Abstract
Background
In severe alcohol withdrawal (AW), benzodiazepines may be inadequate to control symptoms. In many situations, benzodiazepine dosing escalates despite no additional efficacy and introduces potential toxicities. Severe cases of AW may require additional agents to control symptoms. Case reports and studies have shown benefits with dexmedetomidine and propofol in severe AW, but these agents have not been compared with one another. This study compares the effects of dexmedetomidine and propofol on benzodiazepine and haloperidol utilization in patients with AW.
Methods
A retrospective chart review was completed on 41 patients with AW who received adjunctive dexmedetomidine or propofol. The primary objective was to compare benzodiazepine and haloperidol utilization before and after initiation of dexmedetomidine or propofol. Secondary measures included AW and sedation scoring, analgesic use, intensive care unit length of stay, rates of intubation, and adverse events.
Results
Among the dexmedetomidine and propofol groups, significant reductions in benzodiazepine (P≤0.0001 and P=0.043, respectively) and haloperidol (P≤0.0001 and P=0.026, respectively) requirements were observed. These reductions were comparable between groups (P=0.933 and P=0.465, respectively). A trend toward decreased intensive care unit length of stay in the dexmedetomidine group (123.6 hours vs 156.5 hours; P=0.125) was seen. Rates of intubation (14.7% vs 100%) and time of intubation (19.9 hours vs 97.6 hours; P=0.002) were less in the dexmedetomidine group. Incidence of hypotension was 17.6% in the dexmedetomidine group vs 28.5% in the propofol group. Incidence of bradycardia was 17.6% in the dexmedetomidine group vs 0% in the propofol group. No differences were observed in other secondary outcomes.
Conclusion
In patients with severe AW who require sedation, both dexmedetomidine and propofol have unique and advantageous properties. Both agents appear to have equivalent efficacy in reducing AW-related symptoms and benzodiazepine and haloperidol requirements. These results should be validated in a larger, prospective trial.
Introduction
Alcohol dependence is a common concern during hospitalization, affecting an estimated 15%–20% of patients.Citation1 Chronic alcohol use leads to modulation of the central nervous system. N-Methyl-D-aspartate (NMDA) receptors are upregulated, while gamma-aminobutyric acid (GABA) receptors become downregulated.Citation2 Temporary absence of alcohol in this setting results in hyperactivity due to enhanced NMDA function, reduced GABA stimulation, and dysregulation of the dopaminergic system,Citation2 all of which contribute to various alcohol withdrawal (AW) symptoms.
AW manifests from alcohol dependence, and symptomatology varies greatly among patients. The most frequent symptoms result from noradrenergic hyperactivity and include tremulousness, sweating, hypertension, tachycardia, and agitation.Citation3,Citation4 These symptoms typically do not require medical intervention, although severe cases of AW may necessitate medical care. In severe cases of AW, patients may suffer from neuronal excitation, such as epileptiform seizures, delirium tremens, and the possibility of death due to severe pulmonary and cardiovascular toxicities.Citation3,Citation4 Symptom presentation begins after 6–24 hours of abstinence with a typical duration of 48–72 hours, although some patients may experience longer withdrawal periods.Citation1–Citation4
The first-line pharmacologic agents for AW management are benzodiazepines due to their pharmacological action of enhancing GABA-mediated inhibition.Citation1,Citation2 They effectively alleviate symptoms while acting to treat and prevent AW seizures due to influx of chloride ions. The influx of anions causes hyperpolarization and creates an inhibitory effect on action potential firing.Citation5,Citation6 Despite these benefits, patients with severe cases of AW can be refractory to standard symptom-triggered benzodiazepine dosing.Citation7 In such cases, dosing continues to increase as the patient remains symptomatic, a consequence of misunderstanding and non-cognizance of AW severity and pathology, in addition to protocol standardization. Such circumstances produce benzodiazepine overuse,Citation8–Citation10 potentially causing excessive sedation, insomnia, delirium, and respiratory depression.Citation11,Citation12
Propofol is a sedative agent that enhances GABA activity, similar to benzodiazepines. However, propofol binds at a different receptor site compared to benzodiazepines.Citation6,Citation13 In comparison to benzodiazepines for sedation, propofol may decrease time to extubation,Citation14,Citation15 and has displayed antiepileptic properties in refractory seizures,Citation16–Citation20 although in patients with AW has not been shown to shorten duration of AW or hospital length of stay.Citation21
Dexmedetomidine is a centrally acting α-2 adrenergic agonist used for sedation in both intubated and nonintubated patients.Citation22,Citation23 Dexmedetomidine has analgesic properties, does not cause respiratory depression,Citation23–Citation26 and has been shown to decrease duration of mechanical ventilation compared to other sedative agents.Citation27–Citation29
Recent studies and case reports using propofol,Citation18–Citation21,Citation30 and more recently dexmedetomidine, have shown reductions in benzodiazepine requirements and symptom control in patients with AW refractory to benzodiazepine therapy alone.Citation31–Citation37 To date, these agents have not been compared against each other. The goal of this study is to compare the effects of dexmedetomidine and propofol on benzodiazepine and haloperidol requirements in patients with AW.
Methods
Patient selection
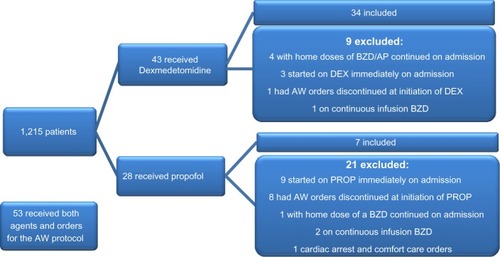
Data were obtained via electronic medical record, identifying 124 patients who had orders for the AW order set and concurrent use of either dexmedetomidine or propofol for sedation from November 2010 to October 2013. Patients were included if they had a diagnosis of AW, received either sedative agent, and were actively receiving the institution’s AW order set. Patients were excluded if they had AW orders discontinued before start of a sedative agent, age less than 18 years, scheduled benzodiazepine or antipsychotic continued from home, concomitant administration of a continuous infusion benzodiazepine, contraindication to using benzodiazepines or antipsychotics, or immediately received either sedative agent within 4 hours of hospital admission. Patients who received both dexmedetomidine and propofol were also excluded from the analysis ().
Study protocol
This retrospective study was Institutional Review Board approved, and informed consent was waived. Data were collected from patients admitted to the medical, cardiac, surgical, or transitional intensive care units (ICUs) (27 beds total). The AW protocol contains intravenous fluids, thiamine, vitamins, haloperidol, and lorazepam. Lorazepam dosing correlates to the Alcohol Withdrawal Assessment Scale (AWAS), which evaluates tremor, tachycardia, blood pressure, diaphoresis, fever, nausea and vomiting, agitation, confusion or disorientation, sleeplessness, and hallucinations, each on a scale of 0–3 (except sleeplessness, which has a scale of 0–2) for a maximum score of 29. Intravenous lorazepam is preferred in patients with an AWAS score more than 11. Scoring is performed every 30 minutes by nursing, and lorazepam dosing is given based on the current score. Haloperidol may be used every 30 minutes as needed for “severe agitation.” Dexmedetomidine and propofol are not listed on the AW protocol, and initiation of therapy is determined by provider judgment. Standard hospital dosing and titration protocols were used for dexmedetomidine and propofol. These protocols do not utilize bolus dosing.
Data collection
Eligible patients were analyzed based on the choice of sedative agent (ie, dexmedetomidine or propofol). Patient data were collected 24 hours before to 24 hours after initiation of the sedative agent (). If a patient was started on sedation within the first 24 hours of admission, data were collected for the same time period following initiation of the sedative. The primary objective was to compare benzodiazepine and haloperidol utilization before and after initiation of dexmedetomidine or propofol. Benzodiazepine use is expressed in lorazepam equivalence ().Citation38 Secondary objectives included ICU length of stay, rates and time of intubation, analgesic usage, AWAS score, Richmond Agitation Sedation Scale (RASS), Confusion Assessment Method for the ICU (CAM-ICU), and incidence of bradycardia and hypotension. Bradycardia was defined as a heart rate less than 60 bpm (beats per minute), and hypotension as a systolic blood pressure less than 90 mmHg.
Table 1 Equipotent doses of various benzodiazepines
Statistical analyses
Statistical analyses were performed using SPSS Statistics 21 (IBM Corporation, Armonk, NY, USA). Two-tailed Wilcoxon signed-rank tests were used to assess significances between 24-hour before and 24-hour after initiation of sedation with benzodiazepine and haloperidol. Two-tailed Mann–Whitney U tests assessed whether benzodiazepine use, haloperidol use, rates and time of intubation, and ICU length of stay differed between the dexmedetomidine and propofol groups. A P-value of less than 0.05 was considered statistically significant.
Results
Of the 124 patients who received orders for the AW protocol and the use of a sedative agent, 41 were included in the analysis. Fifty-three of the patients used dexmedetomidine and propofol concurrently and were excluded. Twelve received dexmedetomidine or propofol immediately on admission, nine had AW protocol discontinued upon initiation of sedation, five had a benzodiazepine or antipsychotic continued from home, three received a continuous infusion of a benzodiazepine, and one was placed on comfort care within 6 hours of AW protocol initiation ().
Benzodiazepine and haloperidol dosing
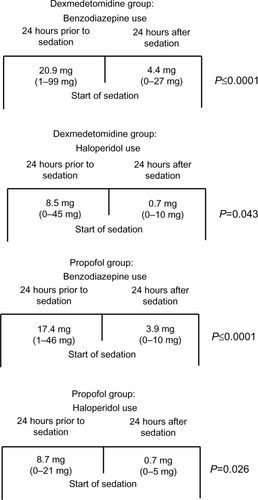
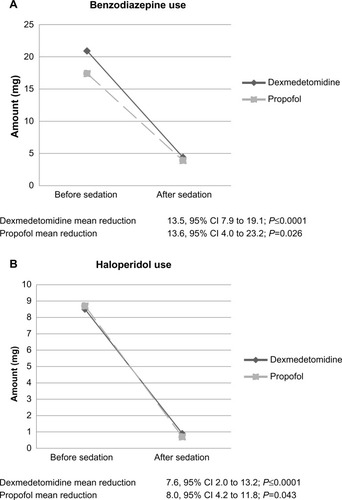
Nine patients (26.4%) received a benzodiazepine other than lorazepam in the dexmedetomidine group, compared to three patients (42.9%) in the propofol group, and were converted to lorazepam equivalency. Mean benzodiazepine and haloperidol use during the 24-hour period before initiation of sedation in the dexmedetomidine group was 20.9 mg and 8.5 mg, respectively. This was similar to the propofol group, where mean benzodiazepine and haloperidol use was 17.4 mg and 8.7 mg, respectively, prior to initiation of sedation (). In the 24-hour period following initiation of sedation, benzodiazepine and haloperidol requirements were decreased to 4.4 mg and 0.9 mg in the dexmedetomidine group (P≤0.0001 and P≤0.0001), and 3.9 mg and 0.7 mg (P=0.043 and P=0.026) in the propofol group (). Differences in benzodiazepine and haloperidol use between the two groups were not statistically significant (P=0.933 and P=0.465, respectively).
Secondary measures
Mean AWAS scores before initiation of sedation were 11.4 (n=24) in the dexmedetomidine group and 12.8 (n=2) in the propofol group. The AWAS average after initiation of dexmedetomidine was 7.5 (n=18). AWAS scores were not available in 15 patients. No patients in the propofol group received follow-up scoring. Average RASS scores were 0.11 and 0.87 in the dexmedetomidine and propofol groups, which decreased to −0.7 and −2.5, respectively, after initiation of sedation. RASS scores were not available in 12 patients. Of the six patients who had Confusion Assessment Method for the ICU scores performed, all six were positive before initiation of sedation. Only one patient had resolution of delirium after 24 hours of dexmedetomidine. There were no differences in analgesic usage between the groups.
Intubation and ICU length of stay
Five patients (14.7%) in the dexmedetomidine group and all seven patients (100%) in the propofol group were intubated. When intubation was required, intubation times were shorter in the dexmedetomidine group (19.9 hours vs 97.6 hours; P=0.002). Four of the five intubated patients in the dexmedetomidine group were extubated during the infusion. There was no statistically significant reduction in ICU length of stay between the groups (123.6 hours vs 156.5 hours; P=0.125).
Adverse events
Bradycardia and hypotension were the only adverse effects evaluated in the analysis (). Six patients (17.6%) in the dexmedetomidine group developed bradycardia and six (17.6%) developed hypotension. Dexmedetomidine was discontinued due to hypotension in one patient (2.8%). Of the six patients who developed hypotension, none of the patients received a bolus dose, and continuous infusion doses ranged from 0.4 μg/kg/h to 0.8 μg/kg/h. The effects of dexmedetomidine on hypotension were seen immediately upon initiation of the infusion and were typically accompanied by slight improvement within a couple of hours. Among patients who developed bradycardia, a drop in heart rate was seen upon initiation of the infusion, followed by slow progression to bradycardia. Bradycardia did not resolve unless the infusion rate was decreased or the infusion was discontinued. No patients in the propofol group developed bradycardia. Two patients in the propofol group developed hypotension (28.5%) during therapy. Hypotension developed in both patients at a dose of 50 μg/kg/min. Blood pressure improved in both patients after reduction of infusion. Propofol was not discontinued in any patient due to adverse events. Three deaths occurred in study patients, one in the dexmedetomidine group and two in the propofol group. All deaths were related to end-stage liver disease and not attributed to the use of either sedative agent.
Table 2 Adverse events
Discussion
Both dexmedetomidine and propofol significantly decreased benzodiazepine and haloperidol requirements in patients with AW. The overall reduction in benzodiazepine and haloperidol requirements was not different when comparing dexmedetomidine and propofol. Small sample size limits speculation on the disparity of benzodiazepine dosage range between each group. However, mean dosing is similar; therefore, meaningful conclusions may still be drawn. Patients in the dexmedetomidine group trended toward shorter ICU length of stay (P=0.125). Only five (14.7%) patients in the dexmedetomidine group were intubated, with four being extubated while receiving dexmedetomidine. All seven patients in the propofol group were intubated while receiving propofol. When intubated, patients in the dexmedetomidine group were extubated sooner than those in the propofol group (P=0.002). However, it should be noted that type 1 error is possible due to the small number of intubated patients. Patients did experience more bradycardia and hypotension during dexmedetomidine therapy, with one patient requiring discontinuation due to adverse events.
While dexmedetomidine and propofol displayed reductions in benzodiazepine and haloperidol dosing, this may have been mediated through symptom control that reduced AWAS scores, rather than directly alleviating AW symptoms. However, given their therapeutic mechanisms, dexmedetomidine and propofol would provide benefits in patients with AW. Dexmedetomidine acts as a selective α-2 adrenergic receptor agonist,Citation39–Citation42 working via a negative feedback mechanism to regulate norepinephrine release.Citation12,Citation24,Citation25,Citation43 The locus coeruleus, located in the pons, is responsible for regulating sleep and vigilance and contains a high density of α-2 receptors and noradrenergic neurons.Citation12,Citation24 These receptors and neurons are responsible for the antinociceptive and sedative effects.Citation44,Citation45 In addition, dexmedetomidine alleviates symptoms of AW through inhibition of noradrenergic hyperactivity, including tachycardia, hypertension, and tremulousness.Citation25,Citation43 Propofol works similar to alcohol and benzodiazepines as a positive modulator of GABA receptors and inhibition of NMDA receptors.Citation18,Citation46–Citation48 These effects may counteract the up- and downregulation of receptors seen in chronic alcohol abuse and prevent AW-related seizures.Citation16–Citation18,Citation49
Among the limitations of this study is the retrospective design, which occasionally provided us with incomplete data for secondary objectives. The sample size was small (n=41), which prevents definitive conclusions from being drawn. However, the 34 patients in the dexmedetomidine group is one of the largest populations evaluated for the use of dexmedetomidine in AW to date. The sample of seven patients in the propofol group is also too small to draw conclusions, and also limits evaluation with uneven distribution of patients between groups. The utilization of the AWAS, which incorporates a variety of nonspecific symptoms including anxiety, agitation, and sweating, also serves as a limitation to our results. These symptoms can manifest in a variety of settings, leading to increased scores and dosing for symptoms unrelated to AW. Also, despite standardization in scoring systems, there is still the possibility of subjectivity, especially in a retrospective review. Doses were reviewed for patients who developed adverse events, but the average dose for all patients was not reviewed. Dexmedetomidine doses ranged from 0.4 μg/kg/h to 1.2 μg/kg/h, and propofol doses ranged from 20 μg/kg/min to 70 μg/kg/min with reductions in benzodiazepine and haloperidol dosing after initiation of sedation for both higher and lower infusion rates.
This study is unique in the comparison of dexmedetomidine to propofol in patients with severe AW. To date, the efficacy of dexmedetomidine has only been evaluated in case reports, retrospective reviews with no comparison group,Citation31–Citation36 and a single double-blind, placebo-controlled trial.Citation37 Propofol for refractory AW has only been evaluated in case reports.Citation18–Citation21,Citation30 This study confirms these reports and shows the potential benefits of dexmedetomidine and propofol in AW, although larger, more definitive trials should be performed to verify the results.
Conclusion
Severe cases of AW may necessitate the addition of a sedative agent to control symptoms. Although our sample size is too small to draw definitive conclusions, this analysis, in addition to previous studies, supports the use of adjunctive dexmedetomidine and propofol in severe AW. Both dexmedetomidine and propofol appear to significantly reduce AW-related symptoms and benzodiazepine and haloperidol requirements. These results should be validated in a larger, prospective trial.
Acknowledgments
The authors would like to thank Joe Strain, PharmD; Janeen Bucholz, PharmD; Denise Baldwin; Jeremiah Brickhouse; Surachat Ngorsuraches; and Brittany Schaffer for their assistance in performing this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- Mayo-SmithMFBeecherLHFischerTLManagement of alcohol withdrawal delirium: an evidence-based practice guidelineArch Intern Med2004164131405141215249349
- McKeonAFryeMADelantyNThe alcohol withdrawal syndromeJ Neurol Neurosurg Psychiatry20087985486217986499
- HallWZadorDThe alcohol withdrawal syndromeLancet19973499069189719009217770
- RamosRMalletTDiVittisACohenRPredictors of severity of alcohol withdrawal in hospitalized patientsJ Clin Med Res20135537638023976910
- TreimanDMGABAergic mechanisms in epilepsyEpilepsia200142Suppl 381211520315
- RichardsGSchochPHaefelyWBenzodiazepine receptors: new vistasSemin Neurosci19913191203
- HackJBHoffmanRSNeslonLSResistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early?J Med Toxicol200622556018072114
- NolopKBNatowAUnprecedented sedative requirements during delirium tremensCrit Care Med19851342462473979072
- LineaweaverWCAndersonKHingDNMassive doses of midazolam infusion for delirium tremens without respiratory depressionCrit Care Med19881632942953342636
- KahnDRBarnhorstAVBourgeoisJAA case of alcohol withdrawal requiring 1,600 mg of lorazepam in 24 hoursCNS Spectr200914738538919773714
- PandharipandePShintaniAPetersonJLorazepam is an independent risk factor for transitioning to delirium in intensive care unit patientsAnesthesiology20061041212616394685
- PertuzziWTSleep in the intensive care unitPharmacotherapy2005255 pt 234S39S15899747
- GommersDBakkerJMedications for analgesia and sedation in the intensive care unit: an overviewCrit Care200812Suppl 3S418495055
- HallRISandhamDCardinalPPropofol vs midazolam for ICU sedation: a Canadian multicenter randomized trialChest20011191151115911296183
- OstermannMEKeenanSPSeiferlingRASibbaldWJSedation in the intensive care unit: a systematic reviewJAMA2000283111451145910732935
- KofkeWAAnesthetic management of the patient with epilepsy or prior seizuresCurr Opin Anaesthesiol201023339139920421790
- MacKenzieSKapadiaFGrantISPropofol infusion for control of status epilepticusAnaesthesia19904512104310452278326
- McCowanCMarikPRefractory delirium tremens treated with propofol: a case seriesCrit Care Med20002861781178410890619
- CoomesTRSmithSWSuccessful use of propofol in refractory delirium tremensAnn Emerg Med19973068258289398785
- TakeshitaJUse of propofol for alcohol withdrawal delirium: a case reportJ Clin Psychiatry200465113413514974494
- SohrabyRAttridgeRLHughesDWUse of propofol-containing versus benzodiazepine regimens for alcohol withdrawal requiring mechanical ventilationAnn Pharmacother201448445646124436457
- Precedex. [package insert]Lake Forest, ILHospira, Inc2012
- GerlachATMurphyCVDastaJFAn updated focused review of dexmedetomidine in adultsAnn Pharmacother200943122064207419934395
- GertlerRBrownCMitchellDHSilviusENDexmedetomidine: a novel sedative-analgesic agentProc (Bayl Univ Med Cent)200114132116369581
- MuzykAJFowlerJANorwoodDKChilipkoARole of alpha2-agonists in the treatment of acute alcohol withdrawalAnn Pharmacother201145564965721521867
- EbertTJHallJEBarneyJAUhrichTDColincoMDThe effects of increasing plasma concentrations of dexmedetomidine in humansAnesthesiology200093238239410910487
- JakobSMRuokonenEGroundsRMDexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilationJAMA2012307111151116022436955
- PandharipandePPPunBTHerrDLEffect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trialJAMA2007298222644265318073360
- SiobalMSKalletRHKivettRHTangJFUse of dexmedetomidine to facilitate extubation in surgical intensive-care-unit patients who failed previous weaning attempts following prolonged mechanical ventilation: a pilot studyRespir Care200651549249616638158
- HughesDWVanwertELeporiLAdamsBDPropofol for benzodiazepine-refractory alcohol withdrawal in a non-mechanically ventilated patientAm J Emerg Med2014321112.e3112.e424075805
- RaynerSGWeinertCRPengHJepsonSBroccardAFStudy institutionDexmedetomidine as adjunct treatment for severe alcohol withdrawal in the ICUAnn Intensive Care2012211222620986
- DarroujJPuriNPrinceELomonacoASpevetzAGerberDRDexmedetomidine infusion as adjunctive therapy to benzodiazepines for acute alcohol withdrawalAnn Pharmacother200842111703170518780809
- DemuroJPBotrosDGWirkowskiEHannaAFUse of dexmedetomidine for the treatment of alcohol withdrawal syndrome in critically ill patients: a retrospective case seriesJ Anesth201226460160522584816
- FrazeeENPersonettHALeungJGNelsonSDierkhisingRABauerPRInfluences of dexmedetomidine therapy on the management of severe alcohol withdrawal syndrome in critically ill patientsJ Crit Care201429229830224360597
- RovasaloATohmoHAantaaRKettunenEPalojokiRDexmedetomidine as an adjunct in the treatment of alcohol withdrawal delirium: a case reportGen Hosp Psychiatry200628436236316814639
- CrispoALDaleyMJPepinJLHarfordPHBrownCVComparison of clinical outcomes in non intubated patients with severe alcohol withdrawal syndrome treated with continuous-infusion sedatives: dexmedetomidine versus benzodiazepinesPharmacotherapy201434991091724898418
- MuellerSWPreslaskiCRKiserTHA randomized, double-blind, placebo-controlled dose range study of dexmedetomidine as adjunctive therapy for alcohol withdrawalCrit Care Med20144251131113924351375
- KaneSPBenzodiazepine equivalents conversion calculator. ClinCalc [Updated February 11, 2014]. Available from: http://clincalc.com/Benzodiazepine/Accessed April 23, 2014
- SavolaJMVirtanenRCentral alpha 2-adrenoreceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medomidineEur J Pharmacol199119521931991678707
- FurstSRWeingerMBDexmedetomidine, a selective alpha 2-agonist, does not potentiate the cardiorespiratory depression of alfentanil in the ratAnesthesiology19907258828881971163
- TobiasJDDexmedetomidine: are there going to be issues with prolonged administration?J Pediatr Pharmacol Ther20101514922477787
- BischoffPKochsEAlpha 2-agonists in anesthesia and intensive medicineAnasthesiol Intensivmed Nofallmed Schmerzther1993281212
- JaatinenPRiihiojaPHaapalinnaAHeinonenEKiianmaaKHervonenAPrevention of ethanol-induced sympathetic overactivity and degeneration be dexmedetomidineAlcohol19951254394468519439
- HunterJCFontanaDJHedleyLRAssessment of the role of alpha2-adrenoceptor subtypes in the antinociceptive, sedative, and hypothermic action of dexmedetomidine in transgenic miceBr J Pharmacol19971227133913449421280
- Correa-SalesCRabinBCMazeMA hypnotic response to dexmedetomidine, an alpha 2 agonists, is mediated in the locus coeruleus in ratsAnesthesiology19927669489521350889
- OrserBABertlikMWangLYMacDonaldJFInhibition by propofol (2,6 diisopropylphenol) of the N-methyl D-aspartate subtype of glutamate receptor in cultured hippocampal neuronsBr J Pharmacol19951162176117688528557
- UchidaILiLYangJThe role of the GABA(A) receptor alpha1 subunit N-terminal extracellular domain in propofol potentiation of chloride currentNeuropharmacology199736161116219517432
- HaraMKaiYIkemotoYPropofol activates GABA-A receptor-chloride ionophore complex in dissociated hippocampal pyramidal neurons of the ratAnesthesiology1993797817888214758
- MarikPEVaronJThe management of status epilepticusChest2004126258259115302747