Abstract
Background
Elevated cardiovascular disease risk is observed in patients with COPD. Non-invasive assessments of endothelial dysfunction and arterial stiffness have recently emerged to provide mechanistic insight into cardiovascular disease risk in COPD; however, the reproducibility of endothelial function and arterial stiffness has yet to be investigated in this patient population.
Objectives
This study sought to examine the within-day and between-day reproducibility of endothelial function and arterial stiffness in patients with COPD.
Methods
Baseline diameter, peak diameter, flow-mediated dilation, augmentation index, augmentation index at 75 beats per minute, and pulse wave velocity were assessed three times in 17 patients with COPD (six males, eleven females, age range 47–75 years old; forced expiratory volume in 1 second =51.5% predicted). Session A and B were separated by 3 hours (within-day), whereas session C was conducted at least 7 days following session B (between-day). Reproducibility was assessed by: 1) paired t-tests, 2) coefficients of variation, 3) coefficients of variation prime, 4) intra-class correlation coefficient, 5) Pearson’s correlations (r), and 6) Bland–Altman plots. Five acceptable assessments were required to confirm reproducibility.
Results
Six out of six within-day criteria were met for endothelial function and arterial stiffness outcomes. Six out of six between-day criteria were met for baseline and peak diameter, augmentation index and pulse wave velocity, whereas five out of six criteria were met for flow-mediated dilation.
Conclusion
The present study provides evidence for within-day and between-day reproducibility of endothelial function and arterial stiffness in patients with COPD.
Introduction
COPD, the third leading cause of morbidity and mortality worldwide, is a progressive disease of the lungs that is characterized by a persistent airflow limitation.Citation1 Common phenotypes observed in patients with COPD are chronic inflammation and oxidative stress, systemic consequences that appear to contribute to an augmented cardiovascular mortality risk in these patients.Citation2–Citation4
More patients with COPD die from cardiovascular disease and coronary heart disease than of direct pulmonary complications.Citation5 Although the pathogenic mechanism that links COPD to vascular related diseases is poorly understood, data have supported the potential contribution of vascular endothelial dysfunction.Citation4,Citation6 In fact, the degree of endothelial dysfunction is proportional with disease severity measured by airway obstruction.Citation2,Citation7 Accordingly, the assessment of vascular function represents an important clinical outcome in the ongoing investigation of the pathophysiology of COPD.
The flow-mediated dilation (FMD) test is the most widely utilized, non-invasive assessment of nitric oxide bioavailability and vascular endothelial function in humans. Additionally, augmentation index (AIx) and pulse wave velocity (PWV) represent two non-invasive methods for assessing aortic and central arterial stiffness, respectively. A lower FMD strongly correlates with vascular damage,Citation8 and both FMD and PWV have been shown to be independent predictors of cardiovascular disease.Citation9,Citation10 In fact, numerous investigations have described how the FMD test can be utilized to provide prognostic information of cardiovascular disease related risk.Citation11–Citation15 Unsurprisingly, both FMDCitation2,Citation16–Citation18 and PWVCitation10,Citation19 have been reported to be attenuated in patients with COPD compared with age-matched controls. In addition, our group has recently identified oxidative stress as a contributing mechanism to this associated vascular dysfunction.Citation18
Since the awareness of cardiovascular disease risk in patients with COPD is growing, we are anticipating more investigations of endothelial function and arterial stiffness in this patient population. There are however, several biological and environmental aspects of COPD that may impact the reliability of these non-invasive techniques.Citation20 Thus, the purpose of this study was to evaluate the within-day and between-day reproducibility of FMD and arterial stiffness in patients with COPD. We hypothesized that repeated assessments of FMD and arterial stiffness in the same day and on different days would be similar in patients with COPD.
Methodology
Experimental design
All patients with COPD reported to the Laboratory of Integrative and Vascular Exercise Physiology on two experimental visits separated by at least 1 week apart. On the first visit, a pulmonary function testing (PFT) was performed and FMD, PWV, and AIx were assessed twice, 3 hours apart, to determine within-day reproducibility of endothelial function and arterial stiffness. At least 1 week following visit 1, patients reported back to the Laboratory of Integrative and Vascular Exercise Physiology where the same assessments were repeated to determine between-day reproducibility of endothelial function and arterial stiffness. Patients were asked to abstain from physical activity, tobacco products for at least 12 hours, and vitamin supplementation for 72 hours prior to each session visit.
Subjects
Seventeen patients with COPD (six males, eleven females, age range 47–75 years) volunteered to participate in the study. Patients were excluded from the study if: 1) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) was >0.7, 2) FEV1 was >80%, 3) they had evidence of overt heart disease, diabetes, gastrointestinal bleeding or pulmonary hypertension, or 4) they were taking any medications known to affect the FMD response. All participants were informed of the objectives and possible risks of the investigation before written consent for participation was obtained. The study followed the principles of the Declaration of Helsinki and was approved by the Institutional Review Board at Georgia Regents University.
Pulmonary function testing
A PFT using closed circuit spirometry (ParvoMedics, Sandy, UT, USA) was performed to determine FVC, FEV1, FVC/FEV1, and forced expiratory flow (FEF25–75) in all patients according to the American Thoracic Society standards.Citation21 Additional PFT methodology can be found in the Supplementary materials.
Brachial artery FMD
Endothelial function was determined via brachial artery FMD in accordance with the latest tutorial on the ultrasound assessment of FMD.Citation22 Additional FMD methodology can be found in the Supplementary materials.
Arterial tonometry
Pulse wave analysis
AIx was determined by applanation tonometry (SphygmoCor; AtCor Medical, West Ryde, Australia) of the left radial artery, calibrated with the brachial systolic and diastolic pressure. Since AIx varies with heart rate, it is adjusted to 75 beats per minute (AIx75).Citation23
PWV
Carotid-femoral PWV was determined in duplicate using the SphygmoCor system by sequentially recording electrocardiographic-gated carotid and femoral artery waveforms by applanation tonometry.Citation24,Citation25 Additional arterial tonometry methodology can be found in the Supplementary materials.
Assessment of reproducibility and statistical analysis
All measurements are expressed as mean ± standard error of mean. All statistical analyses were performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA) and significance was set at P<0.05. Within-day reproducibility was determined by comparing session A vs session B, whereas between-day reproducibility compared session A vs session C. In order to demonstrate reproducibility, five out of the six following criteria were required for each outcome: 1) a non-significant (P>0.05) paired t-test, 2) a significant intra-class correlation coefficient (ICC), 3) a significant Pearson’s correlation coefficient (r), 4) a coefficient of variation (CV= SD/mean ×100) of <20% and 35% for all variables and FMD, respectively, 5) a CV prime (CV′= [100× SD]/[mean +100]) of <3%,Citation26 and 6) a Bland–Altman plot with only two values accepted outside of the 95% CI. In addition, analysis of covariance (ANCOVA) for endothelial function and arterial stiffness was conducted using age, race, sex, and cotinine as covariates.
Results
Study participants
Demographic and clinical characteristics of all patients with COPD who participated in this study are presented in . Majority of the patients (53%) were classified as Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 2, whereas 23% and 24% of patients met criteria for stage 1 and 3, respectively. No significant differences in demographic or clinical variables, including changes in PFT, were identified between visits.
Table 1 Characteristics and blood chemistry of patients with COPD
Reproducibility of endothelial function
Within-day (session A and session B) and between-day (session A and session C) assessments of reproducibility of endothelial function are presented in . Parameters obtained from the FMD test included baseline diameter (cm), peak diameter (cm), and FMD (%). Individual reproducibility assessments that meet the aforementioned criteria are in bold.
Table 2 Within-day (session A vs session B) and between-day (session A vs session C) reproducibility of endothelial function
FMD is reproducible within the same day in patients with COPD
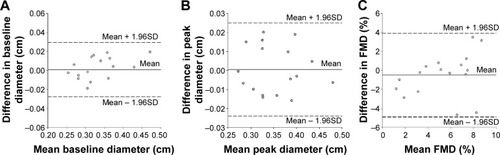
Mean values of baseline diameter, peak diameter, and FMD were all similar (P>0.05) between session A and session B. In addition, all endothelial function testing variables exhibited acceptable CV, CV′, and the ICC and Pearson correlation coefficients were all significant (P<0.03). illustrates the within-day (session A and session B) Bland–Altman plots for baseline diameter (), peak diameter (), and FMD (). All points fall within the 95% CI for peak diameter and FMD; however, there is only one point that falls outside of the CI for baseline diameter. Taken together, all six within-day reproducibility criteria were met for baseline diameter, peak diameter, and FMD.
Figure 1 Bland–Altman analysis plots illustrating no systematic bias and good within-day reproducibility.
FMD is reproducible between-days in patients with COPD
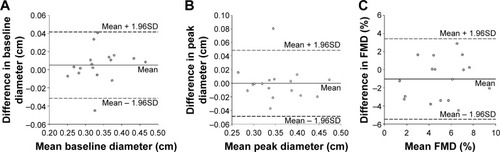
Mean values of baseline diameter, peak diameter, and FMD were all similar (P>0.05) between session A and session C. An acceptable CV and CV′ was observed for baseline and peak diameter, but not for FMD (%). In addition, the ICC and Pearson correlation coefficients were all significant (P≤0.01) for all of the endothelial function testing variables. illustrates the between-day (session A and session C) Bland–Altman plots for baseline diameter (), peak diameter (), and FMD (). All points fall within the 95% CI for FMD; however, there is only one point each that falls outside of the CI for baseline diameter and peak diameter. All six between-day reproducibility criteria were met for baseline diameter and peak diameter, whereas five out of the six criteria were met for FMD.
Figure 2 Bland–Altman analysis plots illustrating no systematic bias and good between-day reproducibility.
Reproducibility of arterial stiffness
Within day (session A and session B) and between day (session A and session C) reproducibility of arterial stiffness are presented in . Parameters of arterial stiffness testing included AIx (%), AIx75 (%), and PWV (m/s). Individual reproducibility assessments that meet the aforementioned criteria are in bold.
Table 3 Within-day (session A vs session B) and between-day (session A vs session C) reproducibility of arterial stiffness
Arterial stiffness is reproducible within the same day in patients with COPD
Mean values of AIx, AIx75, and PWV were all similar (P>0.05) between session A and session B. In addition, all parameters of arterial stiffness exhibited acceptable CV, CV′, and significant ICC and Pearson correlation coefficients (P≤0.01). Furthermore, all points fall within the 95% CI on the Bland–Altman plot for AIx and AIx75; however, there is one point that falls outside of the CI for PWV (Figure S1). All six within-day reproducibility criteria were met for AIx, AIx75, and PWV.
Arterial stiffness is reproducible between-days in patients with COPD
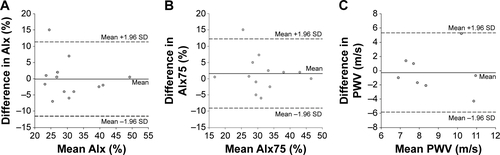
No differences in mean AIx, AIx75, and PWV were observed between session A and session C (all P>0.05). In addition, all arterial stiffness testing variables exhibited acceptable CV, CV′, and significant ICC and Pearson correlation coefficients (P≤0.01). Furthermore, all points fall within the 95% CI on the Bland–Altman plot for PWV; however, there is one point each that falls outside of the CI for AIx and AIx75 (Figure S2). All six between-day reproducibility criteria were met for AIx, AIx75, and PWV.
Discussion
Due to elevated cardiovascular disease risk associated with lung disease, there is emerging interest in conducting clinical trials that target vascular health outcomes in patients with COPD. The present study not only supports within-day, but also between-day reproducibility of vascular health assessments using FMD and arterial tonometry in patients with COPD. These findings are particularly important when evaluating the potential mechanisms that contribute to cardiovascular disease risk in COPD, either in a single testing day or over a longer treatment period.
Patients with COPD present a high risk of cardiovascular disease associated with the disturbance of the vascular endothelium.Citation2,Citation7,Citation18 Local inflammation in respiratory airways induces the over production of reactive oxygen species, chemokines, and cytokines which evoke a systemic perturbation that reduces nitric oxide bioavailability and contributes to systemic vascular dysfunction.Citation18,Citation27 In fact, the degree of endothelial impairment is not only related with disease severity assessed by FEV1 (% predicted), but is also associated with a worse overall prognosis.Citation2,Citation17,Citation28 In an effort to control for the impact of pulmonary exacerbation throughout the present investigation, it is important to emphasize that no changes in pulmonary function were observed in the patients across the multiple testing sessions.
Vascular health reproducibility criteria
Studies in the field suggest the use of three variability indices as an approach to establish consistency and standardization of the technique and provide an accurate assessment of reproducibility. For the current study we included the mean differences between sessions, the CV, and Pearson’s correlations which have been previously recommended,Citation29,Citation30 however, we also included CV′, ICC, and Bland–Altman plots to provide a more robust assessment of vascular health reproducibility in patients with COPD.
Reproducibility of endothelial function in COPD
Since 1992, the FMD test has been widely utilized as a non-invasive method to evaluate systemic vascular endothelial functionCitation31 and predict future cardiovascular disease and events in humans.Citation22 The assessment of endothelial function with FMD has been shown to correlate with other invasive techniques,Citation32 thus asserting the utility that this procedure presents for a non-invasive and fast peripheral artery function evaluation. Indeed, our group and others have contributed to the overall consensus that the FMD test is in fact, a reproducible technique in healthy adults.Citation26,Citation33,Citation34 It is important to note; however, that some studies have described low consistency in the stability of brachial artery FMD,Citation35,Citation36 whereas others have reported high reproducibility.Citation20,Citation30,Citation37–Citation39 Nonetheless, this is the first study to investigate reproducibility of the FMD test in patients with COPD.
The present findings document similar average values for within-day (sessions A and B) and between-day (sessions A and C) reproducibility assessments. In fact, the overall differences between means are considerably lower than the acceptable reproducibility value previously proposed by Corretti et al.Citation29 In addition, we report reproducibility assessments of CV, CV′, Pearson correlation coefficients, ICC, and Bland–Altman plots. In the current study, the mean CVFMD reported for within-day and between-day reproducibility is 31% and 38%, respectively. However, it has to be noted that the expression of FMD as a percentage makes the data very sensitive to small changes. In fact, the range of CV described in the literature for FMD varies from 2% to 84%Citation40 with acceptable overall FMD variations of 35%Citation22,Citation29 indicating reproducibility.Citation22,Citation29 In addition, utilizing a CV′ criteria of <3% for determining reproducibility of FMD (%) over time is considered acceptable.Citation29 In an effort to not only create a robust assessment, but also reinforce the within-day and between-day reproducibility of endothelial function in patients with COPD, the present study included ICC as a reliability parameter, and Bland–Altman plots as a way to estimate the 95% limits of agreement between measurements. The statistically significant and high ICC values reported here constitute reliability in endothelial function within-day and between-day. Additionally, the Bland–Altman plots emphasize the general concept of agreement supported by the other FMD reproducibility indices, both in the same day and on different days. Collectively, our findings provide evidence that assessment of endothelial function using the FMD test is reproducible within the same day as well as between days in patients with COPD.
It is worth noting that, in spite of the increasing interest in this technique, several practical challenges affect the reproducibility of this method, restricting its clinical use.Citation37 Expensive equipment, high technical standardization, considerable sonographer experience, and a precise analysis of the images have limited a wider application of this technique. For those reasons, we have been consistent with the technical aspects of the FMD method by following the recommendations for the ultrasonic assessment of FMDCitation22 to minimize both physiological and technical concerns that influence validity and reproducibility of this procedure. Hence, to avoid variability in the FMD technique and get reliable results presented here, a highly-skilled operator had performed all the analysis, utilizing arterial images to identify internal landmarks and the same arterial segment in each participant throughout the three sessions of the present investigation. We also believe that the use of a stereotactic probe-holder and an automatic software analysis system (Brachial Analyzer Software) have reduced the variability of the FMD test.
Aside from the technical aspects, the instability of the brachial artery dilation has been also related with the spontaneous changes of the arterial walls. Those variations not only impact the hyperemic response, but introduce fluctuations in blood pressure, blood flow, blood viscosity, and arterial stiffness,Citation30,Citation41 all of which can potentially increase the variability of the FMD technique. For that reason, to rule out the interference of other possible factors in the FMD reproducibility assessment and make the present results more robust, we also considered the inclusion of other FMD-related parameters such as baseline and peak diameters.Citation22 Accordingly, these additional results not only complement, but reinforce the low variation of FMD within the same day and between-days and confirm reproducibility.
Reproducibility of arterial stiffness
There is a link between vascular endothelial function and arterial stiffnessCitation42–Citation44 that is mainly due to a degeneration of the media, an increase in collagen content, and a decrease in vascular dispensability.Citation10 Similar to the FMD test for the assessment of endothelial function, arterial stiffness has also been shown to predict cardiovascular eventsCitation10 as well as disease severity in patients with COPD.Citation45 In addition, central arterial stiffness (AIx) has been associated with the severity of airway obstruction during COPD exacerbationsCitation4,Citation46 and there has been increasing utilization of measuring AIx and PWV to assess arterial stiffness over time in patients with COPD.Citation10,Citation18,Citation19,Citation28 The reproducibility of arterial stiffness in patients with COPD; however, has yet to be investigated until now.
No differences in AIx, AIx75, or PWV were observed between sessions A and B or between sessions A and C. Additionally, we report acceptable CV, CV′, Pearson correlation coefficients, and ICC for all arterial stiffness variables. Furthermore, the Bland–Altman plots emphasize the agreement of AIx, AIx75, or PWV on multiple testing sessions (Figures S1 and S2). Collectively, the present data indicate within-day and between-day reproducibility of arterial stiffness in patients with COPD.
There are many different factors that can impact and limit the reproducibility assessment of both endothelial function and arterial stiffness including sex, age and race.Citation20,Citation29,Citation30 Specific to patients with COPD, pulmonary exacerbation and smoking can impact endothelial function and arterial stiffness.Citation47 In order to rule out the possible effects of these variables on our results, an ANCOVA was performed with age, sex and race as covariates. Additionally, we have also included the possible effects of two major metabolites of nicotine (cotinine and trans-3-hydroxycotinine)Citation48 as covariates due to the close relationship among continued tobacco use and worsening prognosis of COPD.Citation49 The covariate analyses confirmed that neither of the aforementioned factors influenced the conclusion of within-day or between-day reproducibility of endothelial function or arterial stiffness in patients with COPD.
Clinical perspectives
Patients with COPD often present with cardiovascular disease, likely related to impaired vascular health and function which lead to a high mortality and morbidity among this patient population. Based on this fact, addressing cardiovascular risk in patients with COPD could improve their survival rate. In this line and for the first time, the present study has provided a robust assessment of within-day and between-day reproducibility of both endothelial function and arterial stiffness in patients with COPD. Assessments of endothelial function met at least five out of the six reproducibility criteria, whereas six out of six criteria were met for arterial stiffness. Our findings corroborate previous conducted studies in patients with COPD that have utilized FMD and PWV. Perhaps more importantly, FMD and arterial tonometry represent novel, non-invasive methods that can be utilized to evaluate the impact of therapeutic interventions on cardiovascular disease risk in patients with COPD.
Authors’ contributions
RAH conceived and designed the study; NS, LB, and RAH performed experiments; PR-M and RAH analyzed data, interpreted the results of experiments, prepared figures, and drafted the manuscript; PR-M, NS, LB, TD, and RAH edited and revised the manuscript and provided final approval; and RAH is the guarantor of the final version of the manuscript. All authors contributed toward data analysis, drafting, and revising the manuscript.
Acknowledgments
The authors would like to thank the patients for their commitment and participation in this study. We also would like to acknowledge Dr Betty Wray for her support in patient recruitment to this study. This study was supported by the American Heart Association 10SDG3050006 (RAH).
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Methodology
Pulmonary function testing
A minimum of three reproducible trials were completed by each participant, and the best of three acceptable forced expiratory maneuvers were selected to represent the pulmonary function values. The National Health and Nutrition Examination Survey (NHANES) III spirometric reference standards were used to determine the percentage predicted data set.
Brachial artery flow-mediated dilation
Endothelial function was determined via brachial artery flow-mediated dilation (FMD) in accordance with the tutorial on the ultrasound assessment of FMD.Citation1 Briefly, measurements took place in a quiet, temperature-controlled (22°C–24°C) room, where patients were instructed to lie supine with their right arm laterally extended for 20 minutes to establish a hemodynamic steady state. The brachial artery was imaged longitudinally in Duplex mode (simultaneous B-mode and blood velocity profiles) by a Doppler ultrasound (Logiq 7; GE Medical Systems, Milwaukee, WI, USA) using a 12-MHz linear transducer placed 2 cm to 10 cm above the antecubital fossa. Blood velocity was obtained with the sample volume set at a depth between 1 cm and 3 cm. The average diameter and blood velocity for 30 cardiac cycles were recorded and analyzed to represent baseline values. Subsequently, a 5 cm ×84 cm forearm occlusion cuff (D.E. Hokanson, Bellevue, WA, USA) was placed immediately distal to the medial epicondyle and rapidly inflated to 250 mmHg for 5 minutes (E-20 rapid cuff inflator; D.E. Hokanson) to induce arterial occlusion and reactive hyperemia of the brachial artery. ECG gaiting (Accusync 72; Accusync Medical Research, Milford, CT, USA) was used to capture end-diastolic arterial diameters, triggered by the QRS complex, for automated offline analysis of brachial artery vasodilatation (Brachial Analyzer Software, Medical Imaging Applications, Coralville, IA, USA). FMD is expressed as a percent increase in peak diameter from baseline diameter.
Arterial tonometry
Pulse wave analysis
Augmentation index (Aix) was determined by applanation tonometry (SphygmoCor; AtCor Medical, West Ryde, NSW, Australia) of the left radial artery, calibrated with the brachial systolic and diastolic pressure measured with an inflated cuff at the brachial artery in accordance with the manufacturer’s recommendations. The SphygmoCor system transforms the peripheral waveform to a central waveform using a mathematical algorithm. Since AIx varies with heart rate, it is usually adjusted to 75 beats per minute (AIx75).Citation2 At least two independent waveform analyses were obtained from each subject, with reproducible measurements only accepted on meeting SphygmoCor quality control criteria.
Pulse wave velocity
Carotid-femoral pulse wave velocity (cfPWV) was determined in duplicate using the SphygmoCor system by sequentially recording electrocardiographic-gated carotid and femoral artery waveforms by applanation tonometry as described previously.Citation3,Citation4 Using a Rosscraft segmometer, straight line distance measurements were taken from the suprasternal notch to the carotid sampling site and from the suprasternal notch to the site where the femoral artery was measured. The time interval (t, in seconds) between the onset of femoral and carotid waveforms was determined as the mean from 10 consecutive cardiac cycles. High quality measurements were confirmed by the standard deviation of time intervals corresponding to the patient’s ECG and femoral and carotid artery waveforms. Standard deviations greater than 10% of the cfPWV value were not accepted. The cfPWV was calculated from the distance between measurement points (D, in meters) and the measured time delay between the peak of the ECG P-wave and the trough of a waveform (t) as follows: cfPWV = D/t (m/sec).
Figure S1 Bland–Altman analysis plots illustrating no systematic bias and good within-day reproducibility for (A) AIx, (B) AIx75, and (C) PWV. Notes: Solid lines represent systematic bias and dashed lines represent the 95% CI at two SD of the differences.
Abbreviations: AIx, augmentation index; PWV, pulse wave velocity; AIx75, AIx at 75 bpm.
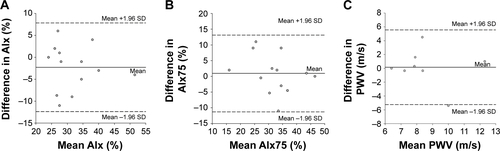
Figure S2 Bland–Altman analysis plots illustrating no systematic bias and good between-day reproducibility for (A) AIx, (B) AIx75, and (C) PWV.
Note: Solid lines represent systematic bias and dashed lines represent the 95% CI at two SD of the differences.
Abbreviations: AIx, augmentation index; PWV, pulse wave velocity; AIx75, AIx at 75 bpm.
References
- HarrisRANishiyamaSKWrayDWRichardsonRSUltrasound assessment of flow-mediated dilationHypertension2010551075108520351340
- WilkinsonIBMacCallumHFlintLThe influence of heart rate on augmentation index and central arterial pressure in humansJ Physiol2000525Pt 126327010811742
- LaurentSCockcroftJVan BortelLExpert consensus document on arterial stiffness: methodological issues and clinical applicationsEur Heart J2006272588260517000623
- RobertsCKLeeMMKatiraieMStrength fitness and body weight status on markers of cardiometabolic healthMed Sci Sports Exerc2015471211121825251047
References
- QureshiHSharafkhanehAHananiaNAChronic obstructive pulmonary disease exacerbations: latest evidence and clinical implicationsTher Adv Chronic Dis20145521222725177479
- EickhoffPValipourAKissDDeterminants of systemic vascular function in patients with stable chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2008178121211121818836149
- SchunemannHJDornJGrantBJWinkelsteinWJrTrevisanMPulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health StudyChest2000118365666410988186
- PatelARKowlessarBSDonaldsonGCCardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201318891091109924033321
- AnthonisenNRConnettJEEnrightPLManfredaJHospitalizations and mortality in the Lung Health StudyAm J Respir Crit Care Med2002166333333912153966
- TakahashiTKobayashiSFujinoNIncreased circulating endothelial microparticles in COPD patients: a potential biomarker for COPD exacerbation susceptibilityThorax201267121067107422843558
- ClarenbachCFSennOSieviNADeterminants of endothelial function in patients with COPDEur Respir J20134251194120423429917
- HalcoxJPDonaldAEEllinsEEndothelial function predicts progression of carotid intima-media thicknessCirculation200911971005101219204308
- YeboahJCrouseJRHsuFCBurkeGLHerringtonDMBrachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health StudyCirculation2007115182390239717452608
- VivodtzevITamisierRBaguetJPBorelJCLevyPPepinJLArterial stiffness in COPDChest2014145486187524687708
- GhiadoniLMagagnaAVersariDDifferent effect of antihypertensive drugs on conduit artery endothelial functionHypertension20034161281128612719441
- PlantingaYGhiadoniLMagagnaASupplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patientsAm J Hypertens200720439239717386345
- CharakidaMMasiSLoukogeorgakisSPDeanfieldJEThe role of flow-mediated dilatation in the evaluation and development of antiatherosclerotic drugsCurr Opin Lipidol200920646046619851104
- HadiHACarrCSAl SuwaidiJEndothelial dysfunction: cardiovascular risk factors, therapy, and outcomeVasc Health Risk Manag20051318319817319104
- BrunnerHCockcroftJRDeanfieldJEndothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of HypertensionJ Hypertens200523223324615662207
- OzbenBEryukselETanrikuluAMPapila-TopalNCelikelTBasaranYAcute exacerbation impairs endothelial function in patients with chronic obstructive pulmonary diseaseTurk Kardiyol Dern Ars20103811720215835
- BarrRGMesia-VelaSAustinJHImpaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) StudyAm J Respir Crit Care Med2007176121200120717761614
- IvesSJHarrisRAWitmanMAVascular dysfunction and chronic obstructive pulmonary disease: the role of redox balanceHypertension201463345946724324045
- VivodtzevIMinetCTamisierRArterial stiffness by pulse wave velocity in COPD: reliability and reproducibilityEur Respir J20134241140114224081763
- DonaldAEHalcoxJPCharakidaMMethodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilationJ Am Coll Cardiol200851201959196418482664
- No authors listedStandards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic SocietyAm J Respir Crit Care Med19951525 Pt 2S77S1217582322
- HarrisRANishiyamaSKWrayDWRichardsonRSUltrasound assessment of flow-mediated dilationHypertension20105551075108520351340
- WilkinsonIBMacCallumHFlintLCockcroftJRNewbyDEWebbDJThe influence of heart rate on augmentation index and central arterial pressure in humansJ Physiol2000525Pt 126327010811742
- LaurentSCockcroftJVan BortelLExpert consensus document on arterial stiffness: methodological issues and clinical applicationsEur Heart J200627212588260517000623
- RobertsCKLeeMMKatiraieMStrength fitness and body weight status on markers of cardiometabolic healthMed Sci Sports Exerc20154761211121825251047
- HerringtonDMFanLDrumMBrachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameterJ Cardiovasc Risk20018531932811702039
- GuzikTJGrodzickiT“Radical” link between chronic obstructive pulmonary disease and cardiovascular disease?Hypertension201463344444624324039
- MaclayJDMcAllisterDAMillsNLVascular dysfunction in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009180651352019542477
- CorrettiMCAndersonTJBenjaminEJGuidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task ForceJ Am Coll Cardiol200239225726511788217
- HarrisRAPadillaJHanlonKPRinkLDWallaceJPReproducibility of the flow-mediated dilation response to acute exercise in overweight menUltrasound Med Biol200733101579158517590500
- CelermajerDSSorensenKEGoochVMNon-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosisLancet19923408828111111151359209
- AndersonTJUehataAGerhardMDClose relation of endothelial function in the human coronary and peripheral circulationsJ Am Coll Cardiol1995265123512417594037
- WelschMAAllenJDGeaghanJPStability and reproducibility of brachial artery flow-mediated dilationMed Sci Sports Exerc200234696096512048322
- HarrisRAPadillaJRinkLDWallaceJPVariability of flow-mediated dilation measurements with repetitive reactive hyperemiaVasc Med20061111616669406
- HijmeringMLStroesESPasterkampGSierevogelMBangaJDRabelinkTJVariability of flow mediated dilation: consequences for clinical applicationAtherosclerosis2001157236937311472736
- De RoosNMBotsMLSchoutenEGKatanMBWithin-subject variability of flow-mediated vasodilation of the brachial artery in healthy men and women: implications for experimental studiesUltrasound Med Biol200329340140612706191
- CharakidaMde GrootELoukogeorgakisSPVariability and reproducibility of flow-mediated dilatation in a multicentre clinical trialEur Heart J201334453501350723821401
- GhiadoniLFaitaFSalvettiMAssessment of flow-mediated dilation reproducibility: a nationwide multicenter studyJ Hypertens20123071399140522525207
- KanaharaMHaradaHKatohAIkedaHNew methodological approach to improve reproducibility of brachial artery flow-mediated dilatationEchocardiography201431219720223909753
- WoodmanRJPlayfordDAWattsGFImproved analysis of brachial artery ultrasound using a novel edge-detection software systemJ Appl Physiol (1985)200191292993711457812
- ThijssenDHBlackMAPykeKEAssessment of flow-mediated dilation in humans: a methodological and physiological guidelineAm J Physiol Heart Circ Physiol20113001H2H1220952670
- NakaKKTweddelACDoshiSNGoodfellowJHendersonAHFlow-mediated changes in pulse wave velocity: a new clinical measure of endothelial functionEur Heart J200627330230916267075
- KinlaySCreagerMAFukumotoMEndothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivoHypertension20013851049105311711496
- WilkinsonIBQasemAMcEnieryCMWebbDJAvolioAPCockcroftJRNitric oxide regulates local arterial distensibility in vivoCirculation2002105221321711790703
- LeeHMLeeJLeeKLuoYSinDDWongNDRelation between COPD severity and global cardiovascular risk in US adultsChest201214251118112522518027
- AlbuAFodorDBondorCSuciuOCarotid arterial stiffness in patients with chronic obstructive pulmonary diseaseActa Physiol Hung201198211712721616770
- MaclayJDMacNeeWCardiovascular disease in COPD: mechanismsChest2013143379880723460157
- LlaquetHPichiniSJoyaXBiological matrices for the evaluation of exposure to environmental tobacco smoke during prenatal life and childhoodAnal Bioanal Chem2010396137939919466395
- ZuoLHeFSergakisGGInterrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatmentsAm J Physiol Lung Cell Mol Physiol20143073L205L21824879054
