/ERS Statement on respiratory muscle testingAm J Respir Crit Care Med2002166451862412186831
- ManfrediniFConconiFMalagoniAMSpeed rather than distance: a novel graded treadmill test to assess claudicationEur J Vasc Endovasc Surg20042830330915288635
- Puente-MaestuLVillarFde MiguelJClinical relevance of constant power exercise duration changes in COPDEur Respir J200934234034519251787
- BorgGAPsycho-physical bases of perceived exertionMed Sci Sports Exerc1982143773817154893
- ClarenbachCFSennOBrackTKohlerMBlochKEMonitoring of ventilation during exercise by a portable respiratory inductive plethysmographyChest20051281282129016162719
- VergesSBoutellierUSpenglerCMEffect of respiratory muscle endurance training on respiratory sensations, respiratory control and exercise performance: a 15-year experienceRespir Physiol Neurobiol2008161162218182333
- BernardiLPassinoCWilmerdingVBreathing patterns and cardiovascular autonomic modulation during hypoxia induced by simulated altitudeJ Hypertens200119594795811393679
- BernardiLSpadaciniGBellwonJHajiricRRoskammHFreyAWEffect of breathing rate on oxygen saturation and exercise performance in chronic heart failureLancet199835130813119643792
- PomidoriLCampigottoFAmatyaTMBernardiLCogoAEfficacy and tolerability of yoga breathing in patients with chronic obstructive pulmonary disease: a pilot studyJ Cardiopulm Rehabil Prev20092913313719305239
- PrioriRAlivertiAAlbuquerqueALQuarantaMAlbertPCalverleyPMJThe effect of posture on asynchronous chest wall movement in COPDAppl Physiol2013114810661075
- SharpJTDrutzWSMoisanTFosterJMachnachWPostural relief of dyspnea in severe chronic obstructive pulmonary diseaseAm Rev Respir Dis198012222012117416599
- GosselinkRAWagenaarRCRijswijkHSargeantAJDecramerMLDiaphragmatic breathing reduces efficiency of breathing in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19951514113611427697243
- TerzanoCCeccarelliDContiVGrazianiERicciAPetroianniAMaximal respiratory static pressures in patients with different stages of COPD severityRespir Res2008219818208602
- TudoracheVOanceaCMlădinescuOFClinical relevance of maximal inspiratory pressure: determination in COPD exacerbationInt J Chron Obstruct Pulmon Dis2010511912320461143
- HollandAEHillCJRasekabaTLeeANaughtonMTMcDonaldCFUpdating the minimall important difference for six-minute walk distance in patients with chronic obstructive pulmonary diseaseArch Phys Med Rehabil20109122122520159125
- PolkeyMISpruitMAEdwardsLDSix minute-walk test in chronic obstructive pulmonary disease: minimal critically important difference for death or hospitalizationAm J Respir Crit Care Med201318748286
- TroosterTGosselinkRDecramerMShort- and long- effects of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trialAm J Med2000109320721210974183
- VergesSLenherrOHanerACSchulzCSpenglerCMIncreased fatigue resistance of respiratory muscles during exercise after respiratory muscle endurance trainingAm J Physiol Regul Integr Comp Physiol2007292R1246R125317068160
