Abstract
Long-acting bronchodilators are the mainstay of the treatment of COPD. With the advent of several combination inhalers with long-acting antimuscarinic agents (LAMAs) and long-acting beta agonists (LABAs), the choice of therapy in the treatment of COPD has been ever expanding. With the focus of COPD management shifting from FEV1-based treatment escalation to symptoms and risk-based treatment, we are seeing a paradigm shift in COPD treatment with early introduction of LAMA–LABA combination as a single inhaler. Glycopyrronium/formoterol fumarate fixed-dose combination formulated in a familiar metered-dose inhaler format using proprietary co-suspension technology is a new option on the market. We purport to discuss the evidence behind the approval of the drug combination and its place in therapy.
Introduction
COPD is a common cause of morbidity and mortality in both developing and developed countries. It is the fourth leading cause of death in the world according to WHO statisticsCitation1 and is predicted to be the third leading cause of death in the world by 2020.Citation2 It presents a significant economic burden to the society. The mainstay of COPD management has been long-acting bronchodilators including long-acting antimuscarinic agents (LAMAs) and long-acting beta agonists (LABAs), the latter frequently combined with inhaled corticosteroid (ICS). The initial 2001 Global initiative in Obstructive Lung Diseases (GOLD) guidelines recommended short-acting bronchodilators in patients with mild obstruction, long-acting bronchodilators (LAMA) in patients with moderate obstruction, and a combination of long-acting bronchodilators and ICS in those with severe obstruction and a history of frequent or severe exacerbations. However, physicians have increasingly realized that grading COPD severity and determining treatment based on spirometric values are simplistic, as there are patients across the spectrum of spirometric values who are very symptomatic and at high risk of frequent exacerbations. The most recent GOLD Report acknowledges this by categorizing COPD into different risk groups (A–D), and now recommends tailoring therapy to the patient’s overall symptom burden and risk of adverse outcomes.Citation3 Evidence suggests that ICS is beneficial in reducing frequent exacerbations but can probably be safely withdrawn in patients who are not at high risk of exacerbations.Citation4 It is in this context that LAMA–LABA combinations are emerging, and a few studies have shown superiority of this combination to LABA–ICS combination in symptom control. There are five fixed-dose LAMA–LABA combination currently available, including formoterol/aclidinium, indacaterol/glycopyrronium, olodaterol/tiotropium, umeclidium/vilanterol, and formoterol/glycopyrronium. Formoterol/glycopyrronium is a new LAMA–LABA combination that is recently approved in a few countries around the world, including the USA. Herein, we discuss the efficacy and safety data behind this unique LABA–LAMA combination and its role in therapy of COPD. We will summarize the evidence behind the use of long-acting bronchodilator therapy in COPD and will then focus on this particular combination in the treatment of COPD.
Formoterol in COPD
The treatment of symptomatic COPD patients historically involved short-acting bronchodilators, most commonly beta agonists. With the advent of salmeterol, and subsequently more than half-a-dozen LABA compounds, LABA has had a center stage in the treatment of COPD. A Cochrane database systematic review of 23 studies concluded in 2000 that LABA improves FEV1 and health-related quality-of-life while reducing the use of short-acting bronchodilators and the number of exacerbations.Citation5
Formoterol was initially demonstrated to produce effective and sustained bronchodilation in COPD in open-label trials in the late 1980s.Citation6 It was shown to have more sustained bronchodilation up to 12 hours compared to salbutamolCitation7 and ipratropium.Citation8 Subsequent double-blind randomized controlled trials have demonstrated its efficacy in the COPD population with improvements reported in FEV1 and exercise capacity.Citation9–Citation11
Formoterol has subsequently been used either alone as a Foradil Aerolizer™ or in combination with ICSs for the management of COPD. It was subsequently formulated in a proprietary porous lipid particle co-suspension metered-dose inhaler (MDI) technology by Pearl Therapeutics (Morristown, NJ, USA; now a subsidiary of AstraZeneca) and has been shown to have comparable efficacy to formoterol delivered in an Aerolizer device.Citation12
Glycopyrronium in COPD
Ipratropium bromide was the first short-acting antimuscarinic agent used in the management of COPD symptoms, based on a few small-scale studies in the last century. With the advent of long-acting form in tiotropium, LAMAs have been used extensively in the management of COPD, either as monotherapy in moderate COPD or as combination with LABA–ICS in more advanced form of the disease. Several newer antimuscarininc agents have since been developed in the last decade, including aclidinium, umeclidinium, and glycopyrronium.
Interest in glycopyrrolate (glycopyrronium bromide) as a useful drug for relieving bronchospasm dates back to the 1980s, with case reports of its efficacy with intravenous use in the literature.Citation13 The first randomized controlled trial demonstrating the efficacy of inhaled glycopyrrolate nebulization in addition to albuterol was performed in the 1990s.Citation14 Subsequent studies of inhaled glycopyrronium demonstrated effective bronchodilation with early onset of action and sustained effect for 24 hours after use in patients with COPD at a dose of 50 μg daily in a dry powder Breezhaler™ device (non-US version of Neohaler™ device).Citation15 Several randomized controlled trials have since been conducted demonstrating efficacy of the compound in COPD patients.
GEM1 study was a 12-week randomized, double-blind, placebo-controlled study that randomized 441 patients with moderate-to-severe obstruction to glycopyrronium 15.6 μg or placebo via Neohaler device; it demonstrated clinically significant improvement in lung function, dyspnea score, St George’s Respiratory Questionnaire (SGRQ) score, COPD Assessment Test score, and rescue medication use with comparable side effects. The median time of onset of action of the medication was 18.8 min (for a change in FEV1 of at least 100 mL), which might be beneficial for rapid symptom relief. A twice daily dosing was chosen in this study due to dose-ranging study showing increased efficacy in comparison to once daily dosing.Citation16
GLOW1 and 2 randomized 1888 patients to glycopyrronium 50 μg once daily in a dry powder inhaler via a Breezhaler device (NVA237), tiotropium 18 μg once daily, or placebo for 26–52 weeks and demonstrated equivalent efficacy of glycopyrronium to tiotropium in terms of improvement in FEV1 and reduction in the rate of moderate-to-severe exacerbation. The effects were sustained through weeks 26 and 52. The onset of action was more rapid compared to tiotropium. Common side effects included dry mouth, nasopharyngitis, cough, bacterial URTI, and headache. Major cardiovascular side effects were similar to placebo and tiotropium.Citation17,Citation18 This efficacy was redemonstrated in a predominantly Chinese population in the GLOW7 study that randomized patients to glycopyrronium or placebo.Citation19
Since one of the major limiting factors in a patient with COPD is dyspnea and reduced exercise capacity, GLOW3 study was conducted to demonstrate the efficacy of glycopyrronium in improving exercise capacity. It demonstrated statistically significant improvement in ergometer exercise time after 21 days of the use of glycopyrronium 50 μg once daily, as well as improvement in inspiratory capacity, thereby denoting decrease in air trapping.Citation20 GLOW5 was a 12-week randomized controlled trial comparing efficacy of glycopyrronium 50 μg daily to tiotropium 18 μg daily. It demonstrated that glycopyrronium was not only as effective as tiotropium in improving FEV1 and symptom scores at 12 weeks but also provided superior bronchodilation and improvement in FEV1 compared to tiotropium on day 1, demonstrating its rapid onset of action.Citation21 GLISTEN study demonstrated the efficacy of glycopyrronium when added to salmeterol/fluticasone combination.Citation22 Finally, results are awaited from two studies (GOLDEN-3 and 4) evaluating the efficacy of nebulized glycopyrrolate (developed by Sunovion Pharmaceuticals Inc®, Marlborough, MA, USA).
Glycopyrronium bromide (glycopyrrolate) was subsequently developed in an inhaled MDI form by Pearl Therapeutics (subsidiary of AstraZeneca), which is referred to by its active ingredient glycopyrronium. It is formulated in a special porous particle comprising phosphatidylinositolcholine and calcium chloride, on which the active medication is adsorbed. This fine-particle formulation allows stability of the MDI formulation of the drug and homogenous delivery of the active medication into the distal airway. In the dose-finding pharmacokinetic study done on the MDI formulation of the drug, BID dosing was found to be most appropriate for sustained improvement in both the 1–12 hours FEV1 and 12–24 hours FEV1, with total recommended daily dose of 57.6 and 115.2 μg.Citation23
Formoterol–glycopyrronium combination in COPD
The mainstay of COPD management for the last one and a half decade has been the use of inhaled LAMA, with addition of the combination of ICS–LABA for more severe COPD or those with frequent exacerbations. Support for this approach comes from multiple trials and meta-analyses. However, we are now beginning to realize that not all patients with COPD need ICSs. The WISDOM study demonstrated this by withdrawing inhaled steroids from the regimen of stable patients with COPD and showed that there was no worsening of symptoms, exacerbation rate, or quality of life.Citation4 However, ICSs come with their own risk. Multiple studies including a Cochrane database review have shown an increased risk of pneumonia with commonly used inhaled steroids.Citation24 It hence seems logical that if the patients could be managed without inhaled steroids, while at the same time providing maximal bronchodilation and improved quality of life, then this should be the best course of action. This is true for patients who have severe activity limitation but are not frequent exacerbators. However, caution should be exercised when withdrawing steroids in patients who have been stable on LABA–ICS combination because a statistically significant decline in FEV1 was demonstrated after withdrawal of inhaled steroid in both the WISDOM studyCitation4 and the GLUCOLD study from the Netherlands.Citation25
The efficacy of LAMA–LABA fixed-dose combination (FDC) was first studied with umeclidinium–vilanterol, which not only showed non-inferiority in control of symptoms but also showed superiority in at least one study.Citation26 A Cochrane database review from 2015 concluded that there was a small increase in mean FEV1 and in health-related quality-of-life with addition of LABA to LAMA therapy.Citation27 Several LAMA–LABA FDCs have since been developed, including glycopyrrolate/indacaterol, tiotropium/olodaterol, and aclidinium/formoterol. All of these FDCs were formulated either as dry-powder inhalers (DPIs) or as soft mist inhaler. Since MDIs remain a popular and widely familiar device for drug delivery, a novel MDI formulation of formoterol fumarate (9.6 μg) and glycopyrronium (18 μg) was developed utilizing the proprietary co-suspension technology. Drug particles were suspended in the hydroxyfluoralkalane (HFA) propellant using distearoyl-phosphatidylcholine particles. This formulation was developed to provide a homogenous distribution of the drug in an HFA medium without deposition of drug crystals. The combination has subsequently received approval in several countries including the USA, where it is currently marketed as the Bevespi Aerosphere™ by AstraZeneca.
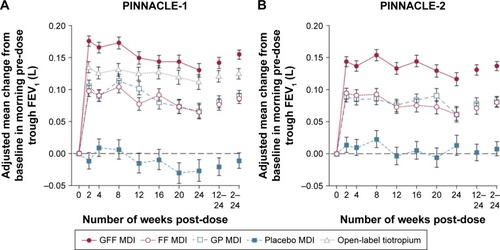
PINNACLE 1 and 2 were both 24-week double-blind, placebo-controlled Phase III trials that compared the safety and side effects of the combination glycopyrronium/formoterol fumarate (GFF) MDI, used two puffs twice daily, to their individual monocomponents (GP MDI, glycopyrrolate 18 μg and FF MDI, formoterol fumarate 9.6 μg), open-label tiotropium DPI (only in PINNACLE-1), and placebo.Citation28 The combined sample size was 3,710 patients; 53% of whom had moderate obstruction (GOLD 2), 41% had severe obstruction (GOLD 3), and 5% had very severe obstruction (GOLD 4). The analysis involved 3,699 patients, of whom 3,001 (81%) completed 24 weeks of treatment. GFF combination demonstrated improvement versus placebo and monocomponents in morning pre-dose trough FEV at 24 weeks (P<0.0001) as well as in 2-hour post-dose FEV1 (P<0.0001) (). Pooled SGRQ data demonstrated a reduction in total score with GFF at week 24 versus placebo (P=0.0051) and GP (P<0.0094). Hazard ratio (HR) of time to first exacerbation was lower with GFF compared to placebo (HR 0.736, P<0.002) and glycopyrronium (HR 0.781, P<0.002), but not formoterol. See for a summary of primary and secondary end points in both studies. Patients also needed less rescue inhaler medication use compared to placebo (P<0.0001) or GP (P<0.0001). Treatment emergent adverse effects were similar across all the active-treatment groups. Those that were reported at a rate higher than the placebo were cough, sinusitis, COPD, back pain, and bronchitis. Adverse effect leading to discontinuation was highest in the placebo group. Cardiovascular side effects were low and comparable in all groups at 0.4%–0.7%. All cause deaths were also comparable in all groups.
Table 1 Summary of primary and secondary end points and safety outcomes from the PINNACLE-1 and PINNACLE-2 study
Figure 1 Mean change from baseline in morning pre-dose trough FEV1 in different treatment groups.
Abbreviations: FF, formoterol fumarate; GFF, glycopyrronium/formoterol fumarate; GP, glycopyrronium; MDI, metered-dose inhaler.
PINNACLE 3 was the extension of the previous two studies where 893 patients who completed the original studies continued the same treatment, including monocomponents and open-label tiotropium for another 52 weeks. There were no unexpected safety findings. Overall incidence of adverse events was similar across all groups (60%–69%), with the most commonly reported events being nasopharyngitis (6.8%), cough (4.3%), and upper respiratory tract infection (3.8%). GFF demonstrated significant improvement in trough and 2-hour post-dose peak FEV1 versus FF (65 and 88 mL, respectively), GP (57 and 129 mL, respectively), and tiotropium (25 and 93 mL, respectively). GFF also demonstrated significant improvement versus GP (P<0.001) and open-label tiotropium (P=0.002) for average daily use of rescue medication.Citation29
Safety
Long-acting bronchodilators have been found to be relatively safe in COPD patients in multiple other studies. In a large study of 1,429 patients treated with LABA arformoterol (Brovana nebulizer; Sunovion Pharmaceuticals; Marlborough, MA, USA) and salmeterol (Serevent inhalation aerosol, GlaxoSmithKline, Research Triangle Park, NC, USA) who underwent Holter monitoring, atrial tachycardia was found to be relatively common at baseline, but there was only marginal increase in atrial tachycardia with the use of beta agonists, and no increased risk of more serious arrhythmias including ventricular tachycardia.Citation30 A randomized placebo-controlled trial was done to assess cardiac safety in COPD patients given formoterol 12 μg twice daily showed very low evidence of ventricular arrhythmia in both groups, with no significant difference between the active and placebo groups.Citation31 Inhaled tiotropium was demonstrated to be safe in multiple studies including the large-scale TIOSPIR trial.Citation32 Similarly, the cardiac safety of glycopyrronium has been demonstrated in multiple randomized controlled trials, with no increase in cardiac side effects over placebo.Citation33,Citation34 The PINNACLE studies demonstrated acceptable safety profile of the GFF combination compared to its monocomponents as detailed above and as detailed in .
Conclusions
In conclusion, the formoterol–glycopyrronium combination MDI appears to be a safe and effective treatment option for moderate-to-severe COPD in terms of symptoms reduction, improvement of quality-of-life, and decreasing the frequency of exacerbations. The newly developed proprietary porous particle technology allows the drug to be formulated in an MDI form, which is a widely familiar device to the COPD patients. This new drug formulation in a familiar delivery system should be a good alternative to maximize bronchodilation in patients with COPD.
Acknowledgments
Dr Criner reports grants from Boehringer-Ingelheim, Novartis, Astra Zeneca, Respironics, MedImmune, Actelion, Forest, Pearl, Ikaria, Aeris, PneumRx, and Pulmonx and consultation fees from Amirall, Boehringer-Ingelheim, and Holaira. Dr Dhungana reports grants from Oncocyte.
Disclosure
The authors report no conflicts of interest in this work.
References
- WHOProjections of mortality and causes of death, 2015–2030WHO2014 Available from: http://www.who.int/healthinfo/global_burden_disease/projections/enaccessed April 17, 2017
- MathersCDLoncarDProjections of global mortality and burden of disease from 2002 to 2030PLoS Med2015311e442
- Global Initiative for Obstructive Lung DiseaseGlobal strategy for the diagnosis, management and prevention of chronic obstructive pulmonary diseaseGOLD2017 Available from: http://www.goldcopd.org2017Accessed April 17, 2017
- MagnussenHDisseBRodriguez-RoisinRWithdrawal of inhaled glucocorticoids and exacerbations of COPDN Engl J Med2014371141285129425196117
- AppletonSPoolePSmithBJLong-acting beta2-agonists for poorly reversible chronic obstructive pulmonary diseaseCochrane Database of Systematic Reviews20063CD001104
- Schultze-WerninghausGMulticenter 1-year trial on formoterol, a new long-acting β2-agonist, in chronic obstructive airway diseaseLung1990168183891974687
- MaesenFPSmeetsJJGubbelmansHLLZweersPGBronchodilator effect of inhaled formoterol versus salbutamol over 12 hoursChest19909735905941968371
- DahlRGreefhorstLANowakDInhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001164577878411549532
- FusoLIncalziRABassoSEffects of formoterol inhaled dry powder on exercise performance in chronic obstructive pulmonary disease: a single-center, randomized, double-blind, placebo-controlled, crossover studyCurr Ther Res Clin Exp200364531732624944380
- AalbersRAyresJBackerVFormoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3-month trialEur Respir J200219593694312030736
- CampbellMElirazAJohanssonGFormoterol for maintenance and as-needed treatment of chronic obstructive pulmonary diseaseRespir Med200599121511152016199148
- QuinnDSealeJPReisnerCA randomized study of formoterol fumarate in a porous particle metered-dose inhaler in patients with moderate-to-severe COPDRespir Med201410891327133525060541
- SlovisCMDanielsGMWhartonDRIntravenous use of glycopyrrolate in acute respiratory distress due to bronchospastic pulmonary diseaseAnn Emerg Med19871688989003619169
- CydulkaRKEmermanCLEffects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary diseaseAnn Emerg Med1995254470473 Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=cctr&NEWS=N&AN=CN-00112786Accessed April 17, 2017.7710150
- FogartyCHattersleyHDiLDrollmannABronchodilatory effects of NVA237, a once daily long-acting muscarinic antagonist, in COPD patientsRespir Med2011105333734221144724
- LaForceCFeldmanGSpangenthalSEfficacy and safety of twice-daily glycopyrrolate in patients with stable, symptomatic COPD with moderate-to-severe airflow limitation: the GEMI studyInt J COPD201611112331243
- D’UrzoAFergusonGTvan NoordJAEfficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trialRespir Res201112115622151296
- KerwinEHébertJGallagherNEfficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 studyEur Respir J20124051106111423060624
- WangCSunTHuangYEffcacy and safety of once-daily glycopyrronium in predominantly Chinese patients with moderate-to-severe chronic obstructive pulmonary disease: the GLOW7 studyInt J COPD2015105768
- BeehKMSinghDDi ScalaLDrollmannAOnce-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trialInt J COPD20127503513
- ChapmanKRBeehKBeierJA blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 studyBMC Pulm Med201414111124387157
- FrithPAThompsonPJRatnavadivelRGlycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study, a randomised controlled trialThorax201570651952725841237
- RennardSFogartyCReisnerCRandomized study of the safety, pharmacokinetics, and bronchodilatory efficacy of a proprietary glycopyrronium metered-dose inhaler in study patients with chronic obstructive pulmonary diseaseBMC Pulm Med201414111825027304
- KewKMSeniukovichAInhaled steroids and risk of pneumonia for chronic obstructive pulmonary diseaseCochrane Database Syst Rev2014103CD010115
- KunzLIZPostmaDSKloosterKRelapse in FEV1 Decline After Steroid Withdrawal in COPDChest2015148238939625836351
- SinghDWorsleySZhuCHardakerLChurchAUmeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trialBMC Pulm Med2015159126286141
- FarneHACatesCJLong-acting beta 2-agonist in addition to tiotropium versus either tiotropium or long-acting beta 2-agonist alone for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20151010CD008989
- MartinezFJRabeKFFergusonGTEfficacy and safety of glycopyrrolate/formoterol MDI formulated using Co-Suspension™ Delivery Technology in patients with COPDChest2016151201734035727916620
- HananiaNATashkinDPKerwinESafety and efficacy of a novel LAMA/LABA co-suspension technology Glycopyrrolate/Formoterol fixed-dose combination delivered by MDI: results of a one-year extension study in patients with COPD (PINNACLE-3)D36. COPD: LABA, LAMA, ICS, and combinations. American Thoracic Society International Conference AbstractsAmerican Thoracic Society2016A6791A6791
- HanrahanJPGroganDRBaumgartnerRAArrhythmias in patients with chronic obstructive pulmonary disease (COPD)Medicine (Baltimore)200887631932819011503
- CampbellSCCrinerGJLevineBECardiac safety of formoterol 12 microg twice daily in patients with chronic obstructive pulmonary diseasePulm Pharmacol Ther200720557157916911869
- WiseRAAnzuetoACottonDTiotropium Respimat inhaler and the risk of death in COPDN Engl J Med2013369161491150123992515
- Van de MaeleBFabbriLMMartinCHortonRDolkerMOverendTCardiovascular safety of QVA149, a combination of Indacaterol and NVA237, in COPD patientsCOPD20107641842721166630
- VogelmeierCVerkindreCCheungDSafety and tolerability of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patientsPulm Pharmacol Ther201023543844420416390
