Abstract
Despite advances in cytotoxic chemotherapy and surgical cytoreduction, disease recurrence continues to be a troubling problem in patients with advanced-stage epithelial ovarian cancer (EOC). Malignant ascites affects approximately 10% of patients with recurrent EOC and is associated with troublesome symptoms, including abdominal pressure, distension, dyspnea, pelvic pain, and bowel/bladder dysfunction. To date, no effective therapy has been identified for the treatment of malignant ascites in patients with recurrent, advanced-stage ovarian cancer. Recently, immune modulation has gained attention as a novel approach to anti-cancer therapy. This review explores the role of epithelial cell-adhesion molecule (EpCAM)-directed immunotherapy, with a specific focus on the mechanism of action of the trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3). In addition, clinical trials exploring the use of catumaxomab in the treatment of malignant ascites in patients with ovarian cancer are reviewed.
Introduction
Epithelial ovarian cancer (EOC) accounts for 25% of all malignancies affecting the female genital tract, and is the most lethal gynecologic malignancy. In 2013 there will be an estimated 22,240 new ovarian cancer cases in the USA, with 14,030 deaths.Citation1 Advanced-stage EOC is traditionally managed with cytoreductive surgery, followed by combination platinum- and taxane-based chemotherapy.Citation2 Despite aggressive treatment, the majority of these patients develop recurrent cancer, with selection of chemotherapy-resistant clones.Citation3 The subset of patients who develop recurrent disease is a population that traditionally faces extended exposure to multiple cytotoxic chemotherapy regimens, dictated by their disease-free interval.Citation4–Citation7 Throughout this period, management of disease-associated morbidities becomes a priority in an effort to improve quality of life.
Malignant ascites, which affects approximately two-thirds of patients with EOC, primary peritoneal cancer (PPC), and fallopian tube cancer (FTC), is common, and to date few effective therapies have been identified.Citation8,Citation9 Importantly, ascites is associated with troublesome symptoms, including abdominal pressure and distension, dyspnea, pelvic pain, and bowel/bladder dysfunction.Citation10 Historically, malignant ascites in patients with EOC was treated utilizing diuretics and salt restriction, as well as intraperitoneal administration of sclerosing agents and radioactive isotopes.Citation11,Citation12 Patients with malignant ascites secondary to EOC rarely suffer from fluid accumulation due to intraparenchymal liver metastasis, and portal hypertension is uncommonly identified. Rather, these patients traditionally exhibit reduced intravascular volume, making diuretic use an unattractive option.Citation13 With respect to the use of radioactive isotopes, poor tumor penetration and intestinal toxicity (necrosis and perforation) due to loculations and prolonged exposure have caused them to fall out of favor. Limited success rates, in combination with significant side effects, have resulted in the infrequent use of these modalities.
Mechanical drainage of accumulated ascitic fluid via therapeutic paracentesis results in relief in up to 90% of patients.Citation14 However, recurrence/reaccumulation of ascites is common, and multiple paracentesis are required, with their associated risks of pain, visceral perforation, infection, and hematoma formation.Citation15,Citation16 Furthermore, these patients are likely to have intra-abdominal adhesions as a result of their extensive surgical cytoreduction, and the resultant fluid loculations limit the therapeutic benefit derived.Citation17 Alternatively, placement of permanent intra-abdominal drains and peritoneovenous shunts has been explored. Experience with these modalities has been poor, as blockage of the shunt, infectious morbidity, as well as embolization and implantation of tumor cells in distant organs, were reported to be relatively common complications.Citation12,Citation18–Citation21
Unlike other solid malignancies, where ascites portends a universally poor prognosis, patients with EOC and ascites at the time of diagnosis can expect 5-year survival rates approaching 40%.Citation15,Citation22 This discrepancy is largely attributable to the biology of ovarian cancer, and the subsequent etiology of the abdominal fluid accumulation. Specifically, malignant ascites in patients with EOC is thought to be attributable to (1) lymphatic obstruction, (2) increased vascular permeability, (3) release of inflammatory cytokines, and (4) direct increase of fluid production by the cancer cells lining the peritoneal cavity.Citation9,Citation23–Citation25
Given the above, exploration into immune modulation as a novel anti-cancer approach for the treatment of malignant ascites in patients with advanced-stage EOC has been a clinical priority.Citation9 Tumor immunology is complex, as activation of the immune system requires presentation of a foreign antigen via antigen-presenting cells (APCs) to T-cells.Citation26 In normal immune responses, the cytotoxic effects are driven by a combination of T-lymphocyte activity, antibody-dependent mechanisms, and natural killer (NK) cell activation.Citation26–Citation28
Tumor cell destruction by tumor antigen-specific T lymphocytes has been demonstrated in vitro for a variety of both solid and hematologic malignancies. Certain cytokines, including interferon-γ and tumor necrosis factor (TNF), are essential components of the above response. Conversely, antibody-mediated cytotoxicity relies on direct antibody binding to tumor cell surfaces, and the subsequent recruitment of granulocytes and macrophages, both of which contain surface receptors for the fragment crystallizable (Fc) portion of the antibody. Analogously, activated NK cells contain surface receptors for antibody Fc regions, and participate in antibody-mediated cytotoxicity. Furthermore, activated NK cells secrete TNF-α, inducing hemorrhage and tumor necrosis.
Unfortunately, tumor cells have adapted to escape immune destruction, utilizing tumor-related and host-related mechanisms. Cancer cells may fail to provide an antigenic target due to lack of an antigenic (foreign) epitope, lack of major histocompatibility complex-I molecule, or via antigenic modulation and tumor masking.Citation29,Citation30 Host-related mechanisms include immune suppression, regulatory T-cell (Treg) suppression of tumor immunity, deficient epitope expression by host APCs, and failure of host immune cells to reach the tumor due to stromal barriers.Citation31
Ovarian cancer immunology
An increasingly robust body of literature supports the contribution of immune cells in both ovarian cancer therapeutics and pathogenesis.Citation32–Citation42 In 1988, immune studies performed on ovarian cancer specimens confirmed the presence of tumor-infiltrating lymphocytes (TILs).Citation43 Cluster of differentiation 3 (CD3)+ TILs have been found to independently predict tumor recurrence and prolonged survival.Citation42 Conversely, lack of TILs has been associated with poor survival.Citation34,Citation36–Citation40
Certain subsets of T-cells exhibit a pro-cancer effect. Treg infiltration has been associated with higher cancer grade and advanced surgical stage.Citation44 Additionally, increasing numbers of circulatory Tregs identified in peripheral blood samples have been linked to disease progression.Citation45 These CD4+CD25+ Tregs are hypothesized to mitigate the immune response via two distinct mechanisms, inhibiting both cell–cell contact and interleukin-2 transcription.Citation31
The impact of B-cell function on cancer immunology has been more difficult to discern as a result of conflicting data.Citation46–Citation49
Epithelial cell-adhesion molecule (EpCAM)
Epithelial cell-adhesion molecule (EpCAM) is a calcium-independent transmembrane, glycoprotein cell-adhesion molecule, with a molecular weight of 39–42 kDa.Citation50 Traditionally, it is expressed in normal epithelium, with the exception of squamous epithelium, epidermal keratinocytes, gastric parietal cells, myoepithelial cells, thymic cortical epithelium, and hepatocytes.Citation51,Citation52 EpCAM is abundantly expressed on human cancers, and was first described as a dominant antigen in patients with colon carcinoma.Citation53,Citation54 Since then, EpCAM overexpression has been associated with poor prognosis in patients with ovarian, breast, prostate, and gallbladder carcinoma, both functioning as an oncogene and suppressing CD4+ T-cell-dependent immune responses.Citation55–Citation58
Initial interest in the utilization of EpCAM as a target for active immunotherapy emerged following a seminal publication reporting anti-tumor effects with the EpCAM-specific monoclonal antibody edrecolomab in patients with metastatic colorectal cancer.Citation58,Citation59 Despite initial promise, the therapeutic impact of this approach was found to be inferior to traditional cytotoxic chemotherapy regimens in colon cancer patients.
With respect to ovarian cancer, EpCAM expression has been demonstrated in the main histotypes, and is well documented in microarray studies.Citation60 In a study of 21 biomarkers in four distinct ovarian cancer subtypes (high-grade serous, clear cell, endometrioid, mucinous), only EpCAM exhibited consistent high expression.Citation60 Furthermore, data indicate that EpCAM appears to be a stable antigen in ovarian cancer patients, with preserved expression levels in primary, recurrent, and metastatic specimens, as well as malignant ascites and effusions.Citation61,Citation62
The trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3)
In an effort to circumvent the limitations initially encountered with monoclonal antibodies (mAb), bispecific antibodies, which allow for simultaneous binding of both T-cell and accessory cells, were designed and tested. Ultimately, the design of trifunctional antibodies allowed for combination of two distinct anti-tumor functionalities (T-cell-mediated death and accessory cells).Citation27,Citation63,Citation64
Catumaxomab is a trifunctional mAb with two different antigen-binding sites and a functional Fc domain ().Citation65,Citation66 It is composed of a mouse κ light chain, a rat λ light chain, a mouse immunoglobulin (Ig)-G2a heavy chain, and a rat IgG2a heavy chain. The two specific antigen-binding sites bind to epithelial tumor cells via the EpCAM and to T-cells via CD3. In addition, catumaxomab activates Fcγ-receptor I-, IIa-, and III-positive accessory cells (dendritic cells [DCs], macrophages, and NK cells) via its functional Fc domain, resulting in a comprehensive and complex immune reaction ().Citation65,Citation66
Figure 1 Schematic of the structure and mode of action of catumaxomab. (A) Catumaxomab is a trifunctional monoclonal antibody with two different antigen-binding sites and a functional Fc domain. (B) The two specific antigen-binding sites bind to epithelial tumor cells via the EpCAM and to T-cells via CD3, while activating Fcγ-receptor I-, IIa-, and III-positive accessory cells (dendritic cells, macrophages, and NK cells) via its functional Fc domain.
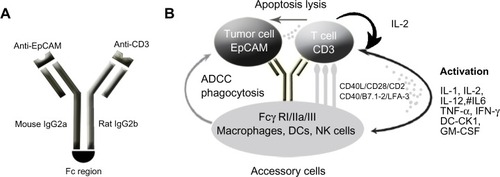
The functionality and selectivity of this novel antibody rely on the fact that tumor cells in malignant ovarian cancer-associated ascites have been shown to express EpCAM in 70%–100% of cases, while the mesothelial cells lining the peritoneal cavity lack expression.Citation67 Following EpCAM binding, catumaxomab results in recruitment and activation of immune effector cells, resulting in its antineoplastic activity. The two unique antibody–antigen binding sites of the trifunctional antibody enable recognition of both T-cells and tumor cells. The functional Fc domain can then activate neighboring Fcγ-receptor-positive macrophages, DCs, and NK cells, as described by Eissler et al in elegant animal model experiments.Citation68 Ultimately, tumor cell death results from cell lysis via perforin/granzyme-B, antibody-mediated cell death, and phagocytosis.Citation66,Citation69,Citation70
Catumaxomab has also been shown to induce anti-tumor immunity in animal models and in patients with peritoneal carcinomatosis. In a study conducted by Ströhlein and Heiss, tumor-reactive CD4+/CD8+ T-cells were induced after an initial treatment course with escalating doses of trifunctional antibodies, followed by re-stimulation 4 weeks after completion of initial treatment.Citation71–Citation73
Catumaxomab in the treatment of ovarian cancer ascites (anti-EpCAM × anti-CD3)
Malignant ascites affects approximately 10% of patients with recurrent EOC and is associated with troublesome symptoms. Intraperitoneal administration of catumaxomab was first studied in the treatment of eight patients (two of whom had ovarian cancer) with malignant ascites in 2005.Citation74 All patients had <2% EpCAM expression via flow cytometry on nuclear ascites cells. Trifunctional antibodies were administered intraperitoneally over 6–8 hours, for at least four cycles. Seven of eight patients required no further paracentesis during follow-up or until death, with a mean paracentesis-free interval of 38 weeks (median 21.5, range 4–136). Clinical response, with disappearance of ascites accumulation, was seen in all patients, which was correlated with elimination of tumor cells (P = 0.0014).Citation74
Following this study, a multicenter phase I/II clinical trial was conducted evaluating the tolerability and efficacy of intraperitoneal catumaxomab in ovarian cancer patients with malignant ascites containing EpCAM-positive tumor cells.Citation75 Twenty-three women with recurrent ascites due to pretreated refractory ovarian cancer were treated with four to five intraperitoneal infusions of catumaxomab in doses of 5, 200 μg within 9 13 days. Treatment with catumaxomab resulted in significant and sustained reduction of ascites. Of the 23 patients, 22 did not require paracentesis between the last infusion and the end of study at day 37.Citation75 The most commonly reported grade 2/3 adverse events in the study included fever, nausea, and vomiting.
Recently, a prospective, randomized phase II/III study was conducted comparing the efficacy of catumaxomab plus paracentesis with paracentesis alone in the treatment of malignant ascites.Citation67 Following paracentesis, catumaxomab was administered at doses of 10, 20, 50, and 150 μg on days 0, 3, 7, and 10, respectively, via an intraperitoneal catheter. The primary efficacy endpoint was puncture-free survival. Secondary efficacy parameters included time to next paracentesis, ascites signs and symptoms, and overall survival (OS). Puncture-free survival was significantly longer in the catumaxomab group (median 46 days) than in the control group (median 11 days) (hazard ratio [HR] 0.254; P < 0.0001), as was median time to next paracentesis (77 versus 13 days; P < 0.0001). Within the ovarian cancer cohort, median puncture-free survival was 52 days in the catumaxomab arm versus 11 days in the placebo arm (HR 0.205; P < 0.0001). In addition, catumaxomab-treated patients had fewer signs and symptoms of ascites than control patients. The most common adverse events included fever, abdominal pain, nausea, and vomiting. One patient had a grade 3 gastric hemorrhage. Findings from the above trials ultimately resulted in the European Medicines Agency approval of catumaxomab for the treatment of malignant ascites in patients with EpCAM-positive tumors for whom no standard therapy is available.Citation23
Immunomonitoring studies performed as part of the clinical trial were notable for a significant decline in EpCAM-positive tumor cells from a median screening value of 6,510 EpCAM-positive cells (165 patients) to a median of 27 cells on day 3 (133 patients), and to 0 cells (115 patients) on day 11 in the catumaxomab-treated arm.Citation76 In the control group, the tumor cell number increased from 9,373 EpCAM-positive tumor cells at screening (85 patients) to 18,929 EpCAM-positive tumor cells (74 patients) at the puncture visit. Furthermore, catumaxomab treatment was associated with a significant decline (63%; P < 0.001) in ascites fluid levels of vascular endothelial growth factor (VEGF), inhibiting vascular permeability, translating into decreased ascites fluid production. Lastly, CD69+ (indicative of lymphocyte proliferation), CD4+, and CD8+ T-cell populations increased more than 2-fold in catumaxomab-treated subjects. The activation of peritoneal T-cells and concomitant decline in EpCAM-positive tumor cells establishes a cellular basis for the anti-tumor immunologic effects of the trifunctional antibody catumaxomab.Citation76
The palliative nature of the treatment of malignant ascites in patients with recurrent ovarian cancer necessitates prioritization of quality of life during treatment. Wimberger et al conducted a post-trial ad hoc analysis of the above-described phase II/III study to determine the impact of catumaxomab on health-related quality of life (HR-QOL).Citation77 Deterioration in QOL scores appeared more rapidly in the control than in the catumaxomab group (median 19–26 days versus 47–49 days). The difference in time to deterioration in QOL between the groups was statistically significant for all scores (P < 0.01).
The chronicity of disease in patients with recurrent malignant ascites related to ovarian carcinoma led to the exploration of intraperitoneal changes resulting from treatment with catumaxomab. In a small retrospective series, ten patients previously treated with intraperitoneal catumaxomab underwent repeat surgical exploration for secondary cytoreduction, treatment of anastomotic leaks or ileus, or for colostomy reversal.Citation78 Catumaxomab treatment was associated with severe intra-abdominal adhesions, grade 3. Conversely, no patients had ascites volume <500 mL despite extensive carcinomatosis in eight of ten subjects.
Given the mouse–rat origin of catumaxomab, limitations in retreatment were anticipated due to formation of human anti-drug antibodies (HADA). However, in 2011, Pietzner et al described a case of successful re-treatment with catumaxomab for the management of malignant ascites.Citation79 A 74-year-old female patient with breast cancer and peritoneal carcinomatosis-associated ascites was treated with catumaxomab, with resolution of her symptoms. The patient remained puncture free for 45 days, and evaluation of HADA levels demonstrated increased levels after cycle 1, followed by a considerable decline and delayed increase in ascites HADA levels for each subsequent cycle. This experience suggested that a repeat cycle of catumaxomab might be feasible and effective in patients suffering from recurrent malignant effusions.
More recently, Ott et alCitation80 conducted a post-trial ad hoc analysis in order to determine the impact of human antimouse antibodies (HAMA) levels 8 days after the last infusion on clinical outcomes in patients treated on the European Union Drug Regulating Authorities Clinical Trials (EudraCT) clinical trial (NCT00836654).Citation81 There was a strong correlation between humoral response and clinical outcome, with HAMA-positive patients showing improvement in median puncture-free survival, median time to next therapeutic puncture, as well as median OS.Citation80 Specifically, median OS in HAMA-positive ovarian cancer patients was 163 days, compared with 82 days in the HAMA-negative patients (P = 0.0123; HR 0.407). This finding remained significant in both the intention-to-treat and per-protocol analysis.
Conclusion
Despite aggressive surgical cytoreduction and adjuvant chemotherapy, disease recurrence continues to be problematic for patients with advanced-stage EOC. Immune modulation has gained significant attention in recent years as a novel anti-cancer approach. Catumaxomab is an innovative trifunctional antibody that relies on direct tumor cell and T-cell binding while simultaneously recruiting accessory immune cells to treat malignant ascites. This complex association results in tumor cell kill and simultaneously induces a humoral immune response. Currently, additional trials are being conducted to explore the safety of a 3-hour infusion of catumaxomab in conjunction with steroid premedication (Catumaxomab Safety Phase IIIb Study With Intraperitoneal Infusion in Patients with Malignant Ascites Due to Epithelial Cancers [CASIMAS]),Citation82 as well as the safety of re-treatment with intraperitoneal catumaxomab (Safety Study of Second Intraperitoneal Infusion Cycle of Catumaxomab in Patients With Malignant Ascites [SECIMAS]).Citation83 Additionally, phase I/II clinical trials exploring combination treatment using catumaxomab and traditional cytotoxic chemotherapy are required, to determine if there is therapeutic efficacy to combined treatment. The ENGOT-ov8 study,Citation84 a multicenter prospective phase II clinical trial, is currently open, and is exploring the feasibility and clinical activity of intraperitoneal catumaxomab followed by systemic intravenous chemotherapy in patients with recurrent ovarian cancer.
Ultimately, as with all novel therapies, symptom relief and treatment goals must be weighed against patient discomfort and adverse events. Careful patient selection, and identification of risk factors, to help reduce significant side effects associated with treatment are required.
Disclosure
The authors have no conflicts of interest to disclose.
References
- SiegelRNaishadhamDJemalACancer statistics, 2013CA Cancer J Clin2013631113023335087
- BookmanMATrials with impact on clinical management: first lineInt J Gynecol Cancer200919Suppl 2S55S6219955916
- MonkBJChoiDCPugmireGBurgerRAActivity of bevacizumab (rhuMAB VEGF) in advanced refractory epithelial ovarian cancerGynecol Oncol200596390290515721449
- CannistraSAEvaluating new regimens in recurrent ovarian cancer: how much evidence is good enough?J Clin Oncol201028193101310320516442
- Pujade-LauraineEWagnerUAavall-LundqvistEPegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapseJ Clin Oncol201028203323332920498395
- CannistraSAIs there a “best” choice of second-line agent in the treatment of recurrent, potentially platinum-sensitive ovarian cancer?J Clin Oncol20022051158116011870154
- EskanderRNRandallLMBevacizumab in the treatment of ovarian cancerBiologics201151521383911
- MalayevYLeveneRGonzalezFPalliative chemotherapy for malignant ascites secondary to ovarian cancerAm J Hosp Palliat Care201229751552122363037
- EskanderRNTewariKSEmerging treatment options for management of malignant ascites in patients with ovarian cancerInt J Womens Health2012439540422927770
- LoggieBWPeriniMFlemingRARussellGBGeisingerKTreatment and prevention of malignant ascites associated with disseminated intraperitoneal malignancies by aggressive combined-modality therapyAm Surg19976321371439012427
- QaziRSavlovEDPeritoneovenous shunt for palliation of malignant ascitesCancer19824936006026174196
- ArielIMOropezaRPackGTIntracavitary administration of radioactive isotopes in the control of effusions due to cancer. Results in 267 patientsCancer1966198109611025912325
- RunyonBACare of patients with ascitesN Engl J Med199433053373428277955
- GotliebWHFeldmanBFeldman-MoranOIntraperitoneal pressures and clinical parameters of total paracentesis for palliation of symptomatic ascites in ovarian cancerGynecol Oncol19987133813859887235
- BarniSCabidduMGhilardiMPetrelliFA novel perspective for an orphan problem: old and new drugs for the medical management of malignant ascitesCrit Rev Oncol Hematol201179214415320708947
- BeckerGGalandiDBlumHEMalignant ascites: systematic review and guideline for treatmentEur J Cancer200642558959716434188
- KeenAFitzgeraldDBryantADickinsonHOManagement of drainage for malignant ascites in gynaecological cancerCochrane Database Syst Rev20101CD00779420091648
- StarkRHSauterKESurgical treatment of adenocarcinoma of the stomach in a community hospitalSurg Gynecol Obstet198516021531563969612
- OstrowskiMJHalsallGMIntracavitary bleomycin in the management of malignant effusions: a multicenter studyCancer Treat Rep19826611190319076182995
- MaatBOosterleeJSpaasJAWhiteHLammesFBDissemination of tumour cells via LeVeen shuntLancet19791812398887670
- PaladineWCunninghamTJSponzoRDonavanMOlsonKHortonJIntracavitary bleomycin in the management of malignant effusionsCancer19763851903190862609
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin201060527730020610543
- BeckerGBlumHEVEGF Trap for the treatment of malignant ascitesLancet Oncol201213211511622192728
- GarrisonRNGallowayRHHeuserLSMechanisms of malignant ascites productionJ Surg Res19874221261322434730
- TsikourasPTsagiasNPinidisPThe contribution of catumaxomab in the treatment of malignant ascites in patients with ovarian cancer: a review of the literatureArch Gynecol Obstet Epub552013
- ThibodeauxSRCurielTJImmune therapy for ovarian cancer: promise and pitfallsInt Rev Immunol2011302–310211921557637
- StaerzUDKanagawaOBevanMJHybrid antibodies can target sites for attack by T cellsNature198531460126286312859527
- MeliefCJFinnOJCancer immunologyCurr Opin Immunol201123223423621292460
- StrainicMGShevachEMAnFLinFMedofMEAbsence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3+ regulatory T cellsNature Immunol201314216217123263555
- HodiFSMihmMCSoifferRJBiologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patientsProc Natl Acad Sci U S A200310084712471712682289
- PasareCMedzhitovRToll pathway-dependent blockade of CD4+CD25+T cell-mediated suppression by dendritic cellsScience200329956091033103612532024
- StumpfMHasenburgARienerMOIntraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: relevance of clonal selection of T lymphocytesBr J Cancer200910191513152119861998
- NagataYHanagiriTMizukamiMClinical significance of HLA class I alleles on postoperative prognosis of lung cancer patients in JapanLung Cancer2009651919719054590
- LeffersNGoodenMJde JongRAPrognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancerCancer Immunol Immunother200958344945918791714
- JangKYKimKSHwangSHExpression and prognostic significance of SIRT1 in ovarian epithelial tumoursPathology200941436637119404850
- ClarkeBTinkerAVLeeCHIntraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 lossMod Pathol200922339340219060844
- AdamsSFLevineDACadungogMGIntraepithelial T cells and tumor proliferation: impact on the benefit from surgical cytoreduction in advanced serous ovarian cancerCancer2009115132891290219472394
- HanLYFletcherMSUrbauerDLHLA class I antigen processing machinery component expression and intratumoral T-cell infiltrate as independent prognostic markers in ovarian carcinomaClin Cancer Res200814113372337918519766
- CallahanMJNagymanyokiZBonomeTIncreased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancerClin Cancer Res200814237667767319047092
- HamanishiJMandaiMIwasakiMProgrammed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancerProc Natl Acad Sci U S A200710493360336517360651
- SatoEOlsonSHAhnJIntraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancerProc Natl Acad Sci U S A200510251185381854316344461
- ZhangLConejo-GarciaJRKatsarosDIntratumoral T cells, recurrence, and survival in epithelial ovarian cancerN Engl J Med2003348320321312529460
- HeoDSWhitesideTLKanbourAHerbermanRBLymphocytes infiltrating human ovarian tumors. I. Role of Leu-19 (NKH1)-positive recombinant IL-2-activated cultures of lymphocytes infiltrating human ovarian tumorsJ Immunol198814011404240493286766
- BarnettJCBeanSMWhitakerRSOvarian cancer tumor infiltrating T-regulatory (T(reg)) cells are associated with a metastatic phenotypeGynecol Oncol2010116355656220006900
- FialováAPartlováSSojkaLDynamics of T-cell infiltration during the course of ovarian cancer: the gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cellsInt J Cancer201313251070107922865582
- MilneKKöbelMKallogerSESystematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factorsPloS One200947e641219641607
- NelsonBHCD20+ B cells: the other tumor-infiltrating lymphocytesJ Immunol201018594977498220962266
- ShahSDivekarAAHilcheySPIncreased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cellsInt J Cancer2005117457458615912532
- YangCLeeHJoveVPrognostic significance of B-cells and pSTAT3 in patients with ovarian cancerPloS One201381e5402923326565
- LitvinovSVBakkerHAGourevitchMMVeldersMPWarnaarSOEvidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell-cell adhesionCell Adhes Commun1994254174287842256
- SchmelzerEReidLMEpCAM expression in normal, non-pathological tissuesFront Biosci2008133096310017981779
- SpizzoGFongDWurmMEpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysisJ Clin Pathol201164541542021415054
- HerlynMSteplewskiZHerlynDKoprowskiHColorectal carcinoma-specific antigen: detection by means of monoclonal antibodiesProc Natl Acad Sci U S A197976314381442286328
- PatriarcaCMacchiRMMarschnerAKMellstedtHEpithelial cell adhesion molecule expression (CD326) in cancer: a short reviewCancer Treat Rev2012381687521576002
- GastlGSpizzoGObristPDünserMMikuzGEp-CAM overexpression in breast cancer as a predictor of survivalLancet200035692461981198211130529
- GutzmerRLiWSutterwalaSA tumor-associated glycoprotein that blocks MHC class II-dependent antigen presentation by dendritic cellsJ Immunol200417321023103215240690
- MunzMKieuCMackBSchmittBZeidlerRGiresOThe carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferationOncogene200423345748575815195135
- SpizzoGWentPDirnhoferSOverexpression of epithelial cell adhesion molecule (Ep-CAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancerGynecol Oncol2006103248348816678891
- FagerbergJHjelmALRagnhammarPFrödinJEWigzellHMellstedtHTumor regression in monoclonal antibody-treated patients correlates with the presence of anti-idiotype-reactive T lymphocytesCancer Res1995559182418277728746
- KöbelMKallogerSEBoydNOvarian carcinoma subtypes are different diseases: implications for biomarker studiesPLoS Med2008512e23219053170
- BelloneSSiegelERCoccoEOverexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer: implications for epithelial cell adhesion molecule-specific immunotherapyInt J Gynecol Cancer200919586086619574774
- ShetyeJFrödinJEChristenssonBImmunohistochemical monitoring of metastatic colorectal carcinoma in patients treated with monoclonal antibodies (MAb 17-1A)Cancer Immunol Immunother19882721541623262013
- ChamesPBatyDBispecific antibodies for cancer therapy: the light at the end of the tunnel?mAbs20091653954720073127
- ChamesPBatyDBispecific antibodies for cancer therapyCurr Opin Drug Discov Devel2009122276283
- RufPLindhoferHInduction of a long-lasting antitumor immunity by a trifunctional bispecific antibodyBlood20019882526253411588051
- ZeidlerRMysliwietzJCsánadyMThe Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cellsBr J Cancer200083226126610901380
- HeissMMMurawaPKoralewskiPThe trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trialInt J Cancer201012792209222120473913
- EisslerNRufPMysliwietzJLindhoferHMocikatRTrifunctional bispecific antibodies induce tumor-specific T cells and elicit a vaccination effectCancer Res201272163958396622745368
- RiesenbergRBuchnerAPohlaHLindhoferHLysis of prostate carcinoma cells by trifunctional bispecific antibodies (alpha EpCAM × alpha CD3)J Histochem Cytochem200149791191711410615
- RufPGiresOJägerMFellingerKAtzJLindhoferHCharacterisation of the new EpCAM-specific antibody HO-3: implications for trifunctional antibody immunotherapy of cancerBr J Cancer200797331532117622246
- CheliusDRufPGruberPStructural and functional characterization of the trifunctional antibody catumaxomabmAbs20102330931920418662
- SeimetzDLindhoferHBokemeyerCDevelopment and approval of the trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3) as a targeted cancer immunotherapyCancer Treat Rev201036645846720347527
- StröhleinMAHeissMMThe trifunctional antibody catumaxomab in treatment of malignant ascites and peritoneal carcinomatosisFuture Oncol2010691387139420919824
- HeissMMStröhleinMAJägerMImmunotherapy of malignant ascites with trifunctional antibodiesInt J Cancer2005117343544315906359
- BurgesAWimbergerPKümperCEffective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM × anti-CD3 antibody: a phase I/II studyClin Cancer Res200713133899390517606723
- JägerMSchoberthARufPImmunomonitoring results of a phase II/III study of malignant ascites patients treated with the trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3)Cancer Res2012721243222044753
- WimbergerPGiletHGonschiorAKDeterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis aloneAnn Oncol20122381979198522734013
- PapanikolaouGFotopoulouCBraicuIFirst surgical experience of intraperitoneal treatment with the trifunctional antibody catumaxomab (anti-EpCam × anti-CD3) for epithelial ovarian cancerAnticancer Res20113182603260821778311
- PietznerKJägerMSchoberthAFirst patient treated with a re-challenge of catumaxomab in recurrent malignant ascites: a case reportMed Oncol20122921391139621544631
- OttMGMarméFMoldenhauerGHumoral response to catumaxomab correlates with clinical outcome: results of the pivotal phase II/III study in patients with malignant ascitesInt J Cancer201213092195220321702044
- Fresenius Biotech GmbHTwo-Arm, Randomized (2:1), Open-Label Phase II/III Study in EpCAM Positive Cancer Patients With Symptomatic Malignant Ascites Using the Trifuncitonal Bispecific Antibody Removab (Anti-EpCAM × Anti-CD3) Versus an Untreated Control Group Available from: http://clinicaltrials.gov/ct2/show/NCT00836654. NLM identifier: NCT00836654Accessed August 22, 2013
- Fresenius biotech GmbHCASIMAS: Catumaxomab safety phase IIIb study with intraperitoneal infusion in patients with malignant ascites due to epithelial cancers Available at http://clinicaltrials.gov/ct2/show/NCT00822809. NLM identifier: NCT 00822809Accessed July 3, 2013
- Fresenius biotech GmbHSafety Study of Second Intraperitoneal (I.P.) Infusion Cycle of Catumaxomab in Patients With Malignant Ascites (SECIMAS) Available at http://clinicaltrials.gov/ct2/show/NCT01065246?term=NCT01065246&rank=1. NLM identifier NCT 01065246Accessed July 3, 2013
- Charité – Universitätsmedizin BerlinSingle–arm, multicenter phase-II trial for catumaxomab and chemotherapy in patients with recurrent ovarian cancer to investigate the feasibility and clinical activity of initial intraperitoneal catumaxomab followed by chemotherapy regimes Available at https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-004585-15/DE. IMP identification Eudra CT number 2011-004585-15Accessed July 3, 2013