Abstract
Objective
The apoptotic DNA levels in blood leukocytes of patients with type 2 diabetes (T2D) and thyroid dysfunctionism were evaluated.
Materials and methods
Single-cell gel electrophoresis (comet assay) detects migration of DNA from individual cell nuclei following alkaline treatment. Comet assay pattern was studied in individuals with T2D, hypothyroid (HT), hyperthyroid (HeT), and patients suffering from both diabetes mellitus and HT (HT + DM). Results were compared with the normal subjects (n = 9 in each group). The percentage apoptotic cell populations were calculated from the tail length.
Results
T2D patients showed 92.24% of cell damage compared to HT or HeT patients (51.04% or 54.64%, respectively). Further, increase in cell damage was also observed in HT + DM subjects (P < 0.05). Pharmacologic therapy significantly influenced cell damage. However, age and duration of disease did not show any definite influence on apoptosis.
Conclusion
Dependence of disease seems to be the major contributor of the cell damage. However, thyroid dysfunction did not show any deleterious effects on individual cells under the study.
Introduction
Apoptosis is an important and inevitable event in the remodeling of tissues during development and aging.Citation1 In most postreplicating cells, the rate of apoptosis increases with age and thus may be a contributor in many age-related diseases.Citation2,Citation3 Nucleic acid levels are generally low in the plasma of healthy adults due to rapid clearance by the liver, kidneys, and lymphoid cells, but they increase with age.Citation4–Citation7 The elevation of cell-free nucleic acids and condensed nuclear fragments (nucleosomes) in plasma may be due to necrotic and apoptotic cells, release from nondividing cells, or inefficient clearance.Citation8
Type 2 diabetes (T2D) accounts for most individuals with nonautoimmune forms of diabetes. The spectacular increase in prevalence of T2D worldwide is well documented.Citation9 Apoptosis appears to play a role in the initiation of diabetes mellitus and the complications manifested in the vasculature, brain, heart, kidneys, joints, and eyes.Citation10 Thyroid tumors are the most common endocrine malignancy, accounting for approximately 1% of all malignant diseases and about 0.4% of deaths related to cancer.Citation11 Hypothyroidism and hyperthyroidism affect the metabolism rate and thereby mitochondrial function. Elevated DNA damage levels or unrepaired DNA damage and suboptimal DNA repair may cause mutations that contribute to dysfunctioning of the thyroid gland.Citation12
Both insulin and thyroid hormones are intimately involved in cellular metabolism and excess or deficit of either of these hormones could result in the functional derangement of the other.Citation13 Symptoms of hypothyroidism are common in patients with T2D, and symptoms of hyperthyroidism may be attributed to poor diabetes control in patients with type 1 diabetes.Citation14,Citation15
The single-cell gel electrophoresis technique or comet assay is widely regarded as a quick and reliable method of analyzing DNA damage in individual cells.Citation16 DNA strand breaks allow DNA to extend from lysed and salt-extracted nuclei, or nucleoids, to form a comet-like tail on alkaline electrophoresis. The comet assay, a technique, capable of detecting DNA damage and repair in individual cells, is a valuable approach for human biomonitoring studies.Citation17 Cells undergoing active cell death or apoptosis demonstrate highly fragmented DNA. Apoptosis results in the extensive formation of double-strand breaks and is readily detected using alkaline electrophoretic conditions. When viewed using the comet assay, only a small percentage of DNA of an apoptotic cell remains associated with the comet head.Citation18,Citation19
In the present study, DNA damage in subjects with T2D and/or thyroid dysfunctioning was assessed with the help of alkaline comet assay. Secondly, the impact of pharmacological therapy upon the study population was also analyzed. Apoptosis and overall extent of damage in individual cells were assessed.
Materials and methods
Chemicals’ purchase
Low-melting point agarose (LMPA), normal melting agarose (NMA), Triton X-100, and phosphate-buffered saline (PBS; Ca++, Mg++ free) were purchased from HiMedia Pvt. Laboratories (Mumbai, India). All other chemicals available were of the highest purity.
Patients
The protocol for the present study was approved by the Human Ethics Committee of L.M. College of Pharmacy, Ahmedabad. All subjects were given verbal and written information about the study prior to providing written consent.
Venous blood samples were drawn by a research nurse from all subjects. Two milliliters of blood samples were collected. A total of 45 subjects of different groups viz, normal, hypothyroid (HT), hyperthyroidism (HeT), diabetes mellitus (DM), and both diabetes and HT complications together (HT + DM) were enrolled in the present study (n = 9 in each group).
Pharmacological treatment profile
All the subjects of different groups received their relevant treatment as follows: HT and HeT groups received levothyroxine and neomercazole® therapy, respectively; subjects with T2D received sulphonamides and/or insulin to control blood glucose levels; and all the subjects with HT + DM received sulphonamides and levothyroxine treatment.
Methodology
Biochemical investigations
Biochemical investigations were determined after a 10-hour overnight fast. Fasting blood sugar (FBS) concentration was measured by finger stick with a glucometer (LifeScan One touch Ultra; Johnson and Johnson Limited, New Brunswick, NJ). Serum concentrations of triiodothyronine (T3), thyroxine (T4), and thyroid stimulating hormone (TSH) were measured by enzymatic electrochemiluminescence immunoassay method (Abbott Architect i2000; Abbott Laboratories, Abbott Park, IL). Total cholesterol (TC) was measured by direct enzymatic method on OLYMPUS AU-400 (Olympus Diagnostics, Tokyo, Japan).
Preparation of base slide: Half-frosted slides were dipped into 1% NMA (prepared in MilliQ water), underside of the slide was wiped, and slide was laid on flat surface to dry (first layer).
Cell treatment: To the coated slide, 75 μL of 0.5% LMPA (prepared in PBS; Ca++, Mg++ free) and 10 μL of blood samples were added. The agarose layer was allowed to harden (second layer). The third agarose layer with 80 μL of 0.5% LMPA then followed (third layer). Slides were kept in the lysing solution (2.5 M sodium chloride, 100 mM EDTA, 10 mM Trizma base, 1% Triton X-100, and 10% dimethyl sulfoxide were added freshly) at 4°C overnight.
Electrophoresis of microgel slides: Electrophoresis was carried under alkaline conditions (pH > 13). After lysis, slides were kept in electrophoresis chamber (Genei Equipments, Bangalore, India) containing electrophoresis buffer (30 mL of 10 N sodium hydroxide, 5 mL of 200 mM EDTA quantity sufficient [q.s.] to 1,000 mL; pH > 13). Slides were allowed to sit in alkaline buffer for 20 minutes to allow unwinding of DNA and the expression of alkali-labile damage. Slides were electrophoresed for 30 minutes with the power supply of 24 V and current to 300 mA. Slides were lifted off the chamber and coated with neutralization buffer (0.4 M Tris in dH2O; pH = 7.5). Slides were then stained with 80-μL 1× ethidium bromide and observed under 20× fluorescent microscope. In addition to this, age, duration of disease conditions, and glucose and thyroid hormone levels were also considered, and distribution was measured accordingly as they do contribute to DNA damage.
Statistics
Instat 3 (GraphPad Inc., La Jolla, CA) was used for the analysis. Kruskal–Wallis’ test (nonparametric analysis of variance) was applied. Tukey–Kramer’s multiple comparison test was then followed. A value of P < 0.05 was considered as statistically significant. Pearson’s correlation coefficient (r) was calculated to find the correlation between two variables. Multiple linear regression was analyzed to study the correlation pattern in different groups.
Results
Biochemical investigations of all the subjects are listed in . As can be seen from , fasting blood glucose levels were found to be raised in T2D group of subjects (133 ± 6.61) and HT + DM group of subjects (116.78 ± 5.54) when compared with the normal group of subjects (88.85 ± 2.21; P < 0.001). Serum TC and TSH levels were found to be high in HT subjects compared with other groups under study (P < 0.05).
Table 1 Biochemical investigations
Slides were subjected to analysis with the help of Comet score 15 image analysis software (TriTek Corp., Sumerduck, VA) (). Tail length was measured in 100 cells. Average number of cells falling in the given range was calculated for 100 cells in each duplicated slide. Percentage population of damaged cells was then calculated to get the individual subject’s value for each cell ().
Figure 1 Cells under fluorescent microscope. Image shows cellular effects under 20× fluorescent microscope when stained with ethidium bromide.
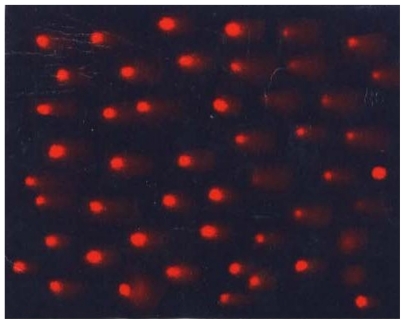
Table 2 Tail length and cellular definition
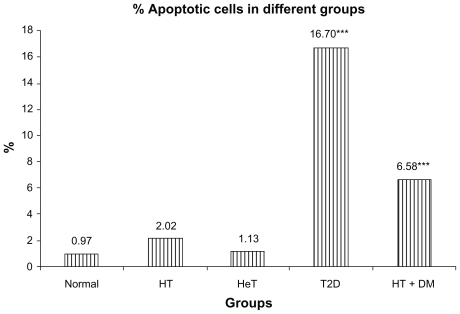
According to the disease condition, specific pattern of comet assay generated in terms of extent of damage in cells has been displayed in . T2D group showed total of 92.24% of cell damage. Cell damage in HT + DM group of subjects was found 95.65%, which could be due to additional effect of thyroid dysfunction. However, presence of apoptotic cells was found significantly high in T2D subjects (16.70%) as compared with 6.58% in HT + DM subjects (). However, the thyroid hormone levels showed negative correlation with the apoptotic cells (r = −0.23 and −0.09 for T3 and T4, respectively). This reflected that thyroid hormone changes do not significantly affect the DNA damage (which is measured in terms of apoptotic cells).
Figure 2 Comparison between disease condition and comet assay.
Abbreviations: HT, hypothyroid patients; HeT, hyperthyroid patients; T2D, type 2 diabetic patients; HT + DM, diabetic hypothyroid patients.
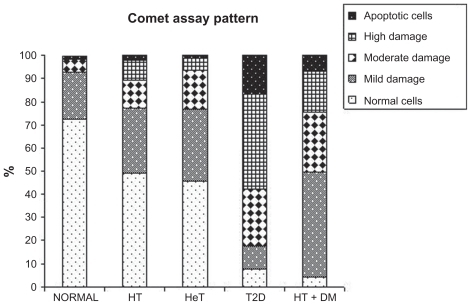
Figure 3 A comparison between disease condition and apoptotic cells.
Abbreviations: HT, hypothyroid patients; HeT, hyperthyroid patients; T2D, type 2 diabetic patients; HT + DM, diabetic hypothyroid patients.
In addition, we have shown Pearson’s correlation coefficient (r) among apoptotic cells, FBS, and thyroid hormone levels (). There was significant correlation observed between apoptotic cells and FBS (r = 0.78; P < 0.001). Further, our data were also stratified based on age and duration of disease conditions ( and ). However, no correlation was observed with reference to these two factors. Thus, it is clear from our observations that these two factors may not contribute any significant role in cellular damage.
Figure 4 Correlation between age and % apoptotic cells.
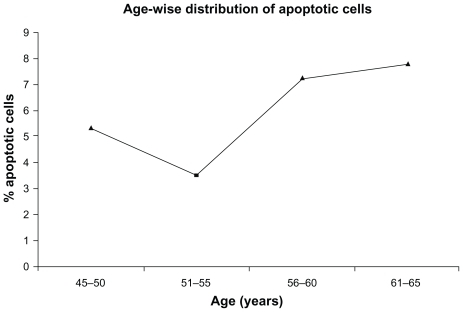
Figure 5 Correlation between duration of disease condition and % apoptotic cells.
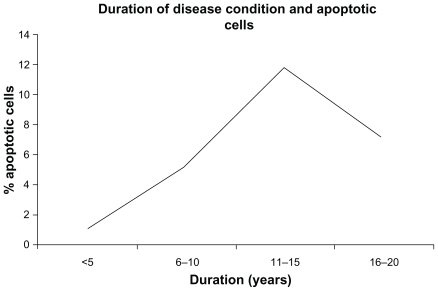
Table 3 Correlations between apoptotic cells and biochemical parameters
Discussion
The importance of studying apoptosis in aging and age-related disorders has been recognized by many scientists.Citation20–Citation22 Subjects with both diabetes and/or thyroid dysfunctioning were included in the present study.
There is a report of direct relationship between the glucose level and amount of apoptosis in diabetic subjects.Citation23 Therefore, the detection of high levels of apoptotic DNA in T2D individuals under the present study might be associated with secondary symptoms of microvascular complications, although there was lower probability of cell damage observed in HT and HeT subjects. In contrast to this, elevated plasma DNA levels and antibodies to DNA have been reported in thyroiditis condition.Citation24,Citation25
Age and long duration of disease are two such factors which contribute to the development of DNA damage and thereby cell damage.Citation7,Citation10 Hence, an effort was made to find the age and duration of disease-dependant distribution of comet assay. However, we could not establish any correlation with these two factors. But, our study is more likely to be dependant on disease type, particularly, diabetic individuals who are as such more susceptible to DNA damage and eventually apoptosis. This hypothesis is further supported by our results of correlation coefficient among apoptotic cells, FBS, and thyroid hormone levels. We strongly confirm and recommend the relation between diseases like T2D and apoptosis based on an r value of 0.0783.
Therapeutic influence may play a role in free radical generation and thereby leading to apoptosis progression. Tolbutamide, a current drug for treating T2D has been shown to trigger apoptosis in pancreatic β cells.Citation26 Glibenclamide has also been reported to be an inducer of apoptotic processes.Citation27 Kang et alCitation28 have reported antiapoptotic action of insulin that is related to the reduction of reactive oxygen species. Further, YamashitaCitation29 already reported decrease in TSH levels with levothyroxine returned the antiapoptotic property of thyroid cells. Neomercazole, being an antithyroid drug, normalizes the basal metabolic rate and thereby reset the cellular apoptosis. Thus, depending upon the pharmacological drug profile of our subjects, role of glibenclamide in inducing and levothyroxine in delaying progression of cell death can not be ruled out at this point of time.
Thus, the present study may help us to identify the extent of cellular damage in individuals suffering from any of the metabolic abnormalities. It can also help to identify the effect of the medication on reactive oxygen species generation and thereby DNA damage. However, this study is associated with certain limitations as comet assay can not differentiate two disease conditions and the status of disease progression.
Conclusion
Our study rules out the dependence of age and duration of disease condition on cellular damage, although dependence of disease conditions seems to be a major contributor of the cell damage as indicated by increase in the percentage of apoptotic cells in diabetic subjects. However, further study is needed to understand the molecular-level mechanisms to establish correlation amongst this population.
Acknowledgments
Funding was received from Rameshwardasji Birla Smarak Kosh, Medical Research Centre, Bombay Hospital Avenue, Mumbai 400 020, India. The authors acknowledge the staff of Gujarat Endocrine Centre, Ahmedabad, especially the guidance of Dr. Parag Shah MD, DM, DNB (Endocrinology) without whose support this work would not have been possible, the authors are also highly thankful to the staff of National Institute of Occupational Health (NIOH), Ahmedabad, for providing guidance to learn comet assay techniques.
Disclosure
The authors report no conflicts of interest in this work.
References
- WangenheimKHPetersonHPControl of cell proliferation by progress in differentiation: clues to mechanisms of aging, cancer causation and therapyJ Theor Biol19981936636789745759
- SabbahHNSharovVGApoptosis in heart failureProg Cardiovasc Dis1998405495629647609
- SinghalPCReddyKFrankiNAge and sex modulate renal expression of SGP-2 and transglutaminase and apoptosis of splenocytes, thymocytes and macrophagesJ Invest Med199745567575
- BennettRMGaborGTMerrittMMDNA binding to human leucocytes. Evidence for a receptor-mediated association, internalization and degradation of DNAJ Clin Invest19857621812190
- LoYMDZhangJLeungTNLauTKChangAMHjelmNMRapid clearance of fetal DNA from plasmaAm J Hum Genet1999642182249915961
- ScottRSMcMahonEJPopSMPhagocytosis and clearance of apoptotic cells is mediated by MERNature200141120721111346799
- FournieGJMartresFPourratJPAlaryCRemeauMPlasma DNA as cell death marker in elderly patientsGerontology1993392152218244049
- StrounMLyauteyJLederreyCOlson-SandAAnkerPAbout the possible origin and mechanism of circulating DNA apoptosis and active DNA releaseClin Chim Acta200131313914211694251
- BertoniAGClarkJMFeeneyPSuboptimal control of glycemia, blood pressure, and LDL cholesterol in overweight adults with diabetes: the Look AHEAD studyJ Diab Comp20082219
- OhsakoSElkonKBApoptosis in the effecter phase of autoimmune diabetes, multiple sclerosis and thyroiditisCell Death Differ19996132110200543
- TrimboliPUlisseSGrazianoFMTrend in thyroid carcinoma size, age at diagnosis, and histology in a retrospective study of 500 cases diagnosed over 20 yearsThyroid2006161151115517123342
- UndegerUSahinTTYukselOAssessment of DNA damage in peripheral blood lymphocytes from patients with benign and malignant thyroid disordersHacettepe Univ J Fac Pharm2008281114
- SatishRMohanVDiabetes and thyroid diseases: a reviewInt J Diabetes Dev Ctries200323 Suppl 4120123
- HanukogluAMizrachiAAlianDAdomniAZvyBSonnekhEExtrapancreatic autoimmune manifestations in type 1 diabetes patients and their first degreeDiabetes Care200326 Suppl 41235124012663603
- WuPThyroid disease and diabetesClinl Diabetes200018 Suppl 11114
- McArtDGMcKerrGHowardCVSaetzlerKWassonGRModelling the comet assayBiochem Soc Trans200937Pt 491491719614618
- KassieFParzefallWKnasmullerSSingle cell gel electrophoresis assay: a new technique for human biomonitoring studiesMutat Res2000463133110838207
- OlivePLDurandReLeRicheJOlivottoIJacksonSMGel electrophoresis of individual cells to quantify hypoxic fraction in human breast cancersCancer Res1993537337368381327
- GopalkrishnaPKharAComet assay to measure DNA damage in apoptotic cellsJ Biochem Biophys Methods19953069737608471
- OrreniusSApoptosis: molecular mechanisms and implications for human diseaseJ Intern Med19952375295367782723
- WarnerHRHodesRJPocinkiKWhat does cell death have to do with aging?J Am Geriat Soc199745114011469288026
- TomeiLDUmanskySRAging and apotosis controlNeurol Clin1998167357459666047
- LangfordMPRedensTBHarrisNRPlasma level of cell free apoptotic DNA ladders and gamma-glutamyltranspeptidase in diabetic childrenExp Biol Med200723211601169
- OhsakoSElkonKBApoptosis in the effector phase of autoimmune diabetes, multiple sclerosis and thyroiditisCell Death Differ19996132110200543
- KotaniTAratakeYHiraiKFukazawaYSatoHOhtakiSApoptosis in thyroid tissue from patients with Hashimotto’s thyroiditisAutoimmunity1995202317578885
- EfanovaIBZaitsevSVZhivotovskyBGlucose and tolbutamide induce apoptosis in pancreatic β cells. A process dependent on intracellular Ca+2 concentrationJ Biol Chem199827333501335079837930
- HambrockAOliveira FranzCBHillerSOsswaldHGlibenclamide induced apoptosis is specifically enhanced by expression of the sulphonylurea receptor isoform SUR1 but not by expression of SUR2B or the mutant SUR1(M1289T)J Pharmacol Exp Therap20063161031103716306272
- KangSSongJKangHKimSLeeYParkDInsulin can block apoptosis by decreasing oxidative stress via phosphatidylinositol 3-kinase and extracellular signal regulated protein kinase dependant signaling pathways in HepG2 cellsEur J Endocrinol200314814715512534368
- YamashitaSEndocrine disease and apoptosisIntern Med19983721941979550607