Abstract
Background
Reloading with high-dose atorvastatin shortly before percutaneous coronary interventions (PCIs) has been proposed as a strategy to reduce periprocedural myonecrosis. There has been a concern that statins that are metabolized by cytochrome P450 3A4 may interfere with clopidogrel metabolism at high doses. The impact of simultaneous administration of high doses of atorvastatin and clopidogrel on the efficacy of platelet inhibition has not been established.
Methods
Subjects (n=60) were randomized to receive atorvastatin 80 mg together with clopidogrel 600 mg loading dose (n=28) versus clopidogrel 600 mg alone (n=32) at the time of PCI. Platelet aggregation was measured at baseline, 4 hours after clopidogrel loading dose, and 16–24 hours after clopidogrel loading dose by light transmittance aggregometry using adenosine diphosphate as agonist.
Results
Platelet aggregation was similar at baseline in both the atorvastatin and the control groups (adenosine diphosphate 10 µM: 57%±19% vs 61%±21%; P=0.52). There was no significant difference in platelet aggregation between the atorvastatin and the control groups at 4 hours (37%±18% vs 39%±21%; P=0.72) and 16–24 hours post-clopidogrel loading dose (35%±17% vs 37%±18%; P=0.75). No significant difference in incidence of periprocedural myonecrosis was observed between the atorvastatin and control groups (odds ratio: 1.02; 95% confidence interval 0.37–2.8).
Conclusion
High-dose atorvastatin given simultaneously with clopidogrel loading dose at the time of PCI does not significantly alter platelet inhibition by clopidogrel. Statin reloading with high doses of atorvastatin at the time of PCI appears to be safe without adverse effects on platelet inhibition by clopidogrel (ClinicalTrials.gov: NCT00979940).
Introduction
High-dose statin therapy has been well established in the medical therapy of acute coronary syndromes (ACS) and in secondary prevention of cardiovascular disease. Beyond the low-density lipoprotein lowering and high-density lipoprotein raising properties, pleiotropic off-target effects of statins have been invoked in the dramatic reduction of thrombotic cardiovascular events. In the PROVE-IT TIMI 22 trial, intensive therapy with atorvastatin 80 mg daily was superior to pravastatin 40 mg daily in reducing ischemic events after ACS with a 16% relative risk reduction for a composite end point of death, myocardial infarction, documented unstable angina requiring hospitalization, revascularization, and stroke in the atorvastatin group.Citation1 Similarly, others have investigated the beneficial properties of pretreatment with statins prior to elective percutaneous revascularization. A meta-analysis including nine trials documented an odds ratio of 0.45 for the occurrence of periprocedural myonecrosis in patients treated with statins prior to coronary intervention.Citation2 Whereas earlier studies included patients who were pretreated with statins for at least 2 days prior to percutaneous coronary intervention (PCI), more recent trials have suggested that shorter pretreatment with statins may provide similar protection.Citation3,Citation4 In the NAPLES II study, atorvastatin 80 mg administered 24 hours prior to PCI significantly reduced the incidence of myocardial infarction in statin-naïve patients.Citation4 Similarly, in the ARMYDA-RECAPTURE study, reloading with atorvastatin 80 mg 12 hours prior to PCI and 40 mg immediately pre-PCI in patients on chronic statin therapy reduced the 30-day major adverse cardiovascular event rate from 9.4% to 3.7% (P=0.037).Citation5
Clopidogrel is a prodrug that requires metabolism by the hepatic cytochrome P450 (CYP) system (2C19, 1A2, 2B6, 3A4, 3A5, and 2C9 isoforms) to provide an active metabolite.Citation6 It has been shown that inhibition of CYP metabolic capacity such as in the case of CYP2C19 by omeprazole and other proton pump inhibitors can lead to inadequate clopidogrel metabolite formation and reduced platelet inhibition. High residual platelet reactivity during treatment with clopidogrel is associated with significantly increased risk for adverse ischemic events after PCI.Citation7
Due to the common CYP3A4 pathway shared by many of the statins (simvastatin, atorvastatin, lovastatin) and clopidogrel, there has been a concern for clinically significant interactions. A documented interaction between atorvastatin and clopidogrel was first described in 2003 by Lau et al,Citation8 but other studies have not been able to confirm this effect using other platelet-testing modalities during maintenance therapy.Citation9,Citation10 Analysis of data from the CHARISMA trial studying 10,078 patients with statin therapy at baseline randomized to dual therapy with clopidogrel and aspirin versus aspirin alone did not demonstrate an effect of CYP3A4-metabolized versus non-CYP3A4-metabolized statins on clinical end points.Citation11 In clinical practice, administration of maintenance statin and antiplatelet agents is often separate, with the former generally being prescribed in the evening and the latter in the morning. However, as early upstream administration of high-dose atorvastatin is being increasingly adopted for patients admitted with ACS, concomitant administration with clopidogrel is more likely to occur. No study has so far investigated the effects of simultaneous administration of high-dose atorvastatin (80 mg) with high loading dose clopidogrel (600 mg) on early platelet inhibition post-PCI.
Methods
Study design and patient population
The ESTATE study (effects of short term, high-dose aorvastatin therapy on periprocedural myonecrosis and platelet inhibition after PCI) is a randomized open-label pilot study to evaluate the effects of high-dose atorvastatin loading immediately before PCI on periprocedural myonecrosis and platelet inhibition. The study was approved by the Indiana University institutional review board. Written informed consent was obtained from all subjects. Subjects were eligible to be enrolled in the study if they were found to have significant coronary artery disease (CAD) with angiographic stenosis of ≥70% in one or more coronary arteries and were undergoing PCI for stable CAD. Patients undergoing catheterization for ST-elevation myocardial infarction (MI), non-ST elevation MI, or high-risk unstable angina were not enrolled in the study. Exclusion criteria included age >85 years and <21 years, recent myocardial infarction within the last month, cancer, renal failure with creatinine >3.0 mg/dL, liver cirrhosis, lymphoproliferative disorder, pregnancy, thrombocytopenia <150,000/mm3, coagulopathy (international normalized ratio [INR] >1.5), abnormal liver function tests, illicit drug use, history of statin intolerance, history of rhabdomyolysis, and planned use of glycoprotein IIb/IIIa inhibitors during PCI. Given that only very few patients who were referred for cardiac catheterization were statin naïve, we allowed subjects who had received simvastatin (which was the primary statin prescribed in our institution) to be enrolled if last simvastatin dose was administered at least 1 day prior. Subjects treated with atorvastatin, pravastatin, lovastatin, fluvastatin, or rosuvastatin were excluded from participation. All patients received aspirin 81–325 mg orally prior to diagnostic catheterization.
Patients were randomized 1:1 to receive either atorvastatin 80 mg orally or no additional atorvastatin, along with standard clopidogrel 600 mg loading dose immediately prior to beginning of ad hoc PCI in the catheterization laboratory. Randomization occurred by opening of previously prepared sealed envelopes containing random allocation. The original sample size of the study (n=88) was calculated based on power 0.8 (two-sided alpha of 0.05) to detect the relative reduction in risk of periprocedural myonecrosis previously demonstrated in a study of moderate-dose atorvastatin pretreatment for several days before PCI.Citation3 The study was stopped after 60 subjects due to slower than expected enrollment.
Blood samples
Blood samples were taken from arterial sheaths in the catheterization laboratory before administration of clopidogrel and atorvastatin. Follow-up blood samples were obtained by venipuncture 4 hours and 16–24 hours after loading dose of clopidogrel. All blood samples were directly transferred into vacutainer tubes containing Na citrate 3.2% and analyzed within 2 hours. Light transmittance aggregometry was performed with a Chronolog 700 aggregometer in platelet-rich plasma using platelet poor plasma as reference as previously described.Citation12,Citation13 Adenosine diphosphate (ADP) at 5 µM, 10 µM, and 20 µM was used as agonist. Platelet inhibition was defined as % change in platelet aggregation from baseline in subjects not on baseline clopidogrel. Cardiac troponin I and creatinine kinase-MB (CK-MB) were measured 8 hours and 16 hours after PCI.
End points
Periprocedural myonecrosis was defined as a rise in cardiac troponin I above the upper limit of normal (ULN) for the assay at any time point after PCI. Periprocedural myocardial infarction was defined as postprocedural rise in CK-MB >3× ULN.Citation14
Statistics
Statistical analysis was performed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA). Statistical significance was defined as P<0.05. Two-sided tests were conducted, and the values are represented as mean ± standard deviation except as otherwise stated. Categorical variables were compared using the χ2-test. Normal distribution of continuous data was assessed by the Kolmogorov–Smirnov test. Unpaired two-sided Student’s t-test was used to compare normally distributed continuous data between the two groups. Multivariable linear regression analysis was performed using on-treatment platelet aggregation as dependent variable to evaluate the effects of assigned treatment arm with forward stepwise adjustment for baseline clinical variables, as well as adjustment for prior treatment with simvastatin and clopidogrel.
Results
Sixty patients who were undergoing elective PCI were randomized to receive either atorvastatin 80 mg together with clopidogrel 600 mg loading dose (n=28) or clopidogrel 600 mg alone (n=32).
One patient in the control (no atorvastatin) group received abciximab during the procedure at the discretion of the operator and was excluded from the analysis in the platelet substudy. Clinical variables of the subjects enrolled in the study are delineated in . There were no significant differences in the prevalence of clinical variables among groups. An average of 1.53 stents were implanted during the PCI, with 91% being drug-eluting stents. The target vessel was the left anterior descending artery in 33%, the circumflex artery in 32%, and the right coronary artery in 45% of cases.
Table 1 Baseline demographics and clinical variables
At baseline prior to clopidogrel and atorvastatin administration, there was no significant difference in baseline platelet aggregation induced by ADP (). Platelet aggregation decreased at 4 hours after clopidogrel administration in both the atorvastatin and the control groups, with only minimal additional decrease in maximal platelet aggregation for all agonists studied at 16–24 hours (). There was a nonsignificant trend toward lower on-treatment maximal platelet aggregation in the atorvastatin group as compared to the control group at both time points. Similarly, there was no significant difference in platelet inhibition between the atorvastatin and control groups (). Multivariable linear regression analysis demonstrated no significant association between atorvastatin treatment arm and final on-treatment platelet aggregation after adjustment for clinical variables and pretreatment with clopidogrel or simvastatin ().
Figure 1 Platelet aggregation.
Abbreviation: ADP, adenosine diphosphate.
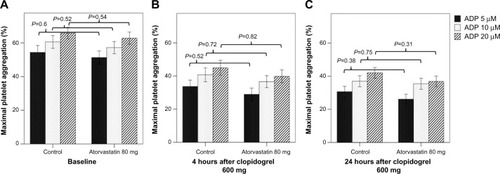
Figure 2 Platelet inhibition 4 hours (A) and 24 hours (B) after clopidogrel loading dose. Periprocedural myonecrosis after PCI (C).
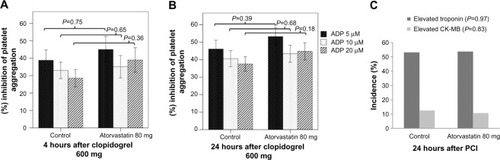
Table 2 Multivariable analysis of atorvastatin treatment with forward stepwise adjustment for baseline clinical variables and baseline clopidogrel and simvastatin treatment
As previously reported by other investigators, a large number of subjects with uncomplicated PCI demonstrated periprocedural myonecrosis, as defined by elevation in cardiac troponin I above the ULN (). A smaller fraction of subjects demonstrated a rise in CK-MB above the ULN (). For both measures, no difference in incidence of periprocedural myonecrosis was observed between atorvastatin and control groups (troponin >1× ULN: odds ratio: 1.02; 95% confidence interval [CI] 0.37–2.8; P=0.97; CK-MB >1× ULN: odds ratio: 0.84; 95% CI 0.17–4.1; P=0.83). Two subjects in the atorvastatin group and one subject in the control group had a periprocedural myocardial infarction as defined by CK-MB rise above 3× ULN (odds ratio: 2.4; 95% CI 0.2–28; P=0.5).
Discussion
The results of our study suggest that simultaneous administration of high-dose atorvastatin 80 mg and clopidogrel 600 mg loading doses does not significantly increase the risk of high posttreatment platelet reactivity. While our study was not powered to demonstrate effectiveness in reducing the risk of periprocedural myocardial necrosis, given early termination of the study prior to targeted enrollment, and its pilot design, there was no difference in rates of periprocedural myonecrosis between subjects with and without concomitant high-dose atorvastatin administration. Previous studies by other investigators have suggested that high-dose atorvastatin given within a short time interval of 12 hours before PCI in patients with ACS may significantly reduce the risk of major cardiac events. In addition, updated guidelines recommend atorvastatin 40–80 mg/d or rosuvastatin 20–40 mg/d for secondary prevention of CAD.Citation15 Thus, high doses of atorvastatin are increasingly being coprescribed in patients undergoing ad hoc PCI who may be started on clopidogrel concomitantly during an admission for an ACS. Many studies have evaluated the pharmacodynamic interactions of CYP3A4-metabolized statins and clopidogrel, with the majority evaluating the effects of chronic statin exposure on platelet reactivity.Citation8–Citation11,Citation16–Citation22 The timing of peak hepatic plasma exposure to the parent drugs may affect the extent of the pharmacodynamic interaction. The majority of studies examining statin–clopidogrel interaction have used traditional timing of dose administration, with statins being prescribed in the evening and antiplatelet agents in the morning. This may significantly influence the degree of interaction given that atorvastatin has a terminal half-life of ∼10 hours.Citation23 The majority of studies have demonstrated no consistent effect of atorvastatin treatment on clopidogrel response or clinical event rates, with few exceptions. Leoncini et alCitation24 demonstrated improved platelet inhibition among patients randomized to high-dose atorvastatin who were previously found to have high platelet reactivity on clopidogrel. Some studies have suggested a modest pleiotropic anti-aggregatory effect of low-dose atorvastatin with reduction of ADP platelet reactivity after treatment for several months as well as improvement in prevalence of aspirin resistance, which could in part counterbalance a reduction in clopidogrel platelet inhibition.Citation25,Citation26 In contrast, Park et alCitation18 showed that changing atorvastatin to a non-3A4-metabolized statin significantly improved platelet inhibition among nonresponders to clopidogrel with high posttreatment platelet reactivity. Pelliccia et alCitation27 recently reported that treatment with low-dose atorvastatin was associated with a small but significant increase in platelet reactivity among patients treated with clopidogrel, but this effect was only observed in subjects with high baseline on-treatment platelet reactivity by Verify Now assay. These conflicting results suggest that among certain individuals, in particular subjects who have high residual platelet reactivity during treatment with clopidogrel, concomitant treatment with high-dose atorvastatin could contribute to differences in pharmacodynamic response to clopidogrel. However, the results of this study suggest that simultaneous administration of high-dose atorvastatin and clopidogrel loading dose does not significantly impair early platelet inhibition after PCI, which is supported by lack of differences in outcomes among patients with statins in large clinical trials of clopidogrel.Citation11,Citation28,Citation29
Limitations of this study include the open-label study design and that not all patients were naïve to statins and clopidogrel at the time of enrollment. Also, no other platelet agonists were studied other than ADP. Additional limitations are the relatively small sample size and the premature termination of the study after slow enrollment, leading to decrease in power to detect significant differences in incidence of periprocedural myonecrosis.
Conclusion
The results of our study indicate that simultaneous administration of high-dose atorvastatin and clopidogrel prior to PCI appears to be safe and does not significantly alter platelet inhibition by clopidogrel.
Acknowledgments
This study was supported in part by the Indiana Clinical and Translational Sciences Institute funded in part by Grant Number (RR025761) from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award, as well as the Indiana University Health Values Grant, the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative, and internal funding from the Department of Medicine, Indiana University School of Medicine, Indianapolis.
Disclosure
The authors report no conflicts of interest in this work.
References
- CannonCPBraunwaldEMcCabeCHIntensive versus moderate lipid lowering with statins after acute coronary syndromesN Engl J Med20043501495150415007110
- MerlaRReddyNKWangFUretskyBFBarbagelataABirnbaumYMeta-analysis of published reports on the effect statin treatment before percutaneous coronary intervention on periprocedural myonecrosisAm J Cardiol200710077077617719318
- PasceriVPattiGNuscaAARMYDA InvestigatorsRandomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (atorvastatin for reduction of myocardial damage during angioplasty) studyCirculation200411067467815277322
- BriguoriCViscontiGFocaccioANovel approaches for preventing or limiting events (Naples) II trial: impact of a single high loading dose of atorvastatin on periprocedural myocardial infarctionJ Am Coll Cardiol200954232157216319664895
- Di SciascioGPattiGPasceriVGaspardoneAColonnaGMontinaroAEfficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (atorvastatin for reduction of myocardial damage during angioplasty) randomized trialJ Am Coll Cardiol200954655856519643320
- KreutzRPFlockhartDAAmlodipine-not a significant contributor to clopidogrel non-response?Heart201399743743923315611
- StoneGWWitzenbichlerBWeiszGADAPT-DES InvestigatorsPlatelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry studyLancet2013382989261462323890998
- LauWCWaskellLAWatkinsPBAtorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interactionCirculation2003107323712515739
- MullerIBestaFSchulzCZhongyanLMassbergSGawazMEffects of statins on platelet inhibition by a high loading dose of clopidogrelCirculation20031082195219714568892
- VinholtPPoulsenTSKorsholmLThe antiplatelet effect of clopidogrel is not attenuated by statin treatment in stable patients with ischemic heart diseaseThromb Haemost200594243844316113837
- SawJBrennanDMSteinhublSRLack of evidence of a clopidogrel-statin interaction in the CHARISMA trialJ Am Coll Cardiol20075029129517659194
- KreutzRPTantryUSBlidenKPGurbelPAInflammatory changes during the ‘common cold’ are associated with platelet activation and increased reactivity of platelets to agonistsBlood Coagul Fibrinolysis20071871371817982310
- KreutzRPOwensJBreallJAC-reactive protein and fibrin clot strength measured by thrombelastography after coronary stentingBlood Coagul Fibrinolysis201324332132623429252
- ThygesenKAlpertJSWhiteHDJoint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial InfarctionUniversal definition of myocardial infarctionCirculation2007116222634265317951284
- StoneNJRobinsonJGLichtensteinAHAmerican College of Cardiology/American Heart Association Task Force on Practice Guidelines2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesCirculation201412925 suppl 2S1S4524222016
- FaridNASmallDSPayneCDEffect of atorvastatin on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel in healthy subjectsPharmacotherapy200828121483149419025429
- PellicciaFRosanoGMarazziGPharmacodynamic effects of atorvastatin versus rosuvastatin in coronary artery disease patients with normal platelet reactivity while on dual antiplatelet therapy – the PEARL randomized cross-over studyEur J Pharmacol2014725182224444439
- ParkYJeongYHTantryUSAccelerated platelet inhibition by switching from atorvastatin to a non-CYP3A4-metabolized statin in patients with high platelet reactivity (ACCEL-STATIN) studyEur Heart J201233172151216222507978
- KimGBKimJKParkSEffect of atorvastatin and clopidogrel co-administration after coronary stenting in Korean patients with stable anginaKorean Circ J2011411283321359066
- MitsiosJVPapathanasiouAIRodisFIElisafMGoudevenosJATselepisADAtorvastatin does not affect the antiplatelet potency of clopidogrel when it is administered concomitantly for 5 weeks in patients with acute coronary syndromesCirculation20041091335133815023882
- WenaweserPWindeckerSBillingerMEffect of atorvastatin and pravastatin on platelet inhibition by aspirin and clopidogrel treatment in patients with coronary stent thrombosisAm J Cardiol20079935335617261397
- GorchakovaOvon BeckerathNGawazMAntiplatelet effects of a 600 mg loading dose of clopidogrel are not attenuated in patients receiving atorvastatin or simvastatin for at least 4 weeks prior to coronary artery stentingEur Heart J2004251898190215522468
- SternRHGibsonDMWhitfieldLRCimetidine does not alter atorvastatin pharmacokinetics or LDL-cholesterol reductionEur J Clin Pharmacol19985364754789551707
- LeonciniMTosoAMaioliMHigh-dose atorvastatin on the pharmacodynamic effects of double-dose clopidogrel in patients undergoing percutaneous coronary interventions: the ACHIDO (atorvastatin and clopidogrel high dose in stable patients with residual high platelet activity) studyJACC Cardiovasc Interv20136216917923428009
- TektenTCeyhanCErcanEOnbasiliAOTurkogluCThe effect of atorvastatin on platelet function in patients with coronary artery diseaseActa Cardiol200459331131515255464
- TirnaksizEPamukcuBOflazHNisanciYEffect of high dose statin therapy on platelet function; statins reduce aspirin-resistant platelet aggregation in patients with coronary heart diseaseJ Thromb Thrombolysis2009271242817917708
- PellicciaFRosanoGMarazziGPharmacodynamic comparison of pitavastatin versus atorvastatin on platelet reactivity in patients with coronary artery disease treated with dual antiplatelet therapyCirc J201478367968424389597
- OjeifoOWiviottSDAntmanEMConcomitant administration of clopidogrel with statins or calcium-channel blockers: insights from the TRITON-TIMI 38 (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38)JACC Cardiovasc Interv20136121275128124239201
- SawJSteinhublSRBergerPBCREDO InvestigatorsLack of adverse clopidogrel-atorvastatin clinical interaction from secondary analysis of a randomized, placebo-controlled clopidogrel trialCirculation200310892192412925453
