Abstract
Multiple sclerosis (MS) is a disease of the central nervous system that is characterized by the demyelination of neuronal axons. Four different patterns of demyelination have been described, showing the heterogeneity in the immunopathologic processes involved in the demyelination. This review will focus on reactive oxygen species (ROS)-related inflammation in MS. Special emphasis will be placed on the nuclear factor erythroid-2-related factor 2 (Nrf2) as it regulates the transcription of ROS-protective genes. In the cytosol, Nrf2 binds to Keap1 (Kelch-like ECH-associated protein 1), and together they are degraded by the 26S proteasome after ubiquitination. If challenged by ROS Nrf2, binding to Keap1 is abrogated, and it translocates into the nucleus. Here it binds to the antioxidant response element and to a small protein termed Maf (musculoaponeurotic fibrosarcoma oncogene homolog). This leads to an enhanced transcription of ROS protective genes and represents the physiological answer against ROS challenge. It has been shown that dimethyl fumarate (DMF) has the same effect and leads to an enhanced transcription of ROS-protective genes. This response is mediated through a reduced binding of Nrf2 to Keap1, thus resulting in a higher level of free Nrf2 in the cytosol. Consequently, more Nrf2 translocates to the nucleus, promoting transcription of its target genes. DMF has been used for the treatment of psoriasis for many years in Germany without the occurrence of major side effects. In psoriasis, DMF reduces ROS-related inflammation in skin. A DMF analog, BG-12, was recently approved for the treatment of relapsing-remitting MS by the European Union and the US Food and Drug Administration. As an oral formulation, it gives patients a convenient and effective alternative to the injectable immune modulators in the long-term treatment of MS.
Keywords:
Multiple sclerosis – a “paradigm shift”
The classic description of multiple sclerosis (MS) indicated a central nervous system (CNS) disease with the main qualifiers being T-cell mediated process, demyelination, relapsing-remitting nature, and inflammation. Immunopathological evidence in MS suggests a variety of pathological types disputing a T-cell “only” mediated disease.Citation1 In fact, what was thought to be the description of an MS lesion involving activated T-cells, macrophage- and cytokine-mediated multifocal damage of myelin is now considered a category of MS (type I), which surprisingly affects less than half of all MS patients. Other pathological types include: type II, caused by complement-mediated multifocal damage via activated B-cells; type III, involving loss of myelin-associated glycoprotein and apoptosis of oligodendrocytes, resulting in diffuse low grade inflammation; and type IV, characterized by non-inflammation-induced degeneration of oligodendroglial cells. Although demyelination remains the most conspicuous pathology of MS, neurons, especially their axonal compartment, are also prominently affected.Citation2–Citation4 Serial magnetic resonance imaging disputes the notion of sharply defined relapses and remissions in MS, with mounting evidence that progressive changes can occur without clinical worsening.Citation5 Finally, the traditional concept of MS being an inflammatory disease is under scrutiny, with evidence suggesting that neurodegeneration initiated by non-inflammatory oligodendroglia cell death resulting in secondary inflammation may contribute to the pathology of MS in some cases.Citation6 Neurodegeneration, likely triggered by microglial activation, is also prominent in secondary-progressive MS after the inflammation of relapsing-remitting MS subsides.Citation7 Damage by free radicals has been associated with many neurodegenerative diseases including Alzheimer’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, and Huntington’s disease. In this review, we present evidence for free-radical damage being a part of the pathogenesis of MS, and we describe the possible involvement of transcription factor Nrf2 (nuclear factor erythroid-2-related factor 2) and its downstream pathways in MS. Finally, therapies which may target Nrf2, implications for disease management, and future directions will be reviewed.
Free radicals: their role, sources, and endogenous antioxidant systems
Free radicals in the human body are ions that have unpaired valence electrons and therefore are “electron acceptors” for other molecules. The process of accepting an electron oxidizes the acceptor molecule, making the donor known as “oxidizing agent.” Free radicals usually originate from oxygen (reactive oxygen species [ROS]) or nitrogen (reactive nitrogen species [RNS]). ROS include the following radicals: O2•−, H2O2, •OH, RO2•, RO•, whereas RNS include: •NO, •NO2, and ONOO−. Free radicals are routinely generated under physiological conditions and are very important in different regulatory functions of the body. Mechanisms involving free radicals include oxidative phosphorylation, detoxification of xenobiotics by cytochrome P450, apoptosis of defective cells, destruction of microorganisms and cancer cells by macrophages and cytotoxic lymphocytes, use by various oxygenases for production of prostaglandins and leukotrienes, use as second messengers and regulation of redox-sensitive transcription factors (for details, see Devasagayam et alCitation8). Reactive oxidants are produced by different mechanisms, in multiple compartments within the cell (for reviews, see MaCitation9 and FinkelCitation10). When deregulated, free radicals are produced in excess in the cellular milieu, where they may oxidize lipids, proteins, and deoxyribonucleic acid (DNA) and may result in degenerative diseases.
Molecular oxygen is used by mitochondria for the oxidative metabolism of glucose and other substrates, leading to the production of adenosine triphosphate (ATP), the universal energy currency of cells. The process of using oxygen to oxidize glucose to produce ATP is known as oxidative phosphorylation. During oxidative phosphorylation, 0.4%–4.0% of oxygen consumed goes into production of free radical superoxide (•O2−).Citation11–Citation16 Superoxide can then be converted into ROS and RNS. Inside the mitochondria, superoxide molecules are converted to H2O2 by superoxide dismutase (SOD)2.Citation11,Citation12,Citation17 H2O2 is then converted to H2O and O2 by glutathione (GSH) peroxidase in mitochondria, or diffuses into the cytosol and is converted by catalase in peroxisomes to H2O and O2, also. In the presence of reduced transition metals such as copper or iron (Cu or Fe), H2O2 can be converted to the highly reactive •OH radical. Although mitochondria are the primary site of ROS production from aerobic respiration, nearly all enzymes that use molecular oxygen (eg, plasma membrane–bound NADPH [reduced nicotinamide adenine dinucleotide phosphate] oxidase [NOX], microsomal cytochrome P450, and cytoplasmic xanthine oxidase) produce ROS directly or indirectly (for details, see MaCitation18). Especially in the brain, ROS are mainly derived from activated macrophages and microglia,Citation19 in which plasma membrane–bound NOX, particularly NOX2, are mainly responsible for the oxidative burst of these cells. Importantly, during a phagocyte’s, such as a macrophage’s, respiratory burst, myeloperoxidase activity generates hypochlorous acid (HOCl) from H2O2 and chloride anion (Cl−), serving as a feature of free-radical damage.Citation18 RNS are formed in cells, starting with the synthesis of nitric oxide (•NO) by NO synthase, which is present in different cell types expressing NO synthases 1–3 in the brain.Citation20 Examples of RNS include •NO, •NO2, and ONOO−. NO• reacts with superoxide (O2•−) to form a stronger oxidant peroxynitrite anion (ONOO−). ONOO− reacts with other molecules to generate other RNS such as •NO2 and N2O3.Citation18
Endogenous antioxidant systems exist within cells, with the purpose of neutralizing excess ROS. This is critical for proper cellular function. Reduced GSH, a major cellular antioxidant, is regenerated by GSH reductase and reduced nicotinamide adenine dinucleotide phosphate (NADP) plus (NADPH)Citation21 and other mechanisms along with other antioxidants.Citation22,Citation23 Reduced GSH levels fall with increased oxidative stress. Other major antioxidants include: low-molecular-weight compounds such as vitamins C and E, bilirubin, and urate; non-catalytic antioxidant proteins, such as thioredoxin (Trx), glutaredoxin, and metallothioneins; enzymes, such as superoxide dismutase 2, catalase, peroxiredoxin, and GSH peroxidase. Ultimately, redox reactions in cells are enabled by nicotinamide pairs NADP+/NADPH and NAD+/NADH. NADPH is used to reduce oxidized Trx and GSH (GSSG) through Trx reductase and GSH reductase, respectively. Sulfiredoxin reduces oxidized peroxiredoxin from sulfinic (inactive) to sulfenic (active) acid in an ATP- and GSH-dependent manner. Antioxidant systems are controlled by regulators at multiple levels that respond to oxidative stress.Citation18 One is via the important transcription factor Nrf2 in concert with the antioxidant response element (ARE) in promoters of target genes (see under the heading “Nrf2 pathways and oxidative damage”).
Mechanisms and evidence for free-radical damage in MS
Excess free-radical activity is detected using biochemical methods including assay of key enzymes or by immunocytochemical identification of oxidized nucleotides, proteins, and lipids.Citation24 Several lines of evidence indicate that free radicals are involved in the pathogenesis of MS either directly or indirectly via mitochondrial damage and activation of excitotoxic mechanisms. Several studies indicate the relationship of free-radical damage associated with neurodegeneration in demyelinating disease by promoting transendothelial leukocyte migration and contributing to oligodendrocyte damage and axonal degeneration,Citation25–Citation27 but there is more evidence specific to MS. One pattern of tissue injury in MS is similar to initial white-matter stroke lesions showing microglia activation, distal oligodendrogliopathy, oligodendrocyte apoptosis, and acute axonal injury, after which demyelination and reactive gliosis occurs, suggesting that energy deficiency may play an important role in the pathophysiology of neurodegenerative processes in MS patients.Citation28,Citation29 These hypoxia-like tissue injury type lesions have been found to have ~8.2 times higher expression of oxidized lipids and oxidized DNA compared with complement-associated demyelinating lesions in MS.Citation29 Additionally, the numerous axonal spheroids and end bulbs seen in these lesions are reactive for oxidized phospholipids.Citation29 Oxidative stress as a contributor to early MS pathology is indicated by increased levels of lipid peroxidation markers in the cerebrospinal fluid of probable MS patients.Citation30 Antioxidant enzymes such as CuZnSOD, MnSOD, catalase, NAD(P) H:quinineoxidoreductase 1 are elevated in astrocytes, as well as in perivascular and myelin-laden macrophages in active lesions.Citation31 The surrogate marker for ROS produced by macrophages, myeloperoxidase, is upregulated in actively demyelinating white matter and cortical lesions,Citation32–Citation34 indicating oxidative burst of macrophages and microglia via the NOX2 NADPH oxidase. In addition, there is increased expression of nitric oxide synthase (NOS) in macrophages and microglia at the edge of active lesions.Citation35,Citation36 Mitochondrial injury in neurons is also a potentially important source of ROS.
Mitochondrial dysfunction produces free radicals causing neurodegenerative changes. This dysfunction is not a consequence of energy failure or a consequence of free-radical damage because NO and ROS target respiratory chain complexes at different levels, thereby inducing mitochondria transition and consecutively triggering apoptotic cascadesCitation26,Citation37 via liberation of apoptosis-induced factor or cytochrome C.Citation38 Mitochondrial disturbance in MS is indicated by microarray-based gene-expression analysis and histological approaches.Citation39–Citation42 The remarkable similarity of the tissue alterations in MS lesions to the lesions seen in acute ischemia of white matter due to strokeCitation28 bears testament to mitochondrial injury associated with energy failure. In active MS lesions, the initial mitochondrial changes are characterized by loss of COX1 (cytochrome C oxidase 1) and loss of complex IV activity in the mitochondrial respiratory chain.Citation42 In chronic MS lesions and re-myelinating axons, compensatory increase in complex IV densities have been noted, indicating initial dysfunction of the respiratory chain.Citation43 In chronic inactive lesions, expression of mitochondrial numbers and activity is increased, possibly indicating the higher energy demand of demyelinated axons as compared with myelinated axons.Citation44 Finally, we will briefly review the findings in experimental autoimmune encephalomyelitis (EAE), the most commonly used experimental model for MS. In the EAE model, intra-axonal mitochondrial dysfunction is known to occur before axonal degeneration or local increase in macrophages and prior to any overt demyelination, further supporting the notion that mitochondrial dysfunction may be an early event in the pathophysiology of MS. The EAE model is also relevant to this discussion because it showed that axonal degeneration is reduced by the administration of exogenous antioxidants.Citation45
Oxidative stress can reduce the efficiency of glutamate transporters, resulting in raised glutamate concentration in the extracellular space,Citation46 contributing to excitotoxic damage. The excitotoxic mechanism for damage in MS is supported by the fact that demyelinating lesions are histologically similar to those in animal models of excitotoxicity.Citation47–Citation49 Moreover, oligodendrocytes, which are the myelin-producing cells of the CNS, are highly vulnerable to glutamate excitotoxicity, mainly via activation of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)/kainate receptors.Citation46,Citation47 Yet more evidence for excitotoxic damage in MS comes from the EAE model of MS, where the deleterious effect of excess glutamate can be decreased via blockade of AMPA/kainate.Citation50
Nrf2 pathways and oxidative damage
Under the influence of ROS, Phase II detoxifying enzymes and antioxidant proteins are produced by cells.Citation51 This upregulation of proteins has to be accounted for on the DNA level. Here, Nrf2 is an important transcription factor to mediate the altered protein expression. There are many pathways involving Nrf2 regulation, and numerous interaction partners have been described (). Under physiological conditions, Nrf2 is located in the cytosol and binds to Keap1 (Kelch-like ECH-associated protein 1).Citation52 Through Cullin-3 binding to ring-box 1, an E3 ubiquitin-ligase complex is formed. After ubiquitination, the entire Keap1/Nrf2 complex is prone to proteasomal degradation. Here, Nrf2 has a half live of 10–40 minutes.Citation53,Citation54 Under the influence of electrophilic oxidative stress, as caused by ROS, Nrf2 is released from Keap1 and translocates into the nucleus. There, after dimerization with small Maf proteins, it binds to ARE and thereby initializes the transcription of genes encoding for ROS-protective proteins.Citation54–Citation57 Of the different small Maf proteins, MafG especially seems to be a primary interaction partner.Citation58
Figure 1 Schematic overview of intracellular Nrf2 turnover. Under physiological conditions, Nrf2 binds to a Keap1 dimer. Keap1 is ubiquitinated and then prone to degradation by the 26S proteasome. If subjected to ROS, the binding of Nrf2 to Keap1 is inhibited, and Nrf2 translocates to the nucleus. This binding to Keap1 can also be inhibited by FAE or BG-12, leading to the same effect of more Nrf2 translocation to the nucleus. In the nucleus, Nrf2 binds to a small Maf protein. The Nrf2/Maf complex binds to the ARE box and initiates the transcription of ROS-protective genes.
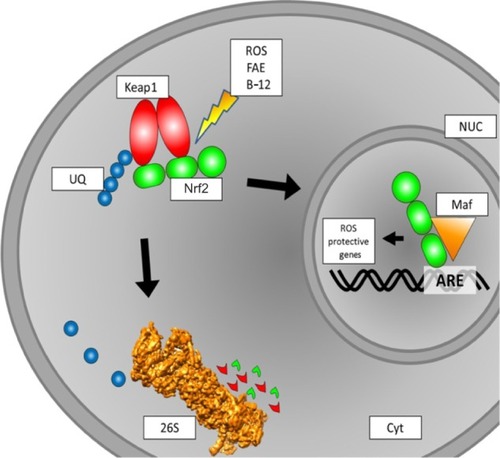
The upregulated proteins belong to two major enzymatic systems responsible for ROS detoxification. These systems are the heme oxygenase system and enzymes mediating GSH synthesis and utilization. Therefore, Nrf2 activation can lead to lower or even no damage related to ROS.Citation59 In CNS lesions, as found in MS patients, ROS and NOS are mainly produced by astrocytes, macrophages, and microglia. The ROS/NOS then damage various parts of the CNS, such as myelin, axons, neurons, and oligodendrocytes. This may lead to the damage of mitochondria and subsequent further accumulation of ROS/NOS. As tissue alterations found in MS patients are similar to those found in white-matter stroke patients,Citation28 the energy supply failure in mitochondria may play an important role in the pathophysiology of MS.Citation39,Citation42,Citation60
Review of therapies which may target Nrf2
Acute attacks (relapses) of MS that result in neurologic symptoms and increased disability or impairments in vision, strength, or cerebellar function are typically treated with glucocorticoids.Citation61 In patients with acute CNS inflammatory demyelinating disease, who do not respond to glucocorticoid therapy, plasma exchange may be beneficial.Citation62,Citation63 Patients with relapsing-remitting MS who manifest current disease activity by either clinical symptoms or recent lesions on magnetic resonance imaging (MRI) should be offered treatment with disease-modifying therapy. Certain immunomodulatory agents, including interferon beta preparations, glatiramer acetate, natalizumab, fingolimod, and teriflunomide, have shown important beneficial effects for patients with relapsing-remitting MS: 1) a decreased relapse rate and 2) a slower accumulation of brain lesions on MRI.
In long-term treatment, interferon beta and glatiramer acetate are commonly used. Whereas interferon beta is a naturally occurring body compound responsible for the regulation of pro- and anti-inflammatory processes,Citation64 glatiramer acetate is an artificial polypeptide. While the exact mode of action for glatiramer acetate is still unknown, clinical studies have proven its beneficial potential in MS.Citation65
As ROS/NOS may play an important role in MS pathogenesis, Nrf2 is an interesting therapeutic target, as reduction of ROS may directly lead to lower inflammation without the necessity of immune suppressors. In vitro studies and in vivo studies in murine systems have shown Nrf2 modulation by various compounds.Citation66–Citation74 Nevertheless, these are not suitable for use in clinical trials, as they are potentially toxic. For fumaric acid esters (FAEs), there is a comprehensive safety profile, and the compound is used to treat psoriasis in German-speaking countries. As in MS, patients diagnosed with psoriasis suffer from (skin) inflammation induced by ROS.Citation75 The most important FAE, namely dimethyl fumarate (DMF), has an immediate metabolite, monomethyl fumarate (MMF).Citation76 Using EAE mice to study MS has revealed the beneficial effects of FAEs. Application of FAEs in the chronic phase of EAE has shown preserving effects on myelin, axons, and neurons.Citation77,Citation78
This preserving effect is due to an alteration of the Keap1 ability to interact with Nrf2, most likely mediated by MMF. The free Nrf2 then translocates to the nucleus, as described, and leads to the transcription of genes encoding for ROS protective proteins. In two large trials, an oral formulation of DMF (BG-12) significantly reduced relapse rates and the development of new brain lesions on MRI in patients with active MS,Citation77,Citation78 and results from one of these trials, suggest that BG-12 reduces the rate of disability progression. DMF was recommended for approval in the European Union as a perioral treatment for MS by the European Medicines Agency in March 2013 under the name Tecfidera® (Biogen Idec, Inc., Weston, MA, USA). On March 27, 2013, the US Food and Drug Administration (FDA) approved Tecfidera capsules to treat adults with relapsing forms of MS in the US. Therefore, treatment with FAEs (especially DMF) is the first approach to actually target the inflammation-causing ROS by activation of an inherent antioxidant response.
Implications for disease management
Based on our previous discussion, the current knowledge of the immunopathology of demyelination justifies two different pharmacologic approaches to MS. One directed against acute relapse and inflammation and one aiming to slow down MS progression.
Based on our current understanding, the treatment of chronic ROS-related human diseases with agents such as FAEs would require long-term use of these agents. The ultimate goal is to maintain constant elevated levels of antioxidant proteins and to lower the absolute amount of ROS, helping to protect myelin, neurons, and axons as seen in the EAE mouse model. This could help to reduce the severity and frequency of inflammatory relapses. As compared with other injectable immunomodulatory drugs used in MS such as interferon beta preparations and glatiramer acetate, DMF has as an orally active agent, which has the benefit of increasing patients’ compliance with the therapy. In comparison with other FDA-approved oral disease-modifying MS drugs such as fingolimod (Gilenya®; Novartis International AG, Basel, Switzerland) and teriflunomide (Aubagio®; Sanofi-Aventis, Bridgewater, NJ, USA), DMF has been used for decades in the treatment of psoriasis. Long-term studies have established the safety of DMF without an increased risk of infections, malignancies, or significant long-term toxic effects.
Future directions
FAEs leads to the release of Nrf2 from the Keap1 complex and the subsequent upregulation of a wide variety of oxidative stress response genes. It would be interesting to see which pathway is responsible for the beneficial effects in MS patients. For primary culture CNS cells, it was found that the FAEs DMF and MMF lead to higher redox potentials in cells and an increase in GSH as well as in ATP levels. Moreover, the mitochondrial membrane potential can be influenced, too. The cell viability in ROS-challenged neurons and astrocytes is increased after DMF or MMF treatment and drastically reduced in Nrf2-deficient cells, thus indicating the role of an Nrf2-dependent oxidative stress response pathway, possibly induced by a better mitochondrial function.Citation79 This is supported by the fact that malfunctioning mitochondria accumulate in axons of MS patients.Citation80
On the other hand, in vitro data suggest reduced levels of GSH in astrocytes after treatment with DMF.Citation81 This unexpected finding illustrates the complexity of the CNS cellular network and the potential and need for future research.
For cardiovascular and renal injury, MG132 was reported to have activating effects on the Nrf-ARE signaling pathway.Citation51 MG132 is an inhibitor for the 26S proteasome reversely binding the chymotrypsin-like active site, thus being a competitive inhibitor. Its activating properties are thought to come from the inhibition of the proteasome and the reduced proteolysis of Nrf2. Unfortunately, MG132 is only for use in in-vitro studies, but there are other proteasome inhibitors that are in clinical trial. One being salinosporamide A, which inhibits the trypsin-like domain of the proteasome core particle.Citation82,Citation83 The first positive results on patients with multiple myeloma were reported in 2011,Citation84 with an unfortunate downside for use in MS patients. The side effects observed were severe, and treatment was only possible for a short period of 21 days. This makes the use of salinosporamide A in long-term medication impossible.
Carfilzomib, a proteasome inhibitor, targeting the chymotrypsin-like catalytic site of the 26S proteasome, was approved by the FDA on July 20, 2012. Nevertheless, there is only data for 22 patients treated with the compound for more than 1 year. Carfilzomib was generated on the basis of the natural antitumor product epoxomicin. This proteasome-inhibiting compound was found to have anti-inflammatory properties in vitro and in vivo in the murine ear edema assay.Citation85 Unfortunately, it was not reported whether or not the Nrf2 system is involved in this response. Finding a “mild” (reversely binding) proteasome inhibitor (possibly for the chymotrypsin-like catalytic domain) that is usable in patients would be highly desirable and could be beneficial for MS patients.
Conclusion and discussion
Multiple subtypes of MS have been described over the last few years, involving different cell types and factors. The most prominent pathophysiological features observed in these various subtypes are demyelination of axons and oligodendrocyte cell death. Inflammation itself can no longer be considered the common feature among MS types, as type IV seems to be a primary form of degeneration without any evidence of inflammation. The very broad description of MS pathophysiology helps to explain why existing treatment options aimed specifically at inflammation and immunological factors have had limited success.
In this review, a specific characteristic of MS is targeted, namely the occurrence of ROS in CNS inflammation. As it was shown that reduction of ROS has a positive impact on the duration and severity of inflammatory processes, the transcription factor Nrf2 came into the focus of research. After binding to ARE, it elevates the transcription of antioxidant response genes, thereby enhancing the oxidative stress response. Increasing Nrf2 levels can be obtained in two ways. Either Keap1, the cytosolic interaction partner of Nrf2 has to be blocked, or the degradation via the 26S proteasome has to be abolished. The inhibition of the proteasomal degradation is limited, as many proteins use the 26S proteasome. A more specific approach is the blockade of Nrf2 binding to Keap1. This blockade can be mediated by FAEs and has shown positive effects in vitro and in vivo. The benefits of reduction of ROS in the management of human diseases is not without precedent. The clinical efficacy of FAEs in reducing ROS-related inflammation is supported by years of experience in psoriasis patients in German-speaking countries. Recently, BG-12, a DMF analog, was approved by the European Union and the FDA for relapsing-remitting MS. Relapse rates were lower, and relapses were less severe in the clinical studies, having a positive effect on patients’ lives. Nevertheless, a long-term treatment in a large number of MS patients has to show the validity of the new treatment. To further improve the understanding of ROS-related MS, a deeper understanding of the ROS-forming cell processes is necessary. This could lead to treatment options preventing the initial formation of ROS and possibly preventing the inflammatory process.
Acknowledgment
This work was supported by a grant from the CH-Foundation (to HW).
Disclosure
The authors report no conflicts of interest in this work.
References
- LucchinettiCBruckWParisiJScheithauerBRodriguezMLassmannHHeterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelinationAnn Neurol200047670771710852536
- BjartmarCTrappBDAxonal degeneration and progressive neurologic disability in multiple sclerosisNeurotox Res200351–215716412832230
- KuhlmannTLingfeldGBitschASchuchardtJBruckWAcute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over timeBrain2002125Pt 102202221212244078
- FergusonBMatyszakMKEsiriMMPerryVHAxonal damage in acute multiple sclerosis lesionsBrain1997120Pt 33933999126051
- SimonJMagnetic resonance imaging in the diagnosis of multiple sclerosis, elucidation of disease, course, and determining prognosisBurksJSJohnsonKPMultiple Sclerosis: Diagnosis, Medical Management, and RehabilitationNew YorkDemos Medical Publishing200099126
- BarnettMHPrineasJWRelapsing and remitting multiple sclerosis: pathology of the newly forming lesionAnn Neurol200455445846815048884
- GonsetteRENeurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicityJ Neurol Sci20082741–2485318684473
- DevasagayamTPTilakJCBoloorKKSaneKSGhaskadbiSSLeleRDFree radicals and antioxidants in human health: current status and future prospectsJ Assoc Physicians India20045279480415909857
- MaQTranscriptional responses to oxidative stress: pathological and toxicological implicationsPharmacol Ther2010125337639319945483
- FinkelTSignal transduction by mitochondrial oxidantsJ Biol Chem201228774434444021832045
- BoverisADetermination of the production of superoxide radicals and hydrogen peroxide in mitochondriaMethods Enzymol19841054294356328196
- ChanceBSiesHBoverisAHydroperoxide metabolism in mammalian organsPhysiol Rev197959352760537532
- HansfordRGHogueBAMildazieneVDependence of H2O2 formation by rat heart mitochondria on substrate availability and donor ageJ Bioenerg Biomembr199729189959067806
- TurrensJFBoverisAGeneration of superoxide anion by the NADH dehydrogenase of bovine heart mitochondriaBiochem J198019124214276263247
- ShigenagaMKHagenTMAmesBNOxidative damage and mitochondrial decay in agingProc Natl Acad Sci U S A1994912310771107787971961
- CadenasEDaviesKJMitochondrial free radical generation, oxidative stress, and agingFree Radic Biol Med2000293–422223011035250
- BoverisACadenasEMitochondrial production of hydrogen peroxide regulation by nitric oxide and the role of ubisemiquinoneIUBMB Life2000504–524525011327317
- MaQRole of Nrf2 in oxidative stress and toxicityAnnu Rev Pharmacol Toxicol20135340142623294312
- BedardKKrauseKHThe NOX family of ROS-generating NADPH oxidases: physiology and pathophysiologyPhysiol Rev200787124531317237347
- LoveSOxidative stress in brain ischemiaBrain Pathol1999911191319989455
- LuSCRegulation of glutathione synthesisCurr Top Cell Regul2000369511610842748
- BiewengaGPHaenenGRBastAThe pharmacology of the antioxidant lipoic acidGen Pharmacol19972933153319378235
- PackerLWittEHTritschlerHJalpha-Lipoic acid as a biological antioxidantFree Radic Biol Med19951922272507649494
- van HorssenJWitteMESchreibeltGde VriesHERadical changes in multiple sclerosis pathogenesisBiochim Biophys Acta20111812214115020600869
- HendriksJJAlblasJvan der PolSMvan TolEADijkstraCDde VriesHEFlavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitisJ Exp Med2004200121667167215611292
- SmithKJKapoorRFeltsPADemyelination: the role of reactive oxygen and nitrogen speciesBrain Pathol19999169929989453
- van MeeterenMEHendriksJJDijkstraCDvan TolEADietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating diseaseBiochem Pharmacol200467596797515104250
- Aboul-EneinFRauschkaHKornekBPreferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseasesJ Neuropathol Exp Neurol2003621253312528815
- HaiderLFischerMTFrischerJMOxidative damage in multiple sclerosis lesionsBrain2011134Pt 71914192421653539
- NaidooRKnappMLStudies of lipid peroxidation products in cerebrospinal fluid and serum in multiple sclerosis and other conditionsClin Chem19923812244924541458583
- van HorssenJSchreibeltGDrexhageJSevere oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expressionFree Radic Biol Med200845121729173718930811
- GrayEThomasTLBetmouniSScoldingNLoveSElevated myeloperoxidase activity in white matter in multiple sclerosisNeurosci Lett2008444219519818723077
- GrayEThomasTLBetmouniSScoldingNLoveSElevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosisBrain Pathol2008181869518042261
- MarikCFeltsPABauerJLassmannHSmithKJLesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity?Brain2007130Pt 112800281517956913
- CrossAHManningPTKeelingRMSchmidtREMiskoTPPeroxynitrite formation within the central nervous system in active multiple sclerosisJ Neuroimmunol1998881–245569688323
- LiuJSZhaoMLBrosnanCFLeeSCExpression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesionsAm J Pathol200115862057206611395383
- SmithKJLassmannHThe role of nitric oxide in multiple sclerosisLancet Neurol20021423224112849456
- VetoSAcsPBauerJInhibiting poly(ADP-ribose) polymerase: a potential therapy against oligodendrocyte deathBrain2010133Pt 382283420157013
- WitteMEBoLRodenburgRJEnhanced number and activity of mitochondria in multiple sclerosis lesionsJ Pathol2009219219320419591199
- DuttaRMcDonoughJYinXMitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patientsAnn Neurol200659347848916392116
- DuttaRTrappBDMechanisms of neuronal dysfunction and degeneration in multiple sclerosisProg Neurobiol201193111220946934
- MahadDZiabrevaILassmannHTurnbullDMitochondrial defects in acute multiple sclerosis lesionsBrain2008131Pt 71722173518515320
- ZamboninJLZhaoCOhnoNIncreased mitochondrial content in remyelinated axons: implications for multiple sclerosisBrain2011134Pt 71901191321705418
- MahadDJZiabrevaICampbellGMitochondrial changes within axons in multiple sclerosisBrain2009132Pt 51161117419293237
- NikicIMerklerDSorbaraCA reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosisNat Med201117449549921441916
- VolterraATrottiDTrombaCFloridiSRacagniGGlutamate uptake inhibition by oxygen free radicals in rat cortical astrocytesJ Neurosci1994145 Pt 1292429327910203
- MatuteCSanchez-GomezMVMartinez-MillanLMilediRGlutamate receptor-mediated toxicity in optic nerve oligodendrocytesProc Natl Acad Sci U S A19979416883088359238063
- MatuteCCharacteristics of acute and chronic kainate excitotoxic damage to the optic nerveProc Natl Acad Sci U S A1998951710229102349707629
- McDonaldJWAlthomsonsSPHyrcKLChoiDWGoldbergMPOligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicityNat Med1998432912979500601
- NoseworthyJHLucchinettiCRodriguezMWeinshenkerBGMultiple sclerosisN Engl J Med20003431393895211006371
- CuiWBaiYLuoPMiaoLCaiLPreventive and therapeutic effects of MG132 by activating Nrf2-ARE signaling pathway on oxidative stress-induced cardiovascular and renal injuryOxid Med Cell Longev2013201330607323533688
- HayesJDMcMahonMNRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancerTrends Biochem Sci200934417618819321346
- AlamJKilleenEGongPHeme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2Am J Physiol Renal Physiol20032844F743F75212453873
- StewartDKilleenENaquinRAlamSAlamJDegradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmiumJ Biol Chem200327842396240212441344
- Dinkova-KostovaATHoltzclawWDColeRNDirect evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidantsProc Natl Acad Sci U S A20029918119081191312193649
- ItohKWakabayashiNKatohYKeap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domainGenes Dev199913176869887101
- ItohKWakabayashiNKatohYIshiiTO’ConnorTYamamotoMKeap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophilesGenes Cells20038437939112653965
- HirotsuYKatsuokaFFunayamaRNrf2-MafG heterodimers contribute globally to antioxidant and metabolic networksNucleic Acids Res20124020102281023922965115
- LeeJMLiJJohnsonDANrf2, a multi-organ protector?FASEB J20051991061106615985529
- TrappBDStysPKVirtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosisLancet Neurol20098328029119233038
- FilippiniGBrusaferriFSibleyWACorticosteroids or ACTH for acute exacerbations in multiple sclerosisCochrane Database Syst Rev20004CD00133111034713
- WeinerHLDauPCKhatriBODouble-blind study of true vs sham plasma exchange in patients treated with immunosuppression for acute attacks of multiple sclerosisNeurology1989399114311492549450
- WeinshenkerBGO’BrienPCPettersonTMA randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating diseaseAnn Neurol199946687888610589540
- KieseierBCThe mechanism of action of interferon-beta in relapsing multiple sclerosisCNS Drugs201125649150221649449
- JohnsonKPBrooksBRCohenJACopolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study GroupNeurology1995457126812767617181
- ChenPCVargasMRPaniAKNrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyteProc Natl Acad Sci U S A200910682933293819196989
- EllrichmannGPetrasch-ParwezELeeDHEfficacy of fumaric acid esters in the R6/2 and YAC128 models of Huntington’s diseasePloS One201161e1617221297955
- KapposLGoldRMillerDHEffect of BG-12 on contrast-enhanced lesions in patients with relapsing – remitting multiple sclerosis: subgroup analyses from the phase 2b studyMult Scler201218331432121878455
- KapposLGoldRMillerDHEfficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb studyLancet200837296481463147218970976
- BittnerSMeuthSGGobelKTASK1 modulates inflammation and neurodegeneration in autoimmune inflammation of the central nervous systemBrain2009132Pt 92501251619570851
- KhodagholiFEftekharzadehBMaghsoudiNRezaeiPFChitosan prevents oxidative stress-induced amyloid beta formation and cytotoxicity in NT2 neurons: involvement of transcription factors Nrf2 and NF-kappaBMol Cell Biochem20103371–2395119844776
- LewerenzJAlbrechtPTienMLInduction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitroJ Neurochem2009111233234319694903
- RachakondaGXiongYSekharKRStamerSLLieblerDCFreemanMLCovalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3Chem Res Toxicol200821370571018251510
- ThiessenASchmidtMMDringenRFumaric acid dialkyl esters deprive cultured rat oligodendroglial cells of glutathione and upregulate the expression of heme oxygenase 1Neurosci Lett20104751566020347008
- KavanaghGMBurtonJLDonnellVOEffects of dithranol on neutrophil superoxide generation in patients with psoriasisBr J Dermatol199613422342378746335
- LitjensNHBurggraafJvan StrijenEPharmacokinetics of oral fumarates in healthy subjectsBr J of Clin Pharmacol200458442943215373936
- FoxRJMillerDHPhillipsJTPlacebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosisN Engl J Med2012367121087109722992072
- GoldRKapposLArnoldDLPlacebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosisN Engl J Med2012367121098110722992073
- ScannevinRHChollateSJungMYFumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathwayJ Pharmacol Exp Ther2012341127428422267202
- LeeDHGoldRLinkerRAMechanisms of oxidative damage in multiple sclerosis and neurodegenerative diseases: therapeutic modulation via fumaric acid estersInt J Mol Sci2012139117831180323109883
- SchmidtMMDringenRFumaric acid diesters deprive cultured primary astrocytes rapidly of glutathioneNeurochem Int201057446046720096739
- ChauhanDCatleyLLiGA novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from BortezomibCancer Cell20058540741916286248
- FelingRHBuchananGOMincerTJKauffmanCAJensenPRFenicalWSalinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinosporaAngew Chem Int Ed Engl200342335535712548698
- PottsBCAlbitarMXAndersonKCMarizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trialsCurr Cancer Drug Targets201111325428421247382
- MengLMohanRKwokBHElofssonMSinNCrewsCMEpoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activityProc Natl Acad Sci U S A19999618104031040810468620
