Abstract
Worldwide, an estimated 200 million people have chronic kidney disease (CKD), the most common causes of which include hypertension, arteriosclerosis, and diabetes. Importantly, ~40% of patients with diabetes develop CKD, yet evidence from major multicenter randomized controlled trials shows that intensive blood glucose control through pharmacological intervention can reduce the incidence and progression of CKD. Standard therapies for the treatment of type 2 diabetes include metformin, sulfonylureas, meglitinides, thiazolidinediones, and insulin. While these drugs have an important role in the management of type 2 diabetes, only the thiazolidinedione pioglitazone can be used across the spectrum of CKD (stages 2–5) and without dose adjustment; there are contraindications and dose adjustments required for the remaining standard therapies. Newer therapies, particularly dipeptidyl peptidase-IV inhibitors, glucagon-like peptide-1 receptor agonists, and sodium-glucose cotransporter-2 inhibitors, are increasingly being used in the treatment of type 2 diabetes; however, a major consideration is whether these newer therapies can also be used safely and effectively across the spectrum of renal impairment. Notably, reductions in albuminuria, a marker of CKD, are observed with many of the drug classes. Dipeptidyl peptidase-IV inhibitors can be used in all stages of renal impairment, with appropriate dose reduction, with the exception of linagliptin, which can be used without dose adjustment. No dose adjustment is required for liraglutide, albiglutide, and dulaglutide in CKD stages 2 and 3, although all glucagon-like peptide-1 receptor agonists are currently contraindicated in stages 4 and 5 CKD. At stage 3 CKD or greater, the sodium-glucose cotransporter-2 inhibitors (dapagliflozin, canagliflozin, and empagliflozin) either require dose adjustment or are contraindicated. Ongoing trials, such as CARMELINA, MARLINA, CREDENCE, and CANVAS-R, will help determine the position of these new therapy classes and if they have renoprotective effects in patients with CKD.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Chronic kidney disease (CKD) can adversely affect the pharmacokinetics (PK) or pharmacodynamics (PD) of some therapies.Citation1 Thus, in order for clinicians to make informed choices, it is important to understand how: 1) the use of glucose-lowering therapies is affected by renal impairment; 2) treatment regimens may need to be modified and how these treatments may impact on CKD outcomes; and 3) the importance of safety across the spectrum of CKD.
Worldwide, an estimated 200 million people have CKD,Citation2 a long-term condition that may lead to renal failure and, if left untreated, to premature death. CKD is characterized by the presence of kidney damage, indicated by albuminuria and/or a gradual loss of kidney function (estimated glomerular filtration rate [eGFR]) over time.Citation2 Common causes of CKD include hypertension,Citation3 atheriosclerosis,Citation4 and diabetes.Citation5 Furthermore, CKD is associated with an elevated risk of death from cardiovascular (CV) disease.Citation6 Kidney Disease: Improving Global Outcomes criteria and associated CKD stages are summarized in and , respectively.Citation7
Table 1 National Kidney Foundation criteria for CKD
Table 2 Staging of CKD
Approximately 40% of patients with diagnosed or undiagnosed diabetes have CKDCitation8 and, in the absence of monitoring for and effective treatment of renal function, CKD can develop insidiously.Citation9 Pathological changes in the kidney include increased glomerular basement membrane thickness, formation of microaneurysms, and mesangial nodules.Citation10 The current estimates show a global prevalence of 415 million adults with diabetes,Citation11 with type 2 diabetes (T2D) accounting for ~90% of these patients;Citation12 CKD in diabetes is, thus, a significant health problem. Atherosclerosis is also an associated condition, leading to narrowing of arterial walls and subsequent high blood pressure.Citation10 Diabetic nephropathy is generally, but not always, preceded by albuminuria, defined as proteinuria of >500 mg/day.Citation10 In fact, there is evidence that intensively improving blood glucose control through pharmacological intervention can reduce the incidence and progression of CKD in people with diabetes. The UK Prospective Diabetes Study found a reduced risk of progressive kidney disease with intensive glycemic control, apparent from a 34% reduction in albuminuria, 67% reduction in the proportion of patients who had a twofold increase in plasma creatinine, and 74% reduction in the proportion of patients who had doubling of plasma urea.Citation13 In addition, the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation trial determined that intensive glucose control significantly reduced the risk of progression to stage 5 CKD by 65%, microalbuminuria by 9%, and macroalbuminuria by 30%.Citation14 In the Steno-2 study, patients with T2D and microalbuminuria receiving intensive therapy (compared with conventional therapy) had a significantly lower risk of nephropathy (hazard ratio: 0.39, 95% confidence interval [CI]: 0.17–0.87; P<0.003).Citation15
A recent meta-analysis demonstrated that combination therapies are effective in improving glycemic control, with some combinations of metformin and newer drug classes (specifically, dipeptidyl peptidase-IV [DPP-IV] inhibitors, glucagon-like peptide-1 receptor agonists [GLP-1RAs], and sodium-glucose cotransporter-2 [SGLT-2] inhibitors) providing glycemic control without increasing the risk of hypoglycemia or weight gain.Citation16 Indeed, many current treatment guidelines now advocate the use of these newer therapies in multiple stepwise combinations.Citation17
As previous reviews have already investigated the impact of conventional glucose-lowering therapies on CKD,Citation18,Citation19 this review will primarily focus on the newer therapies (DPP-IV inhibitors, GLP-1RAs, and SGLT-2 inhibitors), mechanisms by which CKD affects the use of T2D therapies, place of these therapies in CKD treatment, and their potential renoprotective effects.
T2D therapies and CKD
Conventional glucose-lowering therapies
Conventional glucose-lowering therapies include metformin, sulfonylureas, thiazolidinediones, meglitinides, and insulin.
Apart from pioglitazone, a thiazolidinedione,Citation20 other conventional therapies must be reduced or withdrawn as eGFR declines due to increased risk of lactic acidosis (in the case of metformin) and hypoglycemia (in the case of sulfonylureas, meglitinides [such as repaglinide], and insulin).Citation21–Citation33 Their use in CKD is described below and summarized in .
Table 3 The use of conventional glucose-lowering therapies in chronic kidney disease (based on European Union label)
Metformin
Metformin may be used without dose adjustment in patients with stage 2 CKD. With dose adjustment, metformin may be used in patients with stage 3a CKD, but only in the absence of other conditions, such as liver dysfunction,Citation35 CV disease,Citation36 and renal disease,Citation36 which may increase the risk of lactic acidosis. Renal function must be closely monitored every 3–6 months and, if eGFR falls to <45 mL/min/1.73 m2, metformin administration must be stopped.Citation21 Current UK National Institute for Health and Care Excellence guidelines suggest stopping if eGFR falls to <30 mL/min/1.73m2.Citation37 Clinical data on the risk of lactic acidosis in metformin-treated patients with CKD are currently limited.Citation38
Sulfonylureas
Patients with CKD who are treated with glipizide, glibenclamide, gliclazide, and glimepiride require careful monitoring. Moreover, these sulfonylureas are contraindicated in patients with stage 4 CKD.Citation22–Citation25 The PK and/or PD of glipizide may be altered in patients with impaired renal function; initial and maintenance dosing should, therefore, be conservative to avoid hypoglycemic episodes.Citation22 Glibenclamide should be used cautiously in patients with stages 2 and 3 CKD; however, in long-term clinical trials, patients with renal impairment have been treated adequately at reduced doses with frequent monitoring.Citation23 Likewise, in long-term clinical trials, patients with renal impairment have also been treated adequately using gliclazide at reduced doses with frequent monitoring.Citation24 In the case of glimepiride, in patients with low creatinine clearance (CrCl), there is a trend for glimepiride clearance to elevate and serum concentrations to diminish.Citation25
Meglitinides
The meglitinides, such as repaglinide, are short-acting. Patients with CKD ranging from stages 3b to 5 require careful management.Citation26 Repaglinide is mostly metabolized by the liver and, with dose adjustment, may be used in patients with CKD.Citation26 In a clinical trial, after 5 days of treatment with repaglinide, there was a twofold increase in exposure (area under the curve [AUC]) and half-life in patients with stage 4 CKD compared with individuals with normal renal function.Citation26
Thiazolidinediones
Of the thiazolidinedione family, only pioglitazone is generally available due to safety concerns with other agents in this class. No dose adjustment is required with impaired renal function (stages 2–5 CKD), as pioglitazone is metabolized mainly by the liver.Citation20,Citation39 No information is available for patients undergoing dialysis; therefore, pioglitazone should not be used in this setting. Plasma concentrations of pioglitazone and its metabolites in patients with CKD are lower than those observed in individuals with normal renal function, but the parent compound shows comparable apparent clearance.Citation20 Edema occurs in ~5% of patients treated with pioglitazone in monotherapy or combination therapy.Citation40 Although there have been concerns over CV risk,Citation41 a recent study showed that pioglitazone was not associated with any increase in CV adverse events (AEs).Citation41
Alpha-glucosidase inhibitors
The alpha-glucosidase inhibitor acarbose has gastrointestinal side effects associated with its use, including flatulence, soft stools, and abdominal discomfort.Citation34,Citation42 Acarbose is excreted unaltered by the renal system and should not be initiated in patients with stage 4 CKD.Citation34
Amylin analogs
Amylin analogs, such as pramlintide, are commonly used in the US. Pramlintide has limited side effects but a small proportion of patients may have intolerable nausea despite receiving the lowest doses.Citation43 The dosing requirements for pramlintide are not altered in patients with stages 2–4 CKD.Citation43 Pramlintide has not been studied in patients with stage 5 CKD.Citation43
Dopamine agonists
Dopamine agonists, such as bromocriptine, are commonly used in the US. Common side effects of bromocriptine include nausea, asthenia, constipation, dizziness, and rhinitis.Citation44 No PK studies have been conducted in patients with renal impairment.Citation45 Although the kidney is a minor pathway for elimination of bromocriptine, careful monitoring should be undertaken in patients with renal impairment.Citation45
Insulin
Classes of insulin include intermediate-acting (neutral protamine Hagedorn insulin, indicated for basal coverage), long-acting (glargine 100 units/mLCitation28 and 300 units/mL [U300],Citation46 detemirCitation29 and degludecCitation30 indicated for basal coverage), and fast-acting (lispro,Citation47 glulisine,Citation48 and aspart,Citation49 indicated for prandial coverage). CKD may reduce insulin requirements dramatically and increase the risk for hypoglycemia in patients.Citation50 Insulin dose, therefore, needs to be adjusted on an individual basis.
In patients with CKD, glargine requirements may be diminished because of reduced insulin metabolism;Citation28,Citation46 the high concentration of insulin (U300) results in different PK and PD profiles, but both concentrations have been shown to be safe in patients with renal failure.Citation46,Citation51,Citation52 In a clinical study, the PK of detemir did not significantly differ in patients with stages 3–4 CKD compared with healthy individuals.Citation53 Likewise, in one clinical study, renal impairment did not have a significant effect on maximum concentration (Cmax), apparent clearance, or AUC0–120 h of single-dose degludec.Citation30,Citation54
The glucodynamic response to insulin lispro is not affected by renal impairment.Citation31,Citation47 Glulisine requirements may be reduced in the presence of renal impairment.Citation32 In patients with type 1 diabetes, renal impairment (stages 2–5 CKD) did not affect the PK of aspart in a clinically significant manner.Citation33,Citation55
Newer therapies
DPP-IV inhibitors, GLP-1RAs, and SGLT-2 inhibitors are the most recent classes of agents licensed for use in T2D in Europe. The use of newer therapies in CKD is described below and summarized in .
Table 4 Newer glucose-lowering therapies and data regarding their use in CKD (based on European Union label)
DPP-IV inhibitors
DPP-IV inhibitors, such as sitagliptin, vildagliptin, saxagliptin, alogliptin and linagliptin, reduce the physiological breakdown of native GLP-1, which increases the secretion of insulin and promotes satiety, effectively decreasing blood glucose levels.Citation70 A meta-analysis has shown that DPP-IV inhibitors are effective in lowering glycated hemoglobin (HbA1c) in patients with T2D and stages 3–4 CKD (−0.52%, 95% CI: −0.64 to −0.39).Citation71 Despite associations with AEs that include nasopharyngeal symptoms, headaches, angioedema,Citation72,Citation73 and pancreatitis,Citation72 meta-analyses showed low prevalence of such AEs.Citation74,Citation75
DPP-IV inhibitors are metabolized and eliminated in a number of different ways. Their mode of action (MOA) and elimination, as well as PK and safety, are described in .
Table 5 DPP-IV inhibitors: modes of action, modes of excretion, and studies investigating PK
Sitagliptin
Sitagliptin undergoes limited hepatic metabolism; ~80% of the dose is excreted renally, and there is a low, reversible protein binding in the plasma (38%).Citation76
The TECOS study showed that sitagliptin was noninferior to placebo for the primary composite CV outcome of CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina (hazard ratio 0.98, 95% CI: 0.88–1.09; P<0.001).Citation83 In addition, no significant differences between the placebo and sitagliptin group in incidences of pancreatic cancer (P=0.32), acute pancreatitis (P=0.07), or hospitalization for heart failure (P=0.98) were reported.Citation83 In T2D patients, sitagliptin has also been shown to reduce significantly urinary albumin excretion (P<0.0001).Citation84
One PK study in patients without T2D showed that, compared with healthy controls, plasma sitagliptin AUC levels in patients with stages 2–5 CKD were increased by factors of 1.6–4.5.Citation77 Cmax was moderately increased, and concentration at 24 hours increased as severity of CKD increased. Time to Cmax was significantly increased in patients with stage 5 CKD, and the half-life increased with increasing severity of CKD. For patients with stages 2–4 CKD, clearance rates were 0.18–0.71 mL/min.Citation77
In treatment, patients with stage 2 CKD and stage 3 CKD (if eGFR is ≥50 mL/min) require no dose adjustment. For stage 3 CKD and an eGFR of <50 mL/min, the sitagliptin dose should be reduced to 50 mg once daily (od). For patients with stages 4–5 CKD, including those undertaking hemodialysis or peritoneal dialysis, the dose should be decreased to 25 mg od. Treatment can be administered regardless of dialysis time.Citation56
Vildagliptin
Vildagliptin is metabolized via four metabolic pathways before excretion.Citation85 Overall, 77% of the vildagliptin dose is excreted renally (22% as parent, 55% as primary metabolite). In the plasma, vildagliptin shows low (10%), reversible protein binding.Citation76 In patients in early stages of nephropathy, vildagliptin significantly decreased albumin concentrations.Citation86
In a PK study, patients with stages 2–5 CKD, systemic exposure to vildagliptin was increased (Cmax: 8%–66%; AUC extrapolated to infinity: 32%–134%) compared with healthy individuals.Citation78 Changes in exposure to vildagliptin showed no relationship with severity of CKD. No safety data were reported.Citation78 A later study showed that the mean AUC of vildagliptin after 14 days in patients with stages 2–4 CKD increased by 40%–100%. Vildagliptin Cmax increased by 32%–37%.Citation79 Vildagliptin was generally safe and well tolerated in both healthy individuals and patients with different stages of CKD.
In treatment, no dose adjustment of vildagliptin is required in patients with stage 2 CKD. In patients with stages 3–5 CKD, for safety, the recommended dose is 50 mg od.Citation57
Saxagliptin
Saxagliptin is metabolized in the liver, producing an active metabolite. It is eliminated via the kidney (12%–29% as parent, 21%–52% as metabolite).Citation76 In the plasma, saxagliptin shows negligible protein binding.Citation76
The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus – Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) trial confirmed that the CV safety of saxagliptin met US Food and Drug Administration requirements.Citation87 Patients with no renal impairment or stage 2 CKD (P<0.0001) and stages 3–4 CKD (P=0.041) randomized to saxagliptin had greater improvement and less worsening of urine albumin-to-creatinine ratio (UACR) than patients randomized to placebo. Indeed, the relative risk of hospitalization for heart failure was comparable among patients with normal renal impairment or stage 2 CKD and patients with stages 3 and 4 CKD (P=0.43).
One PK study showed that, compared with patients with normal renal function, saxagliptin AUC extrapolated to infinity values were 16%–108% increased in those with stages 2–4 CKD.Citation80 In addition, as CrCl values decreased, AUC values either increased or became more variable.
In treatment, no dose adjustment of saxagliptin is recommended for patients with stage 2 CKD;Citation58 however, to keep the plasma concentration comparable to individuals with normal renal function, the dose should be reduced to 2.5 mg od in patients with stages 3–4 CKD. However, saxagliptin is contraindicated in patients with stage 5 CKD requiring hemodialysis.Citation58
Linagliptin
Linagliptin is metabolized by the liver and does not have active metabolites. It is eliminated by the enterohepatic system (>70% unchanged as parent) and the kidney (<6%).Citation76 Linagliptin binds extensively to plasma proteins in a concentration-dependent manner and it has been calculated that, at the therapeutic dose (5 mg), most of the drug is protein-bound.Citation76 In a study in which linagliptin was administered to patients with T2D and renal dysfunction, there was a significant reduction in albuminuria.Citation88
In one PK study, although there was a trend toward slightly higher (20%–60%) linagliptin exposure in patients with CKD, steady-state AUC and Cmax values showed a substantial overlap and were unaffected by the degree of CKD.Citation81 No deaths or serious AEs occurred, and no AEs led to discontinuation. During treatment, two patients reported AEs considered related to linagliptin by the investigator. No other clinically significant AEs were observed.Citation81
In treatment, in patients with CKD, no dose adjustment is required, and it can be administered across the CKD spectrum.Citation59
Alogliptin
Alogliptin is eliminated slowly, primarily via renal excretion (>70% unchanged as parent).Citation76 After oral administration, alogliptin is absorbed with 100% bioavailability and undergoes ~20% protein binding and limited hepatic metabolism.Citation89 In a study by Sakata et al,Citation90 alogliptin reduced UACR following 12 weeks of treatment.
In a PK study involving patients with various degrees of renal impairment (not stated whether any patients had T2D), Karim et alCitation82 determined that, compared with healthy individuals, increases in alogliptin exposure were 1.7–3.8-fold higher in patients with stages 2–5 CKD. The range of alogliptin AUC was comparable in healthy individuals and patients with stage 2 CKD. Cmax increased from 1.1- to 1.4-fold in patients with CKD compared with healthy individuals. Plasma exposure suggested no accumulation of this metabolite in patients with increasing CKD. Approximately 7.2% of the 50 mg dose was eliminated after 3 hours of dialysis in patients with stage 5 CKD. Protein binding was ~20% for each CKD group.Citation82
In patients with stage 2 CKD (in the CrCl range >50 to ≤80 mL/min), no alogliptin dose adjustment is required. For patients with stage 3 CKD (in the CrCl range ≥30 to ≤50 mL/min), half of the recommended dose should be administered (12.5 mg od) to keep the plasma concentration comparable to healthy individuals.Citation60 Likewise, for patients with stages 4–5 CKD requiring dialysis, administration of a quarter of the recommended dose is advised (6.25 mg od).Citation60
Summary of DPP-IV inhibitor use in renal impairment
DPP-IV inhibitors can be used at all stages of CKD with dose reduction, except for linagliptin, which can be used without dose adjustment.Citation56–Citation60 A potential renoprotective effect (reduction in albuminuria) has been observed with all DPP-IV inhibitors.Citation84,Citation86–Citation88,Citation90 It is not clear whether this renoprotective effect is independent of changes in blood pressure and glycemic markers.
GLP-1 receptor agonists
GLP-1RAs function as incretin mimetics, which enhance the action of the endogenous incretin GLP-1, thereby controlling glycemia via several pathways, including enhancement of insulin secretion, inhibition of glucagon secretion, delay of gastric emptying, and induction of satiety.Citation91 GLP-1RAs reduce HbAlc by 0.55%–1.90%Citation72,Citation92 and have low rates of hypoglycemia,Citation93 with the advantage of promoting weight loss as well as controlling blood glucose.Citation91 Owing to the effect of these agents on gastric emptying, AEs are typically gastrointestinal, that is, nausea and vomiting.Citation91 However, gradually increasing the dose over a few weeks helps build a tolerance to such side effects. One possible, but infrequent, AE is pancreatitis. Available evidence suggests that the rate of pancreatitis among patients using GLP-1RAs is low;Citation94 however, these data are not definitive and ongoing analysis is needed.Citation94
The short-acting compounds (short-lived receptor activation) consist of exenatide twice daily (bid) and lixisenatide od; the longer-acting compounds, which continuously activate receptors at their recommended dose, consist of liraglutide od and once weekly (ow) formulations of exenatide, albiglutide, and dulaglutide.Citation95 ExenatideCitation96 and liraglutideCitation97 have been shown to reduce albuminuria and proteinuria, respectively. A summary of the MOAs, elimination, PK, and safety of the GLP-1RAs is provided in .
Table 6 GLP-1RAs: modes of action, modes of excretion, and studies investigating PK
Exenatide
Exenatide is a synthetic analog of exendin-4, which is extracted from the saliva of Heloderma suspectum.Citation103 Exenatide bid and ow are both eliminated mainly by glomerular filtration with subsequent proteolytic degradation. In addition, Zhang et alCitation96 reported decreased levels of 24-hour urinary albumin after 16 weeks of exenatide treatment (P<0.01).
In a study by Linnebjerg et al,Citation98 exenatide (5 or 10 μg) was injected subcutaneously in 31 patients (including one patient with T2D) stratified by renal function (CrCl): normal and stages 2–5 CKD. PK data were then combined with four previous single-dose studies in patients with T2D to determine the relationship of exenatide clearance and CrCl. The half-life for patients with normal renal function and stages 2–5 CKD groups was 1.5–6.0 hours. After pooling data from several studies, least-squares geometric mean for exenatide clearance in individuals with normal renal function and patients with stages 2–4 CKD were 5.19–8.14 L/h.Citation98 Similar category and incidence of AEs were reported between the healthy and stages 2 and 5 CKD groups. No AEs were reported for the group with stage 3 CKD and no patients discontinued because of AEs.Citation98
No dose adjustment is required at stage 2 CKD to preserve therapeutic exposure. Dose adjustment is required in exenatide bid if CrCl in the patient is in the range 30–50 mL/min, which falls within the definition of stages 3a and 3b CKD;Citation61 within the same CrCl range, exenatide ow is contraindicated.Citation62 However, both exenatide bid and ow are contraindicated in patients with stages 4–5 CKD.Citation61,Citation62
Lixisenatide
As with exenatide, lixisenatide is a synthetic version of exendin-4,Citation104 with resistance to physiological degradation by DPP-IV as a result of C-terminal modification.Citation104 Elimination occurs through glomerular filtration followed by tubular reabsorption and subsequent metabolic degradation.Citation72
A study has shown that stage 2 CKD does not influence lixisenatide PK;Citation63 however, in patients with stages 3–4 CKD, the AUC was increased by 24%–46%.Citation63 In an open-label, nonrandomized, parallel-group study by Liu and Ruus,Citation99 no significant differences in AUC up to the last measurable concentration or Cmax were observed for patients with stage 2 CKD compared with individuals with normal renal function or, indeed, for patients with stage 3 CKD versus normal function. However, in patients with stage 4 CKD, there was a significant increase in AUC up to the last measurable concentration, but not in Cmax.Citation99 Treatment-emergent AEs were reported for one patient in each CKD group and four in the group of patients with normal renal function. AEs included headache (n=4; all with placebo), mild gastrointestinal disturbance (n=4), and mild muscle tightness (n=1).Citation99
No dose adjustment is required at stage 2 CKD to maintain therapeutic exposure. In treatment, monitoring is required with lixisenatide in patients whose CrCl is in the range 30–50 mL/min (which falls within the definition of stages 3a and 3b CKD).Citation63 Additionally, lixisenatide is contraindicated in patients with stages 4 and 5 CKD.Citation63
Liraglutide
Liraglutide has 97% sequence homology to GLP-1.Citation63 Liraglutide is endogenously metabolized in a similar process to large proteins with no specific organ established as a major route of elimination.Citation64 Zavattaro et al have shown a decrease in the proportion of patients with microalbuminuria; five patients in the study had microalbuminuria at baseline, but at 12 months, the levels in three of the patients had returned to normal (P<0.006). Total microalbuminuria levels were improved in patients with normal renal function and stage 2 CKD (P<0.02).Citation105 Investigating the superiority of liraglutide 1.8 mg versus placebo as an add-on to existing oral glucose-lowering agents with T2D and stage 3 CKD, the LIRA-RENAL study showed that liraglutide did not affect renal function and demonstrated better glycemic control (estimated treatment difference in HbA1c from baseline was −0.66% [95% CI: –0.90 to –0.43; P<0.0001]). Moreover, no difference in hypoglycemic AEs was reported between treatment groups; common AEs were gastrointestinal (liraglutide, 35.7%; placebo, 17.5%).Citation106
Jacobsen et alCitation100 investigated the effect of renal impairment on the PK of liraglutide in patients with T2D and normal renal function or stages 2–5 CKD. No defined relationship for change in PK parameters was evident across groups with increasing severity of CKD, and CKD did not increase exposure to liraglutide. Headache and gastrointestinal symptoms were the most commonly reported AEs. Reported AEs were mild to moderate in severity.Citation100
In treatment, no dose adjustment is necessary for patients with stages 2–3 CKD. As there is no therapeutic knowledge in patients with stages 4–5 CKD, liraglutide is contraindicated for use in patients with these diagnoses.Citation64
Dulaglutide
Dulaglutide has 90% sequence homology to GLP-1,Citation92 with the GLP-1 portion of the molecule fused to an immunoglobulin 4 molecule, limiting renal clearance.Citation92 Elimination occurs through degradation into amino acids.Citation92
Loghin et alCitation101 assessed the PK of dulaglutide 1.5 mg in renal impairment compared to healthy individuals. The study included stages 2–5 CKD populations. There was no correlation between PK parameters and renal function based on eGFR. Also, there was no statistically significant correlation between exposure and CrCl. A weak, statistically significant linear correlation was established between CrCl and dulaglutide clearance. There were no prominent differences in safety profiles between patients with CKD and healthy individuals.Citation101
In treatment, no dose adjustment of dulaglutide is necessary in patients with stages 2–3 CKD, but there is still limited experience in patients with stages 4–5 CKD; consequently, dulaglutide is contraindicated in these populations.Citation66
Albiglutide
Albiglutide shares 95% sequence homology with native GLP-1.Citation107 It is composed of a GLP-1 dimer fused to an albumin molecule.Citation108 The expected metabolic pathway for albiglutide is degradation to small peptides and individual amino acids by ubiquitous proteolytic enzymes.Citation65
In their investigation of the PK of albiglutide in patients with T2D stages 2–5 CKD groups, Young et alCitation102 determined modest increases in plasma albiglutide concentration with increased severity of renal impairment and a trend for greater glycemic reductions as eGFR decreased, indicating no need for a dose adjustment. Overall, the incidences of nonserious AEs were comparable in the normal, and stages 2 and 3 CKD groups, whereas the stage 4 CKD group demonstrated lower rates. The serious AEs increased with increasing renal dysfunction up to stage 3 CKD, but the stage 4 CKD group reported no serious AEs.Citation102
In treatment, no dose adjustment of albiglutide is required for patients with stages 2–3 CKD. There is limited experience in patients with stages 4–5 CKD, and, therefore, albiglutide is contraindicated in these patients.Citation65
Summary of GLP-1RA use in renal impairment
Dose adjustment is required in exenatide bid in patients whose CrCl is in the range 30–50 mL/min, which falls within the definition of stages 3a and 3b CKD;Citation56,Citation61 within the same CrCl range, exenatide ow is contraindicated.Citation62 Also, within the same range, monitoring is necessary when dosing lixisenatide.Citation63 By contrast, no dose alteration is necessary for liraglutide, albiglutide, and dulaglutide in stages 2, 3a, and 3b CKD.Citation64–Citation66 All GLP-1RAs are currently contraindicated in stages 4–5 CKD.Citation61–Citation66
SGLT-2 inhibitors
SGLT-2 inhibitors inhibit glucose reabsorption and induce excretion of glucose in the urine.Citation109 Treatment with SGLT-2 inhibitors is associated with reductions in HbA1c levels of 0.4%–1.5% and weight of up to 4.7 kg.Citation110,Citation111
SGLT-2 inhibitors carry a low risk of hypoglycemia, unless combined with sulfonylureas or insulin.Citation112 AEs include urinary and genital tract infections (which are usually not severe), especially in female patients.Citation112 SGLT-2 inhibitors appear to be associated with a small increased risk of euglycemic diabetic ketoacidosis and ketosis.Citation112,Citation113
As their efficacy is reliant on renal function, SGLT-2 inhibitors are generally contraindicated in patients with eGFR <60 mL/min/1.73 m2, mainly because of reduced efficacy.Citation72 A summary of their MOAs, elimination, PK, and safety is provided in .
Table 7 SGLT-2 inhibitors: modes of action, modes of excretion, and studies investigating PK and PD
Dapagliflozin
Dapagliflozin is metabolized in both the kidney and the liver, with active metabolites generated at doses of >50 mg.Citation119 It is primarily eliminated via the renal pathway.Citation67
Assessment of PK by Kasichayanula et alCitation114 in healthy individuals and patients with T2D with normal renal function and stages 2–4 CKD showed that dapagliflozin plasma concentrations and its inactive metabolite dapagliflozin 3-O-glucuronide increased with increasing CKD severity. Steady-state Cmax values for dapagliflozin were 4%–9% higher, and for dapagliflozin 3-O-glucuronide were 20%–52% higher in T2D patients with stages 2–4 CKD, compared with patients with T2D and normal renal function.Citation114 Steady-state renal glucose clearance was reduced by 42%–84% in patients with stages 2–4 CKD, thereby also reducing its efficacy.Citation114
In treatment, dapagliflozin is not recommended for use in patients with stages 3–5 CKD.Citation67 However, no dosage adjustment is suggested in patients with stage 2 CKD.
Canagliflozin
Canagliflozin is primarily metabolized by O-glucuronidation.Citation116 It is eliminated largely unchanged in the feces (41.5%) and as metabolites in the urine (30.5%). Recent clinical data suggest that, in addition to the renal mechanism of action, a nonrenal mechanism partially contributes to glucose lowering for canagliflozin 300 mg but not 150 mg.Citation115 Data from a substudy of the CANagliflozin cardioVascular Assessment Study (CANVAS) showed that 100 and 300 mg doses of canagliflozin significantly reduced the primary outcome of HbA1c levels relative to placebo at week 18 (both P<0.001).Citation120
A study examining the impact of CKD on the PK and PD of canagliflozin 100 and 200 mg in Japanese patients with T2D found no significant effect of stage 3 CKD on the Cmax of either canagliflozin dose.Citation121 The canagliflozin AUC values were higher in patients with stage 3 CKD than in patients with T2D and normal renal function or stage 2 CKD. Changes from baseline in 24-hour urinary glucose excretion increased after administration but, in patients with stage 3 CKD, the increases were ~70% of those in patients with stage 2 CKD or normal.
In treatment, in patients with stage 2 CKD, no dose adjustment is needed.Citation68 Canagliflozin should not be initiated in patients with eGFR <60 mL/min/1.73 m2, but in patients tolerating canagliflozin whose eGFR falls continuously below 60 mL/min/1.73 m2, the dose of canagliflozin should be reduced to or managed at 100 mg od. Canagliflozin is contraindicated in patients with stages 3b–5 CKD, and in patients undertaking dialysis.
Empagliflozin
Empagliflozin is metabolized by glucuronidation without generation of active metabolites and eliminated in feces (41%) and urine (54%).Citation69
In a study comparing patients with normal renal function (and T2D), stage 2 CKD (and T2D), stage 3 CKD (and T2D), stage 4 CKD (four patients with T2D and four without), and stage 5 CKD (without T2D), Macha et alCitation118 showed that the Cmax for empagliflozin was similar for stages 3 and 5 CKD and with normal renal function (and T2D). However, empagliflozin Cmax values were ~20% higher for patients with stages 2 and 4 CKD than for patients with normal renal function.Citation118
In treatment, no dose adjustment of empagliflozin is necessary for patients with stage 2 CKD, but empagliflozin treatment should not be administered in patients with eGFR <60 mL/min/1.73 m2. In patients tolerating empagliflozin whose eGFR falls continuously beneath 60 mL/min/1.73 m2, the dose of empagliflozin should be altered or managed at 10 mg od and stopped when eGFR is continuously beneath 45 mL/min/1.73 m2. Empagliflozin is contraindicated in patients with stages 3b to 5 CKD.Citation69
SGLT-2 effect on proteinuria
Many studies have shown evidence of renoprotection with use of SGLT-2 inhibitors in patients with CKD.Citation120,Citation122–Citation124 SGLT-2 inhibition may mediate their renoprotective effects by decreasing intra-glomerular pressure.Citation125 Indeed, inhibition of SGLT-2 can normalize the NaCl concentration at the macula densa and lower glomerular hyperfiltration, thereby lowering glomerular hyperfiltration and albuminuria.Citation125 This MOA could explain the reduction of albuminuria with SGLT-2 (dapagliflozin, canagliflozin, and empagliflozin) treatment described in clinical studies.Citation120,Citation122–Citation124
In a study by Kohan et al,Citation122 UACR values of >1,800 mg/g during the 104-week treatment period were reported in a higher percentage of patients receiving placebo (13.3%) than patients receiving dapagliflozin 5 (10.8%) or 10 mg (9.5%), indicating decreased albuminuria with dapagliflozin. In Phase III studies, canagliflozin treatment was also associated with decreased albuminuria and an early decrease in eGFR.Citation120,Citation124 Yale et alCitation124 determined a lower proportion of subjects in the canagliflozin 100 and 300 mg groups progressing from normoalbuminuria to micro- or macroalbuminuria, or from micro- to macroalbuminuria compared to those in the placebo group (5.1%, 8.3%, and 11.8%, respectively); likewise, the CANVAS substudy reported decreases in UACR from baseline at canagliflozin 100 mg (−9.6, 95% CI: −13.0 to −6.1) and 300 mg doses (−9.5, 95% CI: −12.9 to −6.1).Citation120
In the Efficacy and Safety of Empagliflozin (BI 10773) in Patients With Type 2 Diabetes and Renal Impairment (EMPA-REG RENAL) placebo-controlled trial, small decreases in eGFR and albuminuria were shown in empagliflozin-treated T2D patients with stages 2–3 CKD.Citation123 UACR was enhanced with empagliflozin (10 and 25 mg) after 52 weeks in patients with stage 2 CKD (empagliflozin 10 mg placebo adjusted mean difference: –184.59, P=0.0831; at 25 mg: –235⋅86, P=0.0257) and stage 3 CKD (empagliflozin 25 mg placebo adjusted mean difference: –183.78, P=0.0031). Moreover, greater proportions of patients with stage 3 CKD shifted from macroalbuminuria at baseline to microalbuminuria, or from microalbuminuria to no albuminuria with empaglifozin compared to placebo (32.6% vs 8.6% and 27.5% vs 21.4%, respectively).
Summary of SGLT-2 inhibitor use in renal impairment
For patients with stage 3 CKD or greater, SGLT-2 inhibitors (dapagliflozin, canagliflozin, and empagliflozin) either require dose adjustment or are contraindicated.Citation67–Citation69 However, a renoprotective effect has been observed with all SGLT-2 inhibitors.Citation120,Citation122–Citation124
Impact of CKD on treatment algorithm
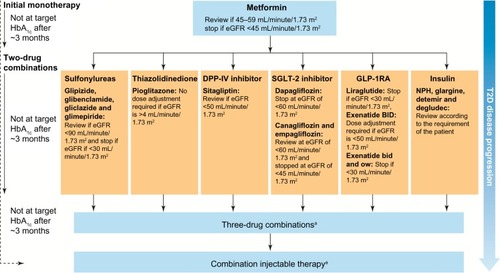
Leading on to the impact of CKD on the T2D treatment algorithm, examples of the dose maintenance and withdrawal of glucose-lowering therapies in the American Diabetes Association/European Association for the Study of Diabetes 2015 guidelines are described in .
Figure 1 The effect of CKD in the European Association for the Study of Diabetes/American Diabetes Association (EASD/ADA) treatment algorithm.
Abbreviations: bid, twice daily; CKD, chronic kidney disease; DPP-IV, dipeptidyl peptidase-IV; eGFR, estimated glomerular filtration rate; GLP-1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated hemoglobin; NPH, neutral protamine Hagedorn; ow, once weekly; SGLT-2, sodium-glucose cotransporter-2.
Ongoing or data pending studies
Clinical investigations of DPP-IV inhibitor, GLP-1RA, and SGLT-2 inhibitor therapies in renal impairment or protection are being carried out in several ongoing studies. A summary is provided in .
Table 8 Ongoing or data pending studies
Incretin-based therapies
The Differential Effects of Diabetes Therapy on Inflammation study aims to determine whether different diabetes treatments have different effects on inflammation, particularly in the kidney, and includesCitation126 patients who need additional glycemic therapy and who are prescribed a DPP-IV inhibitor, GLP-1RA, or insulin.Citation126
Given the relationship between CV safety and T2D and the uncertainty surrounding the CV risk of some therapies, the Cardiovascular and Renal Microvascular Outcome Study with Linagliptin in Patients With Type 2 Diabetes Mellitus (CARMELINA) will compare the CV and renal safety of linagliptin versus placebo, when added to standard care in ~8,000 patients with T2D at high CV risk.Citation127 Clinical data have previously shown that linagliptin reduces albuminuria.Citation88,Citation140 The Efficacy, Safety and Modification of Albuminuria in Type 2 Diabetes Subjects with Renal Disease with Linagliptin (MARLINA) study will investigate this renoprotective effect.Citation128 The Renal Effects of DPP-IV Inhibitor Linagliptin in Type 2 Diabetes (RENALIS) study will further investigate this drug’s action on renal physiology.Citation129 In addition, delayed elimination of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) in renal insufficiency may influence the PK and PD of linagliptin;Citation141 consequently, another study will investigate the effects of linagliptin on active GLP-1 concentrations in patients with renal impairment.Citation130 Examining the safety of a DPP-IV inhibitor in a real-world setting, a postmarketing study will compare the rates of hospitalization for acute kidney injury among patients with T2D who are new initiators of saxagliptin and those patients who are new initiators of other T2D therapies.Citation131
Several studies are currently examining the safety and renoprotective effects of GLP-1RAs. Cardiorenal syndrome type IV is impaired cardiac function due to CKD and is characterized by ventricular hypertrophy, diastolic dysfunction, and/or increased risk of CV AEs.Citation142 The Extended Release Exenatide versus Placebo in Diabetic Patients with Type IV Cardiorenal Syndrome study is evaluating the quantitative impact of 38 weeks of treatment on cardiac biomarkers in patients at high risk of developing cardiorenal syndrome type IV.Citation132 Additionally, the mechanistic and clinical effects of lixisenatide on renal physiology and biomarkers in T2D patients are being explored in the Effect of LIXIsenatide on the Renal System (ELIXIRS) study.Citation133 Meanwhile, the effect of liraglutide on CKD and kidney function in diabetes is being assessed in the Effect of Glucagon-like-peptide 1 (GLP-1) Receptor Agonism on Diabetic Kidney Disease study.Citation134 Dulaglutide has been shown to have similar efficacy and safety to other agents in its class and provide better glycemic control than placebo,Citation92 but there is little experience with its use in populations with renal impairment. The AWARD-7 study will provide important information in this regard by comparing the efficacy and safety of dulaglutide with insulin glargine on glycemic control in patients with T2D and stages 3–4 CKD.Citation135
SGLT-2 inhibitors
Glycemic control and renal safety are being investigated in the Effect of Dapagliflozin on Blood Glucose Level and Renal Safety in Patients With Type 2 Diabetes study.Citation136 In Phase III studies, canagliflozin treatment was also associated with decreased albuminuria and an early decrease in eGFR;Citation120,Citation124 the Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE) trial is a randomized, double-blind, placebo-controlled trial intended to assess whether canagliflozin can delay the development of diabetic nephropathy.Citation138 The renoprotective effect of canagliflozin relative to placebo will be assessed by the composite endpoint of reduction in progression to stage 5 CKD, doubling of serum creatinine, and renal or CV death. Further assessing CV risk, the Effects of Canagliflozin on Renal Endpoints in Adult Participants with Type 2 Diabetes Mellitus (CANVASR) study will recruit 5,875 individuals with T2D.Citation137
The EMPA-REG RENAL trial showed that albuminuria decreased in T2D patients with stages 2–3 CKD treated with empagliflozin.Citation123 To further investigate the impact of empagliflozin on renal kinetics, The Effect of Empagliflozin Kinetics on Renal Glucose Reabsorption in Patients with Type II Diabetes and Healthy Controls study, which has no current published data, investigated the change from baseline of renal tubular maximum reabsorptive capacity for glucose at end of empagliflozin treatment.Citation139
Conclusion
Improved blood glucose control through pharmacological intervention can reduce the incidence and progression of CKD.Citation8,Citation13,Citation14 CKD is a common complication of T2D and should inform the choice of initial and subsequent drug therapy based on individualized glycemic targets.
Apart from the thiazolidinedione pioglitazone,Citation20 which can be used across the spectrum of CKD, there are contraindications and dose adjustments required for all the remaining conventional therapies, including metformin, sulfonylureas, meglitinides, and insulin.Citation21–Citation33 The inclusion of several newer therapy classes in treatment guidelines increases the likelihood that T2D will be well managed,Citation17 with the attendant positive impact on the long-term incidence and progression of renal impairment.
Clinical studies suggest that reductions in albuminuria may be renoprotective and have been observed with all DPP-IV inhibitors,Citation76,Citation84,Citation86,Citation88,Citation90 all SGLT-2 inhibitors, as demonstrated in the EMPA-REG study,Citation120,Citation122,Citation123 and the GLP-1RAs liraglutide and exenatide.Citation96,Citation105 However, as with conventional therapies, not all of these newer therapies can be used when CKD is present, and some require dose adjustment with incident CKD.Citation56–Citation58,Citation60–Citation63,Citation68,Citation69 In contrast to other DPP-IV inhibitors,Citation56–Citation58,Citation60 linagliptin can be used across the spectrum of CKD with no dose adjustment.Citation59 No dose adjustment is required for liraglutide, albiglutide, and dulaglutide in stages 2–3 CKD;Citation64–Citation66 however, they are all currently contraindicated in CKD stages 4–5.Citation61–Citation66 At CKD stages of 3 or more, the SGLT-2 inhibitors dapagliflozin, canagliflozin, and empagliflozin either require dose adjustment or are contraindicated.Citation67–Citation69
Data from ongoing clinical trials with larger populations are awaited to further determine the safety and efficacy profile of DPP-4 inhibitors, GLP-1RAs, and SGLT-2 inhibitors in patients with T2D and CKD.Citation126–Citation139 Ongoing trials, such as CARMELINA, MARLINA, ELIXIRS, A Study Comparing Dulaglutide With Insulin Glargine on Glycemic Control in Participants With Type 2 Diabetes (T2D) and Moderate or Severe Chronic Kidney Disease (CKD) (AWARD-7), CREDENCE, and CANVAS-R, in particular, will help to confirm the position of these new therapy classes in patients with CKD.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors acknowledge support from the UK National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care – East Midlands (NIHR CLAHRC – EM), the Leicester Clinical Trials Unit, and the NIHR Leicester-Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit, which is a partnership between University Hospitals of Leicester NHS Trust, Lough-borough University, and the University of Leicester. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The authors thank Watermeadow Medical for assistance with preparation of this manuscript (funded by Novo Nordisk).
Disclosure
Professor Melanie Davies has acted as a consultant, advisory board member, and speaker for Novo Nordisk, Sanofi- Aventis, Eli Lilly, Merck Sharp and Dohme, Boehringer Ingelheim, AstraZeneca, and Janssen, and as a speaker for Mitsubishi Tanabe Pharma Corporation and Takeda. She has received grants in support of investigator-initiated trials from Novo Nordisk, Sanofi-Aventis, and Eli Lilly. Dr Sudesna Chatterjee has received speaker fees and educational funding from Janssen, Eli Lilly, Novo Nordisk, Astra Zeneca, and Boehringer Ingelheim. Professor Kamlesh Khunti has received funds for research and honoraria for speaking at meetings from, and served on advisory boards for, Astra Zeneca, Boehringer Ingelheim, Lilly, Novartis, Roche, Servier, Sanofi Aventis, MSD, Janssen, and Novo Nordisk. The authors report no other conflicts of interest in this work.
References
- OlyaeiAJStefflJLA quantitative approach to drug dosing in chronic kidney diseaseBlood Purif2011311–313814521228582
- OjoAAddressing the global burden of chronic kidney disease through clinical and translational researchTrans Am Clin Climatol Assoc2014125229243 discussion 243–24625125737
- TedlaFMBrarABrowneRBrownCHypertension in chronic kidney disease: navigating the evidenceInt J Hypertens2011201113240521747971
- Olechnowicz-TietzSGlubaAParadowskaABanachMRyszJThe risk of atherosclerosis in patients with chronic kidney diseaseInt Urol Nephrol20134561605161223483304
- TuttleKRBakrisGLBilousRWDiabetic kidney disease: a report from an ADA Consensus ConferenceDiabetes Care201437102864288325249672
- SavareseGDei CasARosanoGReduction of albumin urinary excretion is associated with reduced cardiovascular events in hypertensive and/or diabetic patients. A meta-regression analysis of 32 randomized trialsInt J Cardiol2014172240341024502877
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work GroupKDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney diseaseKidney Int Suppl2013311163
- PlantingaLCCrewsDCCoreshJPrevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetesClin J Am Soc Nephrol20105467368220338960
- Hippisley-CoxJCouplandCPredicting the risk of chronic kidney disease in men and women in England and Wales: prospective derivation and external validation of the QKidney ScoresBMC Fam Pract2010114920565929
- FowlerMJMicrovascular complications of diabetesClin Diabetes20082627782
- International Diabetes Federation. IDF Diabetes Atlas7th ednBrussels, BelgiumInternational Diabetes Federation2015
- LyssenkoVLaaksoMGenetic screening for the risk of type 2 diabetes: worthless or valuable?Diabetes Care201336Suppl 2S120S12623882036
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) GroupLancet199835291318378539742976
- PerkovicVHeerspinkHLChalmersJIntensive glucose control improves kidney outcomes in patients with type 2 diabetesKidney Int201383351752323302714
- GaedePVedelPLarsenNJensenGVParvingHHPedersenOMultifactorial intervention and cardiovascular disease in patients with type 2 diabetesN Engl J Med2003348538339312556541
- McIntoshBCameronCSinghSRSecond-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysisOpen Med201151e35e4822046219
- InzucchiSEBergenstalRMBuseJBManagement of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of DiabetesDiabetes Care201538114014925538310
- YaleJFOral antihyperglycemic agents and renal disease: new agents, new conceptsJ Am Soc Nephrol200516Suppl 1S7S1015938025
- CavanaughKDiabetes management issues for patients with chronic kidney diseaseClin Diabetes20072539097
- TakedaActos (pioglitazone) Summary of Product Characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/4236Accessed November 2015
- Merck SeronoGlucophage (metformin) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/1043Accessed November 2015
- Pfizer LimitedMinodiab (glipizide) Summary of Product Characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/9851Accessed November 2015
- Wockhardt UK LimitedGlibenclamide Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/30411Accessed November 2015
- Wockhardt UK LimitedGliclazide Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/27762Accessed November 2015
- Accord Healthcare LimitedGlimepiride Summary of Product Characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/25813Accessed November 2015
- Novo Nordisk LimitedPrandin (repaglinide) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/18980Accessed November 2015
- Eli Lilly and Company LimitedHumulin I (isophane insulin) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/3425Accessed November 2015
- Sanofi-aventisLantus (insulin glargine) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/25506Accessed November 2015
- Novo Nordisk LimitedLevemir (insulin detemir) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/14584Accessed November 2015
- Novo Nordisk LimitedTresiba (insulin degludec) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/27360Accessed November 2015
- Eli Lilly and Company LimitedHumalog (insulin lispro) Summary of Product Characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/30005Accessed November 2015
- SanofiApidra (insulin glulisine) Summary of Product Characteristics2013 Available from: https://www.medicines.org.uk/emc/medicine/26476Accessed November 2015
- Novo Nordisk LimitedNovoMix (insulin aspart) Summary of Product Characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/8591Accessed November 2015
- Actavis UK LtdAcarbose Summary of Product Characteristics2013 Available from: https://www.medicines.org.uk/emc/medicine/27829Accessed February 2016
- BrownJBPedulaKBarzilayJHersonMKLatarePLactic acidosis rates in type 2 diabetesDiabetes Care19982110165916639773726
- RojasLBGomesMBMetformin: an old but still the best treatment for type 2 diabetesDiabetol Metab Syndr201351623415113
- National Institute for Health and Care Excellence (NICE)The management of type 2 diabetes (CG87)LondonNational Institute for Clinical Excellence [Published May 2009]. Available from: http://guidance.nice.org.uk/cg87Accessed June 16, 2015
- InzucchiSELipskaKJMayoHBaileyCJMcGuireDKMetformin in patients with type 2 diabetes and kidney disease: a systematic reviewJAMA2014312242668267525536258
- National Kidney FoundationKDOQI clinical practice guideline for diabetes and CKD: 2012 updateAm J Kidney Dis201260585088623067652
- KasliwalRWiltonLVShakirSAMonitoring the safety of pioglitazone: results of a prescription-event monitoring study of 12,772 patients in EnglandDrug Saf2008311083985018759508
- ShayaFTLuZSohnKWeirMRThiazolidinediones and cardiovascular events in high-risk patients with type-2 diabetes mellitus: a comparison with other oral antidiabetic agentsP T200934949050120140111
- RosakCMertesGCritical evaluation of the role of acarbose in the treatment of diabetes: patient considerationsDiabetes Metab Syndr Obes2012535736723093911
- AstraZenecaABSymlin (pramlintide acetate) Prescribing Information2014 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021332s025lbl.pdfAccessed February 2016
- DefronzoRABromocriptine: a sympatholytic, d2-dopamine agonist for the treatment of type 2 diabetesDiabetes Care201134478979421447659
- VeroScienceCycloset (bromocriptine) Prescribing Information2016 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020866s009lbl.pdfAccessed February 2016
- SanofiToujeo (insulin glargine) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/30586Accessed November 2015
- SimpsonDMcCormackPLKeatingGMLyseng-WilliamsonKAInsulin lispro: a review of its use in the management of diabetes mellitusDrugs200767340743417335298
- DreyerMPragerRRobinsonAEfficacy and safety of insulin glulisine in patients with type 1 diabetesHorm Metab Res2005371170270716308840
- OwensDVoraJInsulin aspart: a reviewExpert Opin Drug Metab Toxicol20062579380417014395
- DuckworthWCBennettRGHamelFGInsulin degradation: progress and potentialEndocr Rev19981956086249793760
- GoykhmanSDrincicADesmanglesJCRendellMInsulin glargine: a review 8 years after its introductionExpert Opin Pharmacother200910470571819284367
- IglesiasPDiezJJInsulin therapy in renal diseaseDiabetes Obes Metab2008101081182318248491
- JacobsenLPopescuGPlumAPharmacokinetics of insulin detemir in subjects with renal or hepatic impairmentDiabetologia200245A259A260
- KissIAroldGRoepstorffCBottcherSGKlimSHaahrHInsulin degludec: pharmacokinetics in patients with renal impairmentClin Pharmacokinet201453217518324163264
- HolmesGGalitzLHuPLynessWPharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairmentBr J Clin Pharmacol200560546947616236036
- Merck Sharp and Dohme LimitedJanuvia (sitagliptin) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/19609Accessed November 2015
- Novartis Pharmaceuticals UK LtdGalvus (vildagliptin) Summary of Product Characteristics2016 Available from: https://www.medicines.org.uk/emc/medicine/20734Accessed January 2016
- AstraZeneca UK LimitedOnglyza (saxagliptin) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/22315Accessed February 2016
- Boehringer Ingelheim LimitedTrajenta (linagliptin) Summary of Product Characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/25000Accessed November 2015
- Takeda UK LtdVipidia (alogliptin) Summary of product characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/28513Accessed November 2015
- AstraZeneca UK LimitedByetta (exenatide) Summary of Product Characteristics2016 Available from: https://www.medicines.org.uk/emc/medicine/19257Accessed January 2016
- AstraZeneca UK LimitedBydureon (exenatide) Summary of Product Characteristics2016 Available from: https://www.medicines.org.uk/emc/medicine/24665Accessed February 2016
- Sanofi-aventisLyxumia (lixisenatide) Summary of Product Characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/27405Accessed November 2015
- Novo Nordisk LimitedVictoza (liraglutide) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/21986Accessed November 2015
- GlaxoSmithKline Trading Services LimitedEperzan (albiglutide) Summary of Product Characteristics2016 Available from: https://www.medicines.org.uk/emc/medicine/31399Accessed February 2016
- Eli Lilly and Company LimitedTrulicity (dulaglutide) Summary of Product Characteristics2016 Available from: https://www.medicines.org.uk/emc/medicine/29747Accessed February 2016
- AstraZeneca UK LimitedForxiga (dapagliflozin) Summary of Product Characteristics2014 Available from: https://www.medicines.org.uk/emc/medicine/27188Accessed November 2015
- Janssen-Cilag LtdInvokana (canagliflozin) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/28400Accessed February 2016
- Boehringer Ingelheim LimitedJardiance (empagliflozin) Summary of Product Characteristics2015 Available from: https://www.medicines.org.uk/emc/medicine/28973Accessed November 2015
- MuskietMHSmitsMMMorsinkLMDiamantMThe gut-renal axis: do incretin-based agents confer renoprotection in diabetes?Nat Rev Nephrol20141028810324375052
- ChengDFeiYLiuYEfficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus patients with moderate to severe renal impairment: a systematic review and meta-analysisPLoS One2014910e11154325360775
- GameFNovel hypoglycaemic agents: considerations in patients with chronic kidney diseaseNephron Clin Pract20141261141824434725
- AmoriRELauJPittasAGEfficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysisJAMA2007298219420617622601
- TriccoACAntonyJKhanPASafety and effectiveness of dipeptidyl peptidase-4 inhibitors versus intermediate-acting insulin or placebo for patients with type 2 diabetes failing two oral antihyperglycaemic agents: a systematic review and network meta-analysisBMJ Open2014412e005752
- BrownNJByiersSCarrDMaldonadoMWarnerBADipeptidyl peptidase-IV inhibitor use associated with increased risk of ACE inhibitor-associated angioedemaHypertension200954351652319581505
- DeaconCFDipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative reviewDiabetes Obes Metab201113171821114598
- BergmanAJCoteJYiBEffect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitorDiabetes Care20073071862186417468348
- HeYLFlanneryBWangYThe influence of renal impairment on pharmacokinetics of vildagliptin (Abstract PIII-86)Clin Pharmacol Ther200781Suppl 1S113
- HeYLKulmatyckiKZhangYPharmacokinetics of vildagliptin in patients with varying degrees of renal impairmentInt J Clin Pharmacol Ther201351969370323782585
- BoultonDWLiLFrevertEUInfluence of renal or hepatic impairment on the pharmacokinetics of saxagliptinClin Pharmacokinet201150425326521348538
- Graefe-ModyUFriedrichCPortAEffect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin(*)Diabetes Obes Metab2011131093994621672124
- KarimAFleckPHetmanLSingle-dose pharmacokinetics of the dipeptidyl peptidase-4 inhibitor alogliptin in subjects with renal impairment (Abstract 538-P)Diabetes200857Suppl 1A160
- GreenJBBethelMAArmstrongPWEffect of sitagliptin on cardiovascular outcomes in type 2 diabetesN Engl J Med2015373323224226052984
- MoriHOkadaYAraoTTanakaYSitagliptin improves albuminuria in patients with type 2 diabetes mellitusJ Diabetes Investig201453313319
- HeHTranPYinHAbsorption, metabolism, and excretion of [14C]vildagliptin, a novel dipeptidyl peptidase 4 inhibitor, in humansDrug Metab Dispos200937353654419074975
- WatanabeMFuruyaFKobayashiTDPP-4 inhibitor vildagliptin reduces urinary albumin excretion in type 2 diabetic patients with microalbuminuriaEndocrine Abstracts201229P687
- UdellJABhattDLBraunwaldESaxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR-TIMI 53 TrialDiabetes Care201538469670525552421
- GroopPHCooperMEPerkovicVEmserAWoerleHJvon EynattenMLinagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunctionDiabetes Care201336113460346824026560
- DineenLLawCScherRPyonEAlogliptin (nesina) for adults with type-2 diabetesP T201439318620224790396
- SakataKHayakawaMYanoYEfficacy of alogliptin, a dipeptidyl peptidase-4 inhibitor, on glucose parameters, the activity of the advanced glycation end product (AGE) – receptor for AGE (RAGE) axis and albuminuria in Japanese type 2 diabetesDiabetes Metab Res Rev201329862463023861159
- AhrenBThe future of incretin-based therapy: novel avenues – novel targetsDiabetes Obes Metab201113Suppl 115816621824270
- KuritzkyLUmpierrezGEkoeJMMancillas-AdameLLandoLFSafety and efficacy of dulaglutide, a once weekly GLP-1 receptor agonist, for the management of type 2 diabetesPostgrad Med20141266607225414935
- TrujilloJMNufferWEllisSLGLP-1 receptor agonists: a review of head-to-head clinical studiesTher Adv Endocrinol Metab201561192825678953
- LiLShenJBalaMMIncretin treatment and risk of pancreatitis in patients with type 2 diabetes mellitus: systematic review and meta-analysis of randomised and non-randomised studiesBMJ2014348g236624736555
- MeierJJGLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitusNat Rev Endocrinol201281272874222945360
- ZhangHZhangXHuCLuWExenatide reduces urinary transforming growth factor-beta1 and type IV collagen excretion in patients with type 2 diabetes and microalbuminuriaKidney Blood Press Res201235648348822687869
- ImamuraSHiraiKHiraiAThe glucagon-like peptide-1 receptor agonist, liraglutide, attenuates the progression of overt diabetic nephropathy in type 2 diabetic patientsTohoku J Exp Med20132311576124064677
- LinnebjergHKotharePAParkSEffect of renal impairment on the pharmacokinetics of exenatideBr J Clin Pharmacol200764331732717425627
- LiuYRuusPPharmacokinetics and safety of the GLP-1 agonist AVE0010 in patients with renal impairment (abstract)Diabetes200959A149A150
- JacobsenLVHindsbergerCRobsonRZdravkovicMEffect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutideBr J Clin Pharmacol200968689890520002084
- LoghinCde la PeñaACuiXZhangXGeiserJSChienJYPharmacokinetics of once weekly dulaglutide in special populationsDiabetologia201457Suppl 1S349
- YoungMAWaldJAMatthewsJEYangFReinhardtRREffect of renal impairment on the pharmacokinetics, efficacy, and safety of albiglutidePostgrad Med20141263354624918790
- GedulinBRNikoulinaSESmithPAExenatide (exendin-4) improves insulin sensitivity and {beta}-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weightEndocrinology200514642069207615618356
- BarnettAHLixisenatide: evidence for its potential use in the treatment of type 2 diabetesCore Evid20116677922022289
- ZavattaroMCaputoMSamaMTOne-year treatment with liraglutide improved renal function in patients with type 2 diabetes: a pilot prospective studyEndocrine201550362062625572181
- DaviesMJBainSCAtkinSLEfficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): A randomized clinical trialDiabetes Care201639222223026681713
- GarberAJLong-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerabilityDiabetes Care201134Suppl 2S27928421525469
- MatthewsJEStewartMWDe BoeverEHPharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in patients with type 2 diabetesJ Clin Endocrinol Metab200893124810481718812476
- BaysHFrom victim to ally: the kidney as an emerging target for the treatment of diabetes mellitusCurr Med Res Opin200925367168119232040
- ChaoECSGLT-2 inhibitors: A new mechanism for glycemic controlClin Diabetes201432141126246672
- FerranniniESoliniASGLT2 inhibition in diabetes mellitus: rationale and clinical prospectsNat Rev Endocrinol20128849550222310849
- NauckMAUpdate on developments with SGLT2 inhibitors in the management of type 2 diabetesDrug Des Devel Ther2014813351380
- PetersALBuschurEOBuseJBCohanPDinerJCHirschIBEuglycemic diabetic ketoacidosis: A potential complication of treatment with sodium-glucose cotransporter 2 inhibitionDiabetes Care20153891687169326078479
- KasichayanulaSLiuXPe BenitoMThe influence of kidney function on dapagliflozin exposure, metabolism and pharmacodynamics in healthy subjects and in patients with type 2 diabetes mellitusBr J Clin Pharmacol201376343244423210765
- SteinPBergJKMorrowLCanagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces post-meal glucose excursion in patients with type 2 diabetes by a non-renal mechanism: results of a randomized trialMetabolism201463101296130325110280
- Sarnoski-BrocavichSHilasOCanagliflozin (Invokana), a novel oral agent for type-2 diabetesP T2013381165666624391386
- InagakiNKondoKYoshinariTPharmacokinetic and pharmacodynamic profiles of canagliflozin in Japanese patients with type 2 diabetes mellitus and moderate renal impairmentClin Drug Investig20143410731742
- MachaSMattheusMHalabiAPinnettiSWoerleHJBroedlUCPharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairmentDiabetes Obes Metab201416321522223859488
- KomoroskiBVachharajaniNBoultonDDapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjectsClin Pharmacol Ther200985552052619129748
- NealBPerkovicVde ZeeuwDEfficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetesDiabetes Care201538340341125468945
- InagakiNKondoKYoshinariTPharmacokinetic and pharmacodynamic profiles of canagliflozin in Japanese patients with type 2 diabetes mellitus and moderate renal impairmentClin Drug Investig20143410731742
- KohanDEFiorettoPTangWListJFLong-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic controlKidney Int201485496297124067431
- BarnettAHMithalAManassieJEMPA-REG Renal Trial investigatorsEfficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trialLancet Diabetes Endocrinol20142536938424795251
- YaleJFBakrisGCariouBEfficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney diseaseDiabetes Obes Metab201315546347323464594
- VallonVThe mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitusAnnu Rev Med20156625527025341005
- University College DublinThe differential effects of diabetes therapy on inflammation Available from: https://clinicaltrials.gov/ct2/show/NCT02150707?term=NCT02150707&rank=1 NLM identifier: NCT02150707Accessed September 30, 2015
- Boehringer IngelheimCardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus (CARMELINA) Available from: https://clinicaltrials.gov/ct2/show/NCT01897532?term=NCT01897532&rank=1. NLM identifier: NCT01897532Accessed September 30, 2015
- Boehringer IngelheimMARLINA – T2D : Efficacy, Safety and Modification of Albuminuria in Type 2 Diabetes Subjects With Renal Disease With LINAgliptin Available from: https://clinicaltrials.gov/ct2/show/record/NCT01792518. NLM identifier: NCT01792518Accessed November 30, 2015
- VU University Medical CenterRenal Effects of DPP-4 Inhibitor Linagliptin in Type 2 Diabetes (RENALIS) Available from: https://clinicaltrials.gov/ct2/show/NCT02106104?term=NCT02106104&rank=1. NLM identifier: NCT02106104Accessed November 30, 2015
- Profil Institut für Stoffwechselforschung GmbHEffects of Linagliptin on Active GLP-1 Concentrations in Subjects With Renal Impairment Available from: https://clinicaltrials.gov/ct2/show/NCT01903070?term=NCT01903070&rank=1. NLM identifier: NCT01903070Accessed November 30, 2015
- Bristol-Myers SquibbRisk of Acute Kidney Injury Among Patients With Type 2 Diabetes Exposed to Oral Antidiabetic Treatments Available from: https://clinicaltrials.gov/ct2/show/NCT01377935?term=Comparison+of+Risk+of+Hospitalization+for+Acute+Kidney+Injury+Between+Patients+With+Type+2+Diabetes+Initiating+Saxagliptin&rank=1. NLM identifier: NCT01377935Accessed November 30, 2015
- Baylor Research InstituteExtended Release Exenatide Versus Placebo In Diabetic Patients With Type 4 Cardiorenal Syndrome Available from: https://clinicaltrials.gov/ct2/show/NCT02251431. NLM identifier: NCT02251431Accessed November 30, 2015
- VU University Medical CenterEffect of LIXIsenatide on the Renal System (ELIXIRS) Available from: https://clinicaltrials.gov/ct2/show/NCT02276196. NLM identifier: NCT02276196Accessed November 30, 2015
- NeffKarlUniversity College DublinThe Effect of Glucagon-like-peptide 1 (GLP-1) Receptor Agonism on Diabetic Kidney Disease Available from: https://clinicaltrials.gov/ct2/show/NCT01847313?term=The+Effect+of+Glucagon-like-peptide+1+%28GLP-1%29+Receptor+Agonism+on+Diabetic+Kidney+Disease&rank=1. NLM identifier: NCT01847313Accessed November 30, 2015
- MadirajuAKErionDMRahimiYMetformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenaseNature2014510750654254624847880
- AstraZenecaABStudy to Evaluate the Effect of Dapagliflozin on Blood Glucose Level and Renal Safety in Patients With Type 2 Diabetes (DERIVE) Available from: https://clinicaltrials.gov/ct2/show/NCT02413398?term=Study+to+Evaluate+the+Effect+of+Dapagliflozin+on+Blood+Glucose+Level+and+Renal+Safety+in+Patients+With+Type+2+Diabetes&rank=1. NLM identifier: NCT02413398Accessed November 30, 2015
- Janssen Research Development, LLCA Study of the Effects of Canagliflozin (JNJ-28431754) on Renal Endpoints in Adult Participants With Type 2 Diabetes Mellitus (CANVAS-R) Available from: https://clinicaltrials.gov/ct2/show/record/NCT01989754. NLM identifier: NCT01989754Accessed November 30, 2015
- Janssen Research Development, LLCEvaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE) Available from: https://clinicaltrials.gov/ct2/show/NCT02065791?term=NCT02065791&rank=1. NLM identifier: NCT02065791Accessed November 30, 2015
- Boehringer IngelheimEffect of Empagliflozin Kinetics on Renal Glucose Reabsorption in Patients With Type II Diabetes and Healthy Controls Available from: https://clinicaltrials.gov/ct2/show/NCT01867307?term=Effect+of+Empagliflozin+Kinetics+on+Renal+Glucose+Reabsorption+in+Patients+With+Type+II+Diabetes+and+Healthy+Controls&rank=1. NLM identifier: NCT01867307Accessed November 30, 2015
- GroopPHDel PratoSTaskinenMRLinagliptin treatment in subjects with type 2 diabetes with and without mild-to-moderate renal impairmentDiabetes Obes Metab201416656056824612167
- MeierJJNauckMAKranzDSecretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjectsDiabetes200453365466214988249
- ClementiAVirziGMGohCYCardiorenal syndrome type 4: a reviewCardiorenal Med201331637023946725
