Abstract
Background:
A trough concentration of >20 mg/L is considered the optimal dosage of teicoplanin required to ensure early therapeutic effects against methicillin-resistant Staphylococcus aureus (MRSA) infections including those in patients who develop febrile neutropenia after chemotherapy. This study determines appropriate initial doses during the first 2 days of administration and evaluates the therapeutic target teicoplanin trough concentration.
Method:
A 2-day regimen was evaluated in patients treated with 600 mg and 1200 mg or 1200 mg and 600 mg (total 1800 mg, Group 1), 800 mg and 800 mg (total 1600 mg, Group 2), and 800 mg and 400 mg (total 1200 mg, Group 3) of teicoplanin on Days 1 and 2, respectively. We also compared the efficiency and adverse effects at trough concentrations of 15–20 mg/L (Group A, n = 28) with >20 mg/L (Group B, n = 27) of teicoplanin, and also compared them with those on the similar concentrations of vancomycin (Groups C and D, n = 50 and 34, respectively).
Results:
The mean trough concentrations of teicoplanin on Days 4 or 5 were 22.2, 17.5, and 16.2 mg/L in Groups 1, 2, and 3, respectively. The clinical efficiency was 85.7%, 81.5%, 92.0%, and 91.5%, in Groups A, B, C, and D, respectively. The rates of adverse effects were not high in teicoplanin (nephrotoxicity, 7.1% and 3.7%, and hepatotoxicity, 14.3% and 11.1% in Groups A and B, respectively). However, more adverse effects tended to arise in patients who received vancomycin in nephrotoxicity (14.0% and 11.8%, in Groups C and D, respectively).
Conclusion:
These results suggest that the 2-day regimens with total 1800 mg achieved the most effective therapeutic trough plasma concentration of teicoplanin (20 mg/L). However, 15–20 mg/L might also be an effective trough target for initial teicoplanin treatment. These teicoplanin regimens might be safer in terms of renal function than vancomycin.
Introduction
Teicoplanin is a glycopeptide antibiotic that has been extensively evaluated as a treatment for extremely invasive infection caused by Gram-positive bacteria.Citation1,Citation2 An initial teicoplanin loading procedure has been recommended to promptly achieve the optimal serum concentration.
Recent reports have recommended that therapeutic drug monitoring (TDM) should be used to maintain adequate serum trough concentrations.Citation2,Citation3 Although a teicoplanin trough concentration of 10 mg/L is generally accepted as the standard of care, a trough concentration of >20 mg/L is currently recommended for some extreme situations, such as treating Staphylococcus aureus endocarditis, and bone or prosthetic infections.Citation1,Citation2
A retrospective study found that favorable clinical outcomes of S. aureus-related deep infections treated with teicoplanin are associated with trough concentration values of >20 mg/L.Citation3,Citation4 Weinbren and Struthers, commenting on possible causes of the emergence of methicillin-resistant S. aureus with reduced susceptibility during teicoplanin therapy, proposed that currently recommended dosages of teicoplanin should be increased.Citation5 Targeting a trough concentration of 20 mg/L might help to treat S. aureus septicemia, particularly when less susceptible microorganisms have a minimum inhibitory concentration (MIC) close to the breakpoint for teicoplanin. Although no evidence has yet indicated MIC ‘creep’ in a teicoplanin regimen, an alternative therapeutic regimen to achieve a higher teicoplanin trough concentration of 15 mg/L should also be considered.Citation1,Citation2,Citation6–Citation8
Here, we created a teicoplanin regimen that achieved a target trough concentration of >20 mg/L in patients and retrospectively evaluated its efficacy and toxicity at different concentrations.
Materials and methods
Patients
This study was carried out at Osaka University Hospital, Japan. This 1076-bed, teaching and academic hospital provides tertiary and advanced medical care. We used an electronic record and ordering system to investigate patients over 16 years of age who were admitted to our hospital between January 2009 and December 2011 under diagnoses of methicillin-resistant S. aureus, methicillin-resistant coagulase-negative staphylococci, ampicillin-resistant enterococci bacteremia, or teicoplanin-susceptible pathogens. We also included patients diagnosed with febrile neutropenia, who were ineligible for empirical antimicrobial therapy if the neutropenia had persisted at <500 cells/mm3 for >5 days, and if they had fever of unknown origin at >38.5°C on one occasion or >38°C on at least two occasions.Citation9
Demographics and laboratory markers of inflammation, renal, and hepatic function namely, C-reactive protein (CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine clearance (CLcr), and total bilirubin were obtained from electronic medical records.
All patients or relatives provided written, informed consent to participate in this study, which was approved by the Research Ethics Committee of Osaka University Hospital.
Treatment regimen and measurement of trough concentration
Three treatment regimens were evaluated prospectively (). Group 1 was administered with 600 mg of teicoplanin twice on Day 1 and once on Day 2. A 2-day regimen comprising a loading dose of 600 mg on the afternoon of Day 1 and 600 mg twice on Day 2 was also included (Group 1). This regimen was administered to patients whose laboratory data were completed in the afternoon, and who received teicoplanin in the evening. The total doses (1800 mg) were identical. The 2-day regimen of a 400-mg loading twice on Days 1 and 2 (total 1600 mg) was administered to Group 2. The 1-day regimen (standard regimen) of 2 × 400-mg loading doses on Day 1 followed by 400 mg on Day 2 (total 1200 mg) was administered to Group 3.
Table 1 Dose of teicoplanin administration to the patients
Teicoplanin target trough concentration samples were obtained just before administration on Days 4 or 5. Achieving a trough concentration of 15–20 mg/L or >20 mg/L was evaluated in each group. The dosing regimen after Day 4 was adjusted according to the trough concentration data.
Trough concentration of teicoplanin was measured by Fluorescence Polarization Immunoassay (FPIA; Tagocid TDM kit-IBL, Immuno-Biological Laboratories Co, Ltd, Fujioka, Japan).
Clinical efficiency and adverse effects at each trough concentration
We retrospectively compared the efficiency and adverse effects of 15–20 mg/L and >20 mg/L trough concentrations of teicoplanin (Groups A and B, n = 28 and 27, respectively) and vancomycin (Groups C and D, n = 50 and 34, respectively) to confirm the clinical safety of teicoplanin. We collected not only the cases which showed these trough concentrations at first measurement at Days 4–5 in teicoplanin and Days 3–4 in vancomycin, but also the cases which showed these trough concentrations at other points, such as Day 7 and Day 10.
Clinical efficacy was evaluated by improvement of body temperature and CRP as therapeutic responses, and clinical evaluations were performed 7–10 days after the final dose as previously reported.Citation6,Citation7
The adverse effects of nephrotoxicity and hepatotoxicity were also evaluated on the day that teicoplanin therapy was completed and 7–10 days after the last dose. Nephrotoxicity was defined as a serum creatinine increase of 0.5 mg/dL or 50% from baseline.Citation6,Citation10 Hepatotoxicity was defined as AST or ALT that was three-fold the upper limit of normal (ULN; AST: 13–33 IU/L, ALT: 8–42 IU/L). If the AST or ALT baseline was abnormal, hepatotoxicity was defined as AST or ALT three times increased from the baseline values.Citation6,Citation11
Statistical analysis
Statistical analysis of the survey data was of a descriptive nature, where continuous variables are shown as summarized means and standard deviation (SD).
Data were statistically analyzed using a parametric, paired or unpaired Student’s t-test as appropriate, or the nonparametric Mann–Whitney rank-sum test, for normally or non-normally distributed data, respectively. For multiple comparison, we performed parametric tests, including Tukey–Krammer’s method, Scheffe’s F-test, and Bonferroni–Dunn’s method, and nonparametric Steel–Dwass’ method, Steel’s method, and Shirley–William’s method, respectively. The clinical efficacy and adverse effects were also analyzed using Fisher’s exact test and nonparametric Kruskal–Wallis test.
A value of P < 0.05 was considered to indicate statistical significance.
Results
Achievement of target trough concentration
shows the characteristics of the 28 patients in the study of the teicoplanin loading regimen (Groups 1, 2, and 3, n = 11, 6, and 11, respectively). Age, body weight, serum albumin, and serum creatinine values were similar among the groups, and no statistically significant differences were found regarding the effects (P = 0.50). Maintenance therapy was administered at 400 mg once per day in most of the cases, and the duration of maintenance therapy was also similar within the group. Most patients were diagnosed with febrile neutropenia and the numbers of patients with various types of infection were similar among the three groups (data not shown).
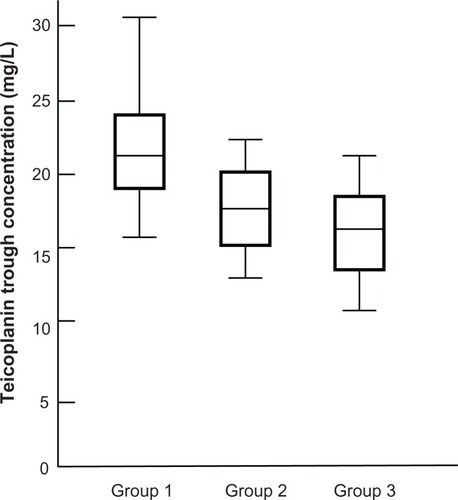
The mean trough concentration in Groups 1, 2, and 3 were 22.2 ± 6.6, 17.5 ± 5.3, and 16.2 ± 3.6 mg/L, respectively (). The >20 mg/L trough concentration was achieved only in Group 1.
Clinical efficiency and adverse effects
shows the results of retrospective analysis for the rates of treatment success and adverse effects in each trough concentration of teicoplanin and vancomycin. The success rates were 85.7%, 81.5%, 92.0%, and 91.2% in Groups A, B, C, and D, respectively (P = 0.50), with no significant differences between Groups A and B that achieved 15–20 mg/L and >20 mg/L of teicoplanin, respectively (P = 0.35), and between Groups C and D that achieved 15–20 mg/L and >20 mg/L of vancomycin, respectively (P = 0.45). The loading regimen of teicoplanin in Groups A and B was also not significantly different (P = 0.50).
Table 2 Treatment success and adverse effects rates in each group
The incidences of nephrotoxicity (Group A vs B, 7.1% vs 3.7%) and hepatotoxicity (Group A vs B, 14.2% vs 11.1%) did not significantly differ among the teicoplanin groups (P = 0.57). However, the groups treated with vancomycin tended to develop nephrotoxicity more frequently (Group C vs D, 14.0% vs 11.7%), compared with the groups treated with teicoplanin (Group B [teicoplanin > 20 mg/L] and C [vancomycin 15–20 mg/L], P = 0.08, respectively).
The incidence of hepatotoxicity in the groups treated with vancomycin (Group C vs D, 18.0% vs 20.6%) was similar to that of the groups treated with teicoplanin (P = 0.50).
Discussion
Recent recommendations suggest that the trough concentration of teicoplanin should be maintained at >20 mg/L to treat severe infection.Citation1,Citation2,Citation5,Citation7–Citation9,Citation12 A high-dose regimen of teicoplanin for the first 3–4 days can achieve a higher target trough concentration.Citation6,Citation7,Citation9,Citation12–Citation14 Brink et al assessed the standard teicoplanin regimen comprising 6 mg/kg every 12 hours on Day 1 followed by 6 mg/kg daily thereafter with a high-dose regimen comprising 6 mg/kg every 12 hours for 4 days among patients with suspected or confirmed Gram-positive infections.Citation8 The mean trough concentration values on Day 4 were 19.1 and 9.6 mg/L for the high-dose and standard regimens, respectively.
Here, we found that only the 1200- and 600- or 600- and 1200-mg regimens (Group 1; total 1800 mg regimen) could achieve a teicoplanin trough concentration of >20 mg/L. The 800- and 800- (Group 2; total 1600 mg regimen) and 800- and 400- (Group 3; total 1200 mg regimen) mg regimens also achieved a sufficient trough concentration of 15–20 mg/L, but the Group 1 regimen might confer the most advantages for rapidly achieving a trough concentration of >20 mg/L. These data suggest that a total dose of 1800 mg might be needed to achieve this trough target. Furthermore, the 600- and 1200-mg regimen could be used for Group 1 if laboratory data are provided in the afternoon/evening as teicoplanin administration twice before midnight might be onerous. This regimen is also more practical than administering two doses on the day of admission.
It has been reported that the trough concentration of teicoplanin achieved by the standard regimen (loading dose of 800 mg on Day 1, followed by maintenance doses of 400 mg) remains <10–15 mg/L.Citation15 Pea et al also compared the standard (400 mg every 12 hours on Day 1 followed by 400 mg daily) with high-dose (800 and 400 mg at an interval of 12 hours on Day 1, 600 and 400 mg at an interval of 12 hours on Day 2, followed by 400 mg every 12 hours) regimens in adult patients (CLcr < 50 mL/minute) with acute leukemia who developed febrile neutropenia after chemotherapy.Citation9 The trough concentration of the high-dose regimen at 72 hours exceeded 20 mg/L in 10 of 22 of their patients, and renal function was not significantly impaired in any of them. These data also supported the necessity and safety of the higher dose at loading in the use of teicoplanin.
It has been suggested that the risk of nephrotoxicity is lower with teicoplanin than vancomycin,Citation1,Citation2,Citation16,Citation17 but high-dose teicoplanin tends to cause hepatotoxicity.Citation18 Therefore, we should confirm teicoplanin safety although we investigated the loading dose regimen to achieve >20 mg/L trough concentration in the current study (Group A–C).
We retrospectively evaluated the efficacy and adverse effects between groups treated with teicoplanin and vancomycin at target trough concentrations of 15–20 mg/L and >20 mg/L. Success rates were acceptable and similar among the four groups, indicating that the clinical efficacy of teicoplanin and vancomycin are essentially equal. Among our patients who received teicoplanin, 60% had febrile neutropenia (data not shown), and teicoplanin trough concentrations of 15–20 mg/L were usually as effective as >20 mg/L.
The incidences of nephrotoxicity and hepatotoxicity did not significantly differ between the 2-day loading regimens among the groups in this study. We also could not find any difference of loading regimen between the groups with 15–20 mg/L and >20 mg/L in this retrospective analysis. Most of these cases were treated by standard (low dose) regimen: ex 400–400 mg or 200–200 mg on Day 1 only. The differences of these achieved trough concentrations might be affected by patients’ clinical characteristics at admission, including complications. Further studies are required.
Although hepatotoxicity occurred at similar rates in both the teicoplanin and vancomycin groups, the incidence of nephrotoxicity was only 7.1% and 3.7% at trough concentrations of 15–20 mg/L and >20 mg/L, respectively, in patients treated with teicoplanin, compared with vancomycin (14.0% and 11.8%, respectively).
Mimoz et al assessed a high-dose teicoplanin regimen (12 mg/kg every 12 hours for two consecutive days, followed by 12 mg/kg daily) in patients (CLcr > 60 mL/minute) with ventilator-associated pneumonia.Citation14 The mean trough concentration on Day 4 was 15.9 mg/L, and no serious clinical or clinically significant biological adverse effects developed. Creatinine values returned to baseline within 2 weeks after discontinuing teicoplanin in one of two patients in that study. In addition, Frye et al reported that decrease of platelets as an adverse effect was found at trough concentration > 40 mg/L,Citation19 and renal dysfunction was reported at trough concentration > 60 mg/L in teicoplanin use.Citation20 These results suggest that the risk of adverse effects, especially renal impairment may be lower with teicoplanin than with vancomycin.
In conclusion, a 2-day regimen which administered total 1800 mg (1200- and 600- or 600- and 1200-mg) of teicoplanin was practical and rapidly achieved a therapeutic trough plasma concentration of >20 mg/L. However, 15–20 mg/L may also be a sufficient initial teicoplanin trough target with which to treat febrile neutropenia. These teicoplanin regimens may be safer in terms of renal function than vancomycin, while offering similar clinical efficacy.
Disclosure
The authors report no conflicts of interest in this work.
References
- GemmellCGEdwardsDIFraiseAPGouldFKRidgwayGLWarrenREJoint Working Party of the British Society for Joint Working Party of the British Society for Antimicrobial Chemotherapy, Hospital Infection Society and Infection Control Nurses AssociationGuidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UKJ Antimicrob Chemother20065758960816507559
- LiuCBayerACosgroveSEClinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summaryClin Infect Dis20115228529221217178
- MacGowanAPPharmacodynamics, pharmacokinetics, and therapeutic drug monitoring of glycopeptidesTher Drug Monit1998204734779780121
- MacGowanAPBowkerKEPharmacodynamics of antimicrobial agents and rationale for their dosingJ Chemother1997964739248964
- WeinbrenMStruthersKEmergence of Staphylococcus aureus (MRSA) with reduced susceptibility to teicoplanin during therapyJ Antimicrob Chemother20025030630712161421
- UedaTTakesueYNakajimaKEvaluation of teicoplanin dosing designs to achieve a new target trough concentrationJ Infect Chemother20121829630222065089
- MatsumotoKKanazawaNIkawaKDetermination of teicoplanin trough concentration target and appropriate total dose during the first 3 days: a retrospective study in patients with MRSA infectionsJ Infect Chemother20101619319920195882
- BrinkAJRichardsGACumminsRRRecommendations to achieve rapid therapeutic teicoplanin plasma concentrations in adult hospitalised patients treated for sepsisInt J Antimicrob Agents20083245545818718742
- PeaFVialePCandoniATeicoplanin in patients with acute leukaemia and febrile neutropenia: a special population benefiting from higher dosagesClin Pharmacokinet20044340541515086277
- RybakMLomaestroBRotschaferJCTherapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases PharmacistsAm J Health Syst Pharm200966829819106348
- SeniorJRMonitoring for hepatotoxicity: what is the predictive value of liver “function” tests?Clin Pharmacol Ther20098533133419129750
- WangJTLiaoHIWu LinFLChangSCLoading dose required to achieve rapid therapeutic teicoplanin trough plasma concentration in patients with multidrug-resistant gram-positive infectionsBasic Clin Pharmacol Toxicol201211041642022309355
- LamontESeatonRAMacphersonMSempleLBellEThomsonAHDevelopment of teicoplanin dosage guidelines for patients treated within an outpatient parenteral antibiotic therapy (OPAT) programmeJ Antimicrob Chemother20096418118719411678
- MimozORollandDAdounMSteady-state trough serum and epithelial lining fluid concentrations of teicoplanin 12 mg/kg per day in patients with ventilator-associated pneumoniaIntensive Care Med20063277577916550370
- PeaFBrolloLVialePPavanFFurlanutMTeicoplanin therapeutic drug monitoring in critically ill patients: a retrospective study emphasizing the importance of a loading doseJ Antimicrob Chemother20035197197512654757
- SvetitskySLeiboviciLPaulMComparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysisAntimicrob Agents Chemother2009534069407919596875
- Van der AuweraPMeunierFIbrahimSKaufmanLDerdeMPTulkensPMPharmacodynamic parameters and toxicity of netilmicin (6 milligrams/kilogram/day) given once daily or in three divided doses to cancer patients with urinary tract infectionAntimicrob Agents Chemother1991356406472069370
- YoshidaMMatznoSNambaHNishikataMMatsuyamaKStatistical analysis of the adverse effects of glycopeptide antibiotics, based on pharmacokinetics and toxicokinetics (PK/TK)J Infect Chemother20061211411816826342
- FryeRFJobMLDretlerRHRosenbaumBJTeicoplanin nephrotoxicity: first case reportPharmacotherapy1992122402421535125
- WilsonAPRGrunegergRSafetyTeicoplanin; The First DecadeAbingdon, UKThe Medicine Group (Education) Ltd1997137144