Abstract
Paget’s disease of bone (PDB) is a progressive monostotic or polyostotic metabolic bone disease characterized by focal abnormal bone remodeling, with increased bone resorption and excessive, disorganized, new bone formation. PDB rarely occurs before middle age, and it is the second most frequent metabolic bone disorder after osteoporosis, affecting up to 3% of adults over 55 years of age. One of the most striking and intriguing clinical features is the focal nature of the disorder, in that once the disease is established within a bone, there is only local spread within that bone and no systemic dissemination. Despite many years of intense research, the etiology of PDB has still to be conclusively determined. Based on a detailed review of genetic and viral factors incriminated in PDB, we propose a unifying hypothesis from which we can suggest emerging strategies and therapies. PDB results in weakened bone strength and abnormal bone architecture, leading to pain, deformity or, depending on the bone involved, fracture in the affected bone. The diagnostic assessment includes serum total alkaline phosphatase, total body bone scintigraphy, skull and enlarged view pelvis x-rays, and if needed, additional x-rays. The ideal therapeutic option would eliminate bone pain, normalize serum total alkaline phosphatase with prolonged remission, heal radiographic osteolytic lesions, restore normal lamellar bone, and prevent recurrence and complications. With the development of increasingly potent bisphosphonates, culminating in the introduction of a single intravenous infusion of zoledronic acid 5 mg, these goals of treatment are close to being achieved, together with long-term remission in almost all patients. Based on the recent pathophysiological findings, emerging strategies and therapies are reviewed: ie, pulse treatment with zoledronic acid; denosumab, a fully human monoclonal antibody directed against RANK ligand; tocilizumab, an interleukin-6 receptor inhibitor; odanacatib, a cathepsin K inhibitor; and proteasome and Dickkopf-1 inhibitors.
Introduction
Paget’s disease of bone (PDB) is a progressive monostotic or polyostotic metabolic bone disease characterized by focal abnormal bone remodeling, with increased bone resorption and excessive, disorganized new bone formation.Citation1 The disease is driven primarily by increased osteoclast activity, but intrinsic defects in other cell types in the bone microenvironment may contribute to disease onset and severity.Citation2 One of the most striking and intriguing clinical features is the focal nature of the disorder, in that once the disease is established within a bone, there is only local spread within that bone and no systemic dissemination.Citation3 Further supporting this focal nature, is the clinical observation of PDB transfer from one part of the skeleton to another as a result of autologous bone grafting after three years’ latency.Citation4 While PDB is classically considered to be a focal disease, there is some evidence to suggest that patients have a mild generalized increase in bone turnover as measured by histomorphometry in nonpagetic sites.Citation5
PDB affects both men and women, with a slight predominance in men.Citation1 PDB rarely occurs before middle age and its prevalence increases steadily with age. It is the second most frequent metabolic bone disorder after osteoporosis, affecting up to 3% of adults over 55 years of age,Citation6 with an unchanged prevalence (2.5%) over the last 1000 years,Citation7,Citation8 although it appears to be declining over the last 50 years,Citation9 which is consistent with a major contribution of environmental triggers for PDB. PDB results in weakened bone strength and abnormal bone architecture, in which the collagen fibers assume a haphazard irregular mosaic pattern (woven bone) instead of the parallel symmetry observed in normal (lamellar) bone. PDB is often asymptomatic, but patients can present with pain, deformity or, depending on the bone involved, fracture in the affected bone.Citation10 Approximately 30%–50% of PDB patients experience disabilities due to bone pain, osteoarthritis secondary to deformities adjacent to weight-bearing joints, fractures, or nerve root compression.Citation11,Citation12 Malignant transformation to osteosarcoma occurs in about 0.3% of patients.Citation11
Despite many years of intense research, the etiology of PDB has still to be conclusively determined. A variety of evidence has implicated members of the Paramyxovirus family as causative agents;Citation13–Citation18 UK researchers have previously shown molecular evidence of canine distemper virus in pagetic bone biopsies,Citation15–Citation18 whereas groups in the US have predominantly identified measles virus.Citation19,Citation20 Although controversial, these data may suggest a slow viral infection in pagetic bone.Citation21,Citation22 Further supporting this viral hypothesis are the frequent associations between the development of PDB and contact with domesticated animals or residency in rural areas.Citation23–Citation25 The high prevalence of PDB in Lancashire (England) and in New Zealand may be related to both environmental and genetic factors. The declining prevalence and severity of PDB in the British population also suggests that PDB is at least somewhat regulated by environmental factors,Citation26,Citation27 although it may be partially due to the influx of migrants from low prevalence regions such as the Indian subcontinent and southeast Asia.Citation28 In contrast, the rural region of Campania (Italy) was recently reported to be a high prevalence area for PDB with an increased clinical severity.Citation29,Citation30
Diagnosis
PDB may present with obvious signs or symptoms or it may be an incidental finding during the investigation of other conditions.Citation10 In a recent study, PDB appears to be less severe, with 34% having a monostotic lesion, and an overall average of 5.5 lesions per patient.Citation31 The diagnosis of PDB is primarily radiological and confirmed with plain radiology of at least one skeletal site.Citation10 The radiological features include initial osteolytic changes (V-shaped lesions in long bones and osteoporosis circumscripta in the skull), followed by sclerotic changes, bone enlargement, and cortical thickening. Plain radiographs are also valuable in the diagnosis of secondary complications of PDB, eg, arthritis or fracture. Total body bone scintigraphy is more sensitive than x-rays and, it is recommended (where available) for patients with asymptomatic PDB and for patients with symptomatic PDB to assess the extent of skeletal involvement.Citation32 In contrast with focal assessment of disease by scintigraphy and radiography, biochemical markers of disease activity provide an integrated index, if not of the focal activity of the underlying disorder, then of its extent.Citation33,Citation34 Measurement of serum total alkaline phosphatase is still the most frequently used and most useful biochemical marker for clinical management of PDB.Citation35 Serum bone alkaline phosphatase and procollagen type 1 N-terminal propeptide, as well as urinary N-terminal telopeptide of type 1 collagen and α-C-terminal telopeptide of type 1 collagen have been demonstrated to be similarCitation36 or slightly superiorCitation37,Citation38 to serum total alkaline phosphatase in assessing disease activity and response to therapy in small cohorts of patients. However, monostotic PDB may be associated with levels of serum total alkaline phosphatase within the reference range, introducing difficulties both in diagnosis and follow-up management of patients.Citation39 PDB patients with serum total alkaline phosphatase within the reference range may be discriminated from normal controls by an increased bone alkaline phosphatase isoform B2 measured by high-pressure liquid chromatography.Citation40 In contrast, serum osteocalcin is not a sensitive marker in PDB, being often in the normal range.Citation41,Citation42
In summary, the assessment of PDB includes serum total alkaline phosphatase, total body bone scintigraphy, skull and enlarged view pelvis x-rays (includes pelvis, 1/3 proximal femurs and L3 to L5 vertebrae), and if needed, additional x-rays. This clinical investigation is associated with very high diagnostic sensitivities for PDB, ie, 85%–91% for skull and enlarged view pelvis x-raysCitation43 and 97%–98% for bone scintigraphy.Citation44
Pathogenesis
Genetic factors
Genetic factors play an important role in PDB.Citation45 One-third of patients with PDB have a familial form transmitted in an autosomal dominant pattern of inheritance with incomplete penetrance.Citation46–Citation48 Genetic heterogeneity has been demonstrated in familial forms of PDB, which have been linked to several chromosomal regions.Citation49 A linkage between the 6p21.3 locus (PDB1) and PDB has been suggested, but not confirmed.Citation50 Four PDB families were linked to markers in the 18q22.1 locus (PDB2), a locus also involved in familial expansive osteolysis, a rare bone disease caused by a mutation in the tumor necrosis factor receptor superfamily, member 11a, NFKB activator (RANK, TNFRSF11A) gene.Citation51,Citation52 However, RANK gene mutations are not a common cause of classical late-onset PDB,Citation53,Citation54 although a genetic association to this gene was recently suggested in PDB patients.Citation55 The 5q35-qter locus (PDB3) was identified by our research group in a genome-wide scan of three large French-Canadian families,Citation48 and replicated in British families.Citation56 Taking advantage of the influence of genetic drift and a strong founder effect of the French-Canadian population, we reported in this population the first and still most common germline mutation, P392L, within the SQSTM1 gene,Citation57 and this was later confirmed in the British population.Citation58 The 5q31 locus (PDB4) was also linked to PDB in two French-Canadian families.Citation48 A genome-wide scan in 62 British families suggested the linkage of PDB with two other loci, 2q36 (PDB5) and 10p13 (PDB6).Citation56 Recently, data from this genome-wide scan were reanalyzed and confirmed a linkage to the 10p13 locus, but not to the 2q36 locus.Citation59 The 18q23 locus (PDB7) was suggested in an Australian family,Citation60 but this locus is more likely to contain a modifier gene rather than a causal gene because a SQSTM1 mutation (L394X) was also found in this pedigree.Citation61 Although no linkage of the osteoprotegerin (OPG, TNFRSF11B) locus (8q24) was suggested with PDB, a British study reported a female sex-restricted association of this gene with PDB.Citation62,Citation63 Mutations of the valosin-containing protein (VCP) gene, located at 9p13-p12, were reported in rare families characterized by an autosomal dominant disorder associating PDB with frontotemporal dementia or inclusion body myopathy.Citation64 However, no mutations were found in pagetic patients in the absence of myopathy or dementia, suggesting that the VCP gene was not a common causal gene of PDB.Citation65 Finally, a recently published genome-wide association study in PDB patients, mostly of British descent, identified a significant association between PDB and six common variants, located at the 1p13 (CSF1 gene) and 10p13 (OPTN gene) loci, and, as previously mentioned, at the 18q21 (RANK gene) locus.Citation55 These genetic associations have been strongly replicated in Belgian and Dutch populations, as well as the association of the dendritic cell-specific transmembrane protein (DC-STAMP, TM7SF4) gene, encoding for a protein involved in cell–cell fusion of osteoclasts.Citation66 Among the seven reported loci, the 5q35qter (PDB3) locus is the only one for which a gene has been identified, namely the sequestosome 1 (SQSTM1) gene that encodes the SQSTM1/p62 protein.Citation57 More than 20 missense or truncating germline mutations of this gene have now been reported, although the P392L mutation is the most frequent.Citation67,Citation68 In the French-Canadian population, the P392L recurrent mutation was involved in 46% of familial forms and 16% of unrelated cases of PDB.Citation57 Sequencing of the SQSTM1 gene in unrelated French PDB patients allowed the identification of two novel mutations, A381V and L413F, and for the first time, the presence of double mutations of SQSTM1 was reported in PDB.Citation69 In the American population, 10% of unrelated PDB patients living in the New York City area carried a SQSTM1 mutation, most frequently the P392L mutation, but also the novel S349T, A390V, and L417Q mutations.Citation70 Almost all of the SQSTM1 mutations are recurrent, and reported in different Caucasian populations on average in 40% of familial forms of PDB and 8% of unrelated patients.Citation61,Citation67,Citation69,Citation71
NF-κB signaling pathway
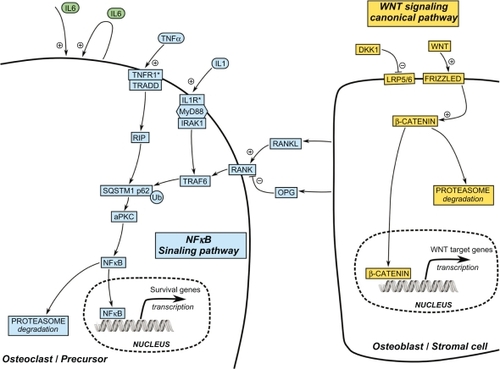
Interestingly, all of the reported SQSTM1 germline mutations result in either missense or truncating mutationsCitation67 enhancing the NF-κB signaling pathway. They are clustered either within or near the C-terminal region of the SQSTM1/p62 protein that embodies the ubiquitin-associated domain. This suggests that an alteration of ubiquitin-chain binding by SQSTM1/p62 is important in the development of PDB,Citation72,Citation73 resulting in an aberrant RANK-NF-κB signaling pathway.Citation74 In osteoclasts, SQSTM1/p62 has been described as a scaffolding protein that interacts with TRAF6 following activation by the RANK ligand ().Citation75 Activation of this complex results mainly in the activation of NF-κB and NFATc1 transcription factors. The overexpression of SQSTM1/p62 in osteoclasts from PDB patients induces major shifts in the pathways activated by the RANK ligand and upregulates osteoclast activity. The P392L mutation may contribute to the overactive state of osteoclasts in PDB,Citation76 and could potentially explain the generalized increase in bone turnover observed in nonpagetic bone sites.Citation5
Ubiquitin-proteasome system, autophagy, and apoptosis
The ubiquitin-proteasome system is involved in the degradation of short-lived, damaged, or misfolded proteins. Target-to-be-degraded proteins are first tagged with ubiquitin then digested by the proteasome.Citation77,Citation78 This system is important for protein degradation and controls various cell functions, including mitosis, signal transduction, gene transcription, immune response, and apoptosis.
Autophagy is another protein degradation system, and includes macroautophagy, microautophagy, and chaperon-mediated autophagy.Citation79,Citation80 Macroautophagy (hereafter termed autophagy) involves the engulfing of a portion of cytoplasm by a double-membrane structure, the autophagosome. The autophagosome fuses with the lysosome, becoming the autolysosome, which undergoes autodigestion.Citation80,Citation81 Autophagy maintains cellular homeostasis and participates in processes including differentiation, remodeling, growth control, cell defense, and adaptation to adverse environments,Citation82 and is involved in eliminating abnormal proteins.Citation83 Loss of autophagy in mice induces inclusion formation in neurons and hepatocytes.Citation84,Citation85
Ubiquitination, through binding of the ubiquitin-associated domain of the p62 protein (encoded by the SQSTM1 gene) to LC3 protein, mediates protein degradation by autophagy and also results in the delivery of p62 itself to autophagosomes for lysosomal degradation.Citation86–Citation88 So far, only one PDB-associated germline missense mutation (D335E) has been shown to affect the LC3-binding region.Citation89 In PDB, autophagy appears to be defective, with impaired p62 clearance that leads to increased levels of p62 regardless of the SQSTM1 mutation status.Citation69,Citation76 p62 not only functions as an adaptor protein that targets substrates to the autophagosome, but also as a scaffold protein interacting with TRAF6 and caspase 8, promoting polyubiquitination of TRAF6 and activation of NF-κB signaling,Citation90,Citation91 as well as the aggregation of cullin-3 modified caspase 8, required for apoptosis, within p62-dependent fociCitation92 which leads to increases in osteoclast survival.Citation76 These increases in osteoclast survival can be induced by artificial overexpression of p62 and appear to be independent of SQSTM1 mutations because they are observed with wild-type and PDB-mutant p62.Citation76 Finally, it is interesting to note that osteoclasts from healthy carriers of germline SQSTM1/p62P392L mutation show an intermediate rate of apoptosis between affected individuals and healthy controls.Citation76 Exploring the precise nature of the potential link between autophagy and PDB has been judiciously proposed as a priority area because autophagy represents a cellular pathway that can be relatively easily manipulated in vivo by pharmacological agents.Citation93
Viral factors
Canine distemper virus
Canine distemper virus can infect and replicate in human osteoclast precursors, raising possible zoonotic implications for canine distemper virus. Canine distemper virus transiently induces osteoclastogenesis in human osteoclast precursor cultures via NF-κB and SQSTM1/p62 activation.Citation94 A variety of other proteins have been shown to be upregulated in PDB, notably Bcl-2,Citation95 leading to an enhanced lifespan of pagetic osteoclasts. Hence, it is possible that the viral effects on ubiquitin and SQSTM1/p62 are only transient, but that the effects on other proteins, such as Bcl-2, lead to an enhanced lifespan of the enlarged osteoclast, with the subsequent recruitment of further precursor cells, thus increasing the size and bone resorbing capacity further.
Measles virus nucleocapsid protein
Osteoclasts in PDB are increased in number and size and express a “pagetic phenotype” that distinguishes them from normal osteoclasts. They contain up to 100 nuclei/osteoclast compared with 3–10 nuclei in normal osteoclasts, their precursors are hyperresponsive to the RANK ligand, tumor necrosis factor alpha, and 1,25(OH)2 vitamin D3,Citation96–Citation98 and form osteoclast at physiologic concentrations of 1,25(OH)2 vitamin D3 (10−11 M) rather than the pharmacologic 1,25(OH)2 vitamin D3 concentrations (10−8 M) required for normal osteoclast formation. The 1,25(OH)2 vitamin D3 hyperresponsivity results from elevated levels of a novel vitamin D receptor coactivator, TAF12 (formerly TAFII-17) in osteoclasts.Citation97 Furthermore, osteoclasts in PDB secrete high levels of interleukin (IL)-6, which are detectable in marrow plasma and peripheral blood from patients with Paget’s disease.Citation99
Both measles virus nucleocapsid (MVNP) and the SQSTM1/p62P392L mutation have been implicated in the pathogenesis of PDB, but their relative contributions are not yet clearly defined. We recently reported that osteoclast from approximately 70% of PDB patients express MVNP, and that normal osteoclast precursors expressing MVNP formed osteoclasts that express the “pagetic phenotype”.Citation100,Citation101 Furthermore, 30% of transgenic mice with targeted expression of MVNP to osteoclasts developed osteoclast and bone lesions characteristic of PDB.Citation102
At least 21 genetic mutations of the SQSTM1/p62 gene are linked to PDB, with p62P392L mutation being the most frequent.Citation57,Citation67,Citation103 However, the role of p62P392L in PDB is unclear because normal osteoclast precursors expressing p62P392L are hyperresponsive to the RANK ligand but not to 1,25(OH)2D3, do not express high levels of IL-6 or TAF12, or form bone lesions or osteoclasts characteristic of PDB.Citation104,Citation105
Therefore, to assess the roles of MVNP and p62P392L in PDB, marrow from clinically involved and uninvolved bones of PDB patients with p62P392L and normals was tested for MVNP, and the effects of antisense MVNP on the osteoclast formed were determined.Citation101 To delineate the mechanism(s) responsible for the abnormal osteoclast activity and bone formation seen with coexpression of MVNP and p62P392L, p62P394L knockin mice (the mouse equivalent of p62P392L) were bred to transgenic mice expressing MVNP in osteoclasts producing the p62P394L knockin/MVNP mice. These mice developed more pagetic osteoclast and pagetic bone lesions than transgenic mice expressing MVNP in osteoclasts.Citation101 The p62P392L gene increased RANK ligand sensitivity of osteoclast precursors while MVNP was responsible for osteoclast hypermultinucleation, increased TAF-12 expression, and IL-6 production through enhanced p38MAPK signaling induced by 1,25(OH)2D3.Citation101 Furthermore, when transgenic mice expressing MVNP in osteoclasts were bred to IL-6 knockout mice, pagetic osteoclast formation no longer occurred.Citation101
In conclusion, studies in mice have demonstrated that the p62P392L mutation leads to some of the phenotypic characteristics of PDB, but this single mutation is seemingly unable to result in the whole PDB phenotype. This mutation may predispose to PDB by increasing the sensitivity of osteoclastic precursors to osteoclastogenic cytokinesCitation104 and/or the osteoclastogenic potential of the bone microenvironment,Citation105 probably in association with other biological mechanisms, such as the presence of MVNP, which is responsible for osteoclast hypermultinucleation, increased TAF-12 expression, and IL-6 production.Citation101,Citation106
SQSTM1/p62, selective autophagy, and measles virus persistence
A unifying hypothesis for SQSTM1/p62, selective autophagy, and measles virus persistence is shown in . It has been recently suggested that successful clearance of viral proteins through p62-mediated selective autophagy may represent an integral component of the normal host antiviral defense response.Citation79 Virus-induced autophagy usually requires viral replicationCitation80 and is then followed by viral persistence. The measles virus is a monotypic virus existing as a single serotype and is among the most infectious viruses.Citation89 Measles virus infections predominantly occur in children, and infection or vaccination with any one strain appears to provide life-long protection from the disease.Citation89 It would be difficult to accept life-long persistence of measles virus RNA or protein in the absence of viral replication and low level gene expression.Citation86 Indeed, intracellular nonreplicating MVNP are inactivated within three days.Citation88 Osteoclasts have a short lifespan (2–3 weeks) and are not self-renewing cells, but are rather formed by fusion of postmitotic precursors of the monocyte-macrophage lineage.Citation90 Immature multipotential hematopoietic progenitors that give rise to granulocytes, erythrocytes, macrophages, and platelets, also express MVNP transcripts, while nonhematopoietic stromal cells do not.Citation87 These cells could easily be the reservoir for measles virus persistence in PDB, although direct evidence is lacking. Toll-like receptors 2 and 4 have been shown to increase susceptibility to measles virus infection,Citation91 suggesting a predisposing role of innate immunity.
Figure 2 Pathogenesis of Paget’s disease of bone: Viral and genetic interactions, unifying hypothesis. Schematic models of cytoplasmic autophagy in A) normal hematopoietic progenitors with adequate clearance of the autolysosome by the proteasome, B) hematopoietic progenitors carrying a germline SQSTM1/p62 mutation leading to defective p62-mediated autophagy, accumulation of p62, further amplifying the process, and p62 aggregates, C) hematopoietic progenitors with persistent measles virus infection and replication leading to impaired autophagy with accumulation of MVNP/p62 aggregates, D) persistent measles virus infection of hematopoietic progenitors carrying a germline SQSTM1/p62 mutation further amplifies the genetically-induced defective p62-mediated autophagy. B–D) These abnormalities in the autophagy process are perpetuated in cells differentiated from the hematopoietic cells with specific functional consequences on mature osteoclasts (see text).
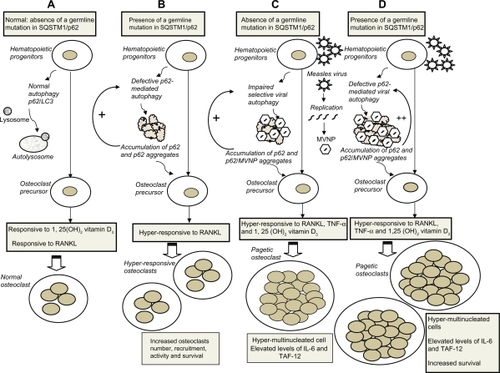
We can then speculate that measles virus persistence could explain the latency between measles virus infection in childhood and PDB development in middle age.Citation86 Measles virus persistence would also explain the presence of MVNP in lifelong immature multipotential hematopoietic progenitors,Citation87 later differentiating into osteoclasts, and would be responsible for osteoclast hypermultinucleation, increased TAF-12 expression, 1,25(OH)2 vitamin D3 responsivity, and IL-6 production.Citation101,Citation106 Defective p62-mediated selective autophagy of MVNP, by germline SQSTM1/p62 mutation or other causes, would lead to accumulation of p62 itself as well as MVNP-p62 aggregates in osteoclasts and antigen-presenting cells, reducing their clearance by the proteasome.Citation79 Mutant p62P392L,Citation76 or any other SQSTM1/p62 mutations associated with PDB and overexpression of native p62, are increasing osteoclast responsiveness to RANK ligandCitation76,Citation104,Citation105 and osteoclast survival,Citation76 which translates clinically into a more severe phenotypic expression of the disease.Citation67
Treatment
Indications for therapy
Guidelines on clinical management of PDB have been published by expert committees from various countries.Citation10,Citation32,Citation107–Citation109 Pain in pagetic bone is the only symptom of PDB for which there is firm evidence that therapy confers a clinical benefit,Citation110 and is, therefore, a definite indication for antipagetic therapy. It is important to distinguish bone pain that occurs as a result of pagetic activity (ie, pain in pagetic bone) from pain in a bone and/or joint deformity that occurs as a consequence of the disease (ie, osteoarthritic pain). The former is usually present at rest, whereas the latter occurs during mobilization of the joint and can, therefore, respond to analgesics, but not to antipagetic drugs.
Pharmacological treatment to prevent future complications, such as osteoarthritis, fracture, hearing loss, or other neurological complications, is more controversial.Citation111 In a 12-year study of 41 patients with PDB, osteoarthritic complications occurred in 62% of patients, in whom serum total alkaline phosphatase levels were halved after therapy compared with 33% of those who had normal serum total alkaline phosphatase following treatment.Citation112 Therefore, a reasonable strategy is to treat pain even when its cause is unclear, because it can often be difficult to distinguish between PDB pain and osteoarthritic pain.Citation10 Both symptomatic and asymptomatic patients with metabolically active PDB requiring therapy include those with involvement of long bones at risk of future bowing deformities, those with extensive skull involvement at risk for future hearing loss, those with pagetic changes in one or more vertebrae with the risk of various neurological complications, and those with PDB in bones adjacent to major joints with the risk of secondary arthritis.Citation32,Citation107 Because current therapies improve radiographic osteolytic lesionsCitation113 and allow normal lamellar bone deposition,Citation114 it is likely that associated complications could be prevented if treatment is administered at an early stage.Citation111,Citation112,Citation115 A recent three-year prospective study known as PRISM (Paget’s Disease: a Randomized Trial of Intensive Versus Symptomatic Management)Citation116 in 1324 patients with long established PDB, concluded that intensive bisphosphonate therapy has no beneficial effect on quality of life, bone pain, or clinical complications (fracture and osteoarthritis) compared with symptomatic management. The negative findings from the PRISM trial could be explained by numerous limitations in the trial design.Citation115,Citation117 Unevenly potent treatment was given late in the disease process, the primary endpoint was inadequate (ideally pain or alternatively fracture in pagetic bone should have been used rather than clinical fracture at any site), and the sample size was too small and the observational period too short to impact on the clinical management of PDB. For preventing complications associated with PDB, initiating pharmacological therapy at the right time (at an early disease stage) is clearly more important than using a highly potent bisphosphonate.
Contraindications to therapy
Elderly asymptomatic patients whose life span would likely limit the chance of future complicationsCitation111 and those with metabolically inactive pagetic lesions (no radiographic osteolytic lesions nor increased uptake on bone scintigraphy) are not candidates for pharmacological therapy. Patients with vitamin D deficiency should be repleted before therapy is initiated to prevent severe hypocalcemia.Citation118
Goals of therapy
Physicians treating PDB should aim for a complete remission, as defined by normalization of serum total alkaline phosphatase and even a nadir value in the lower half of the reference range.Citation119 The ideal therapeutic option would eliminate bone pain, normalize serum total alkaline phosphatase with prolonged remission, heal radiographic osteolytic lesions, restore normal lamellar bone, and prevent recurrence and complications.Citation32
Current pharmacological therapies
The first effective therapy was salmon calcitonin, available in the 1970s as daily subcutaneous formulations, followed by human calcitonin. Calcitonin acts directly on calcitonin receptors expressed on osteoclasts.Citation120 Because calcitonin was associated with partial response, acquired resistance, and a short-lived action, it is not used clinically any more.
Bisphosphonates are a class of drugs related to the naturally occurring mineralization inhibitor, inorganic pyrophosphate.Citation121 In biological systems, they are able to bind to the surface of hydroxyapatite crystals within bone, especially on those surfaces undergoing active osteoclastic resorption. Bisphosphonates work according to one of two main mechanisms of action, depending on the chemical nature of the side chain attached to the basic bisphosphonate core. The relatively weak, non-nitrogen, simple bisphosphonates (ie, etidronate, clodronate, and tiludronate) inhibit bone resorption by generating a toxic analog of adenosine triphosphate, which then targets the mitochondria.Citation122 For the more potent, nitrogen-containing bisphosphonates (ie, alendronate, ibandronate, pamidronate, risedronate, and zoledronic acid), the direct intracellular target in osteoclasts is the farnesyl pyrophosphate synthase enzyme in the mevalonate pathway.Citation122 Its inhibition suppresses a process called protein prenylation, which is essential for the basic cellular processes required for osteoclastic bone resorption and cell survival.
Nitrogen-containing bisphosphonates are the treatment of choice for PDB. The advent of ever more powerful bisphosphonates has led to an aim for a more stringent definition of biochemical response to therapy, ie, a reduction of serum total alkaline phosphatase into the normal range and even a nadir value in the lower half of the reference range.Citation119
Comparative trials have been published evaluating the relative efficacy of the bisphosphonates in the treatment of PDB. These trials typically use extent of suppression of serum total alkaline phosphatase and duration of remission as evidence of superior treatment. provides a summary of the clinical trials assessing the efficacy of bisphosphonates in PDB, as measured by the proportion of patients achieving normalization of serum total alkaline phosphatase.Citation42,Citation123–Citation128 This table reports the results from independent studies without any attempt to compare efficacy between therapies that have not been compared in a head-to-head trial. Although of somewhat differing protocols, these trials demonstrate that alendronate and risedronate are superior to etidronate. In a small comparative study of previously untreated patients, oral alendronate (40 mg/day in three-month blocks) and intravenous pamidronate (60 mg every three months) were given until normalization of serum total alkaline phosphatase, which was observed at one year in 86% and 56% of patients, respectively.Citation129 In previously treated patients, alendronate resulted in normalization of serum total alkaline phosphatase in 79% compared with 14% for pamidronate.Citation129 At one year, nonresponders to pamidronate were crossed over to alendronate treatment, and 71% achieved normalization of serum total alkaline phosphatase.Citation129 In another comparative trial, normalization of serum total alkaline phosphatase was achieved at six months in 93% of patients treated with intravenous zoledronic acid 4 mg and in 35% of patients treated with intravenous pamidronate 60 mg every three months.Citation130 At six months, nonresponders to pamidronate were treated with intravenous zoledronic acid 4 mg or intravenous neridronate 100 mg, and normalization of serum total alkaline phosphatase was achieved in more than 90%.Citation130 A once-weekly alendronate 280 mg oral buffered solution was recently compared with an alendronate 40 mg/day tablet. While both were similarly effective (percentage of patients with serum total alkaline phosphatase normalization not provided), the 40 mg daily tablet was better tolerated.Citation131 Recent comparison of intravenous zoledronic acid 5 mg and oral risedronate 30 mg daily for 60 days in 357 patients after six months showed normalization of serum total alkaline phosphatase in 89% of zoledronic acid-treated patients and 58% of risedronate-treated patients.Citation128
Table 1 Summary of clinical trials assessing bisphosphonate efficacy in Paget’s disease of bone as measured by the proportion of patients with normalization of serum total alkaline phosphatase
In the zoledronic acid group, mean scores for each of the eight components of the SF-36 trended upward at both three and six months, suggesting improvements in quality of life, whereas the responses were more mixed in the rise-dronate group.Citation128 Patients in remission at six months were followed for duration of response and, after two years,Citation132 zoledronic acid 5 mg extended remission in 98% of patients with one single dose, compared with 57% with risedronate, and at 5–6 years these figures were 87% and 38%, respectively.Citation133 Acquired resistance has been commonly observed with etidronate and pamidronate, but not with alendronate, risedronate, or zoledronic acid.Citation134 Upper gastrointestinal intolerance and abdominal pain have been reported as the most frequent adverse events associated with oral bisphosphonates.Citation135 Postinfusion syndrome (a flu-like illness) occurs in about 15% of patients treated with intravenous bisphosphonates (pamidronate, ibandronate, and zoledronic acid), and this almost exclusively at the first infusion.Citation119,Citation128 Oral bisphosphonates should not be used in patients with esophageal stricture or dysmotility. All bisphosphonates should be avoided in patients with renal insufficiency and severe vitamin D deficiency. Osteonecrosis of the jaw and subtrochanteric fractures are very rare events and their pathophysiology remains unclear.Citation135 However, overall, only a very small proportion of patients treated with bisphosphonates experience adverse events and the overall benefits have consistently outweighed their potential risks.
Monitoring and retreatment
Serum total alkaline phosphatase is the most commonly used method of monitoring disease activity.Citation10 It should be measured every three months for the first six months after therapy and every six months thereafter.Citation10 Pretherapeutic serum total alkaline phosphatase is often within the normal range in monostotic disease, and it cannot be used for monitoring.Citation136 Bone scintigraphy (normal uptake) or plain radiographs (filling of osteolytic lesionsCitation113) performed six months after treatment would constitute the ideal monitoring. Retreatment is usually indicated when there are persistent symptoms of PDB or biochemical relapse.Citation10 Although there is no clinical trial evidence to guide clinicians, it is generally accepted that an increase of 25% above nadir indicates significant relapse requiring retreatment.Citation10
Mechanisms of action of bisphosphonates in PDB
Recent in vitro studies suggest that pulse treatment with zoledronic acid, achieving micromolar concentrations (rather than the nanomolar concentrations usually observed in clinical use) similar to what is observed with a single intravenous infusion of 5 mg, causes inhibition of proliferation and induction of apoptosis in human mesenchymal stem cells and enhances differentiation through the osteoblastic lineage.Citation137 Emerging preclinical and clinical evidence suggests that, in addition to their selected inhibition of osteoclastic activity, bisphosphonates exert anticancer activity by interacting with monocytes, macrophages, and tumor cells, and by stimulating the expansion of γδ T cells, a subset of human T cells with antitumor activity.Citation138 Focal high bone turnover lesions like PDB or bone metastases do enrich bisphosphonates in the surrounding bone. Only under those circumstances may it be envisaged that bisphosphonate concentrations in the microenvironment exceed micromolar concentrations for a longer period of time and thus propagate apoptosis of pluripotential hematopoietic progenitors, leading to the long-term remissions observed in PDB after a single intravenous zoledronic acid infusion.Citation133
Potential therapeutic targets
Although bisphosphonates are currently the treatment of choice for the management of PDB, uncertainty about the long-term health consequences of these drugs may now lead to consideration of potential alternative therapies, particularly targeted therapies already designed and used, or about to be used, in clinical practice for the management of other bone disorders.
RANK ligand inhibition
RANK ligand inhibition by the use of a fully human monoclonal antibody (denosumab) induces, in clinical trials, a profound but reversible inhibition of bone resorption. This targeted therapy may be considered for the treatment of OPG/RANK/RANK ligand pathway-mediated diseases, mainly postmenopausal osteoporosis, bone erosion in inflammatory arthritis, and cancer-induced bone disease.Citation139 In PDB, the OPG/RANK/RANK ligand system is usually normal, although enhanced RANK ligand expression and responsivity in bone marrow cells have been reported.Citation98 Moreover, the pathophysiology of several PDB-related diseases involves the OPG/RANK/RANK ligand system, such as mutation in the signal peptide region of the RANK gene in familial expansile osteolysis and a mutation in the OPG gene in juvenile Paget’s disease.Citation140
Interleukin-6 inhibition
Almost 20 years ago, osteotropic factors, such as 1,25(OH)2 vitamin D3, parathyroid hormone, and IL-1, were shown to stimulate osteoblast release of IL-6 which, at low concentrations (<10 ng/mL), stimulates osteoclast formation from precursors, and at higher concentrations, stimulates mature osteoclasts to resorb bone.Citation141 IL-6 plays a central role in the development of the abnormal phenotype of osteoclast in PDB, mainly in the multinucleation and hypersensitivity to 1,25(OH)2 vitamin D3.Citation90 IL-6 was found to be overexpressed in pagetic osteoblasts, and may be involved in both stimulation of osteoclast proliferation and inhibition of osteoblast growth.Citation2 However, a recent study did not find any association of common polymorphisms in IL-6, IL-8, and tumor necrosis factor alpha genes with PDB.Citation142 Tocilizumab, an IL-6 receptor inhibitor, has recently been approved for the treatment of rheumatoid arthritis.Citation143 Although IL-6 plays a key role in causing joint damage in rheumatoid arthritis through possible indirect effects on osteoclastogenesis and bone resorption,Citation144 no clinical trials have been initiated to date in metabolic bone disorders associated with high levels of IL-6.
Dickkopf-1 inhibition
Dickkopf-1 is a natural secreted antagonist of the Wnt/β-catenin signaling interacting with the LRP5/6 coreceptor (). Surprisingly, Dickkopf-1 RNA and protein levels are increased in pagetic osteoblast and stromal cells,Citation2 giving new insights into the role of the osteoblast in PDB. A later independent study reported increased circulating Dickkopf-1 levels in serum from PDB patients,Citation145 and suggested Dickkopf-1 as a potential therapeutic target in PDB.Citation146 Indeed, high levels of Dickkopf-1 have also been reported in multiple myeloma, osteosarcoma, and rheumatoid arthritis, and Dickkopf-1 targeted therapy gave preliminary promising results in multiple myeloma and rheumatoid arthritis.Citation146
Strategies for novel therapeutic target identification
Relevant strategies for the identification of novel therapeutic targets in PDB may rely mostly on the investigation of novel targets developed for the management of other bone disorders and of the results from genetic studies.
Investigation of novels targets developed in other bone disorders
Several metabolic disorders share common pathophysiological features with PDB, such as multiple myeloma, osteoporosis, rheumatoid arthritis-induced bone erosions, and bone metastases of cancer with high affinity for bone, such as prostate and breast cancers. In both PDB and bone metastases, increased osteoclast formation and the increased osteoclastogenic nature of the bone microenvironment are mediated by common factors, namely IL-6 and RANK ligand.Citation147 Available data suggest that, in the case of PDB, there is increased RANK ligand and IL-6 production, and IL-6 enhances responsivity of the osteoclast precursors to RANK ligand, contributing to the elevated numbers of osteoclasts. In patients with multiple myeloma, 95%–100% of whom develop osteolytic bone lesions, both IL-6 and RANK ligand levels are increased.Citation147 We will mainly focus the remaining discussion on therapeutic targets for multiple myeloma and osteoporosis
Bone destruction in multiple myeloma is associated with increased osteoclast formation and activity like in PDB, but with decreased or absent osteoblast differentiation and activity.Citation148 The impairment of osteoblast activity in multiple myeloma results primarily from blockade of osteogenic differentiation of mesenchymal progenitors into mature osteoblasts. Multiple myeloma patients have low to normal levels of bone formation markers, such as alkaline phosphatase and osteocalcin, in the setting of increased bone resorption. In contrast, multiple myeloma patients without bone lesions display balanced bone remodeling with increased osteoclastogenesis and normal or increased bone formation rates. Both soluble factors and cell-to-cell contact between multiple myeloma cells and osteoblast progenitors are responsible for the suppression of osteoblast differentiation in multiple myeloma. Current approaches for the development of target-specific treatment in multiple myeloma concern mainly second-generation proteasome inhibitors, new immunomodulating drugs or thalidomide derivatives, histone deacetylase, and heat shock protein 90 inhibitors.Citation149–Citation151
Other potential targets are represented by inhibitors of Akt and of PI3K/Akt signaling (rapamycin inhibitors), Bcl2 inhibitors and other promoters of apoptotic pathways, MAPK and telomerase inhibitors, to name a few.Citation151 Antibodies have also been designed in multiple myeloma to inhibit IL-6, CD56 (neuronal cell adhesion molecule), CD138 (syndecan-1, a receptor for endothelial growth factor ligands) and Cs1, a cell surface glycoprotein.Citation151 In addition to bisphosphonates, novel therapies are considered for the treatment of bone disease in multiple myeloma, such as denosumab, which specifically inhibits RANK ligand-RANK interaction, bortezomib which is a proteasome antagonist inducing myeloma cell apoptosis, and immunomodulating drugs, which inhibit osteoclast activity by decreasing the expression of cathepsin K.Citation152 Other inhibitors targeting natural antagonists of Wnt signaling, such as Dickkopf-1 and secreted frizzled-related proteins, have been targeted, as well as inhibitors of IL-3 and Il-7.Citation152
Osteoporosis is characterized by a generalized increase in bone resorption, whereas PDB has both focal excesses of bone resorption and many unaffected bones that preserve normal bone remodeling. Both antiresorptive and anabolic agents have being designed as potential novel therapies in osteoporosis. In addition to denosumab, another antiresorptive agent called odanacatib, which is an inhibitor of cathepsin K, is currently being investigated in osteoporosis, as well as glucagon-like peptide 2, an intestinal hormone which may act as an antiresorptive agent with no reduction in bone formation.Citation153 Novel anabolic agents targeting the Wnt signaling pathway designed for future osteoporosis management should be considered with caution, and may probably be contraindicated in PDB, considering the increased risk of osteosarcoma in this disorder.
Results of genetic studies
Gene expression profiling in RNA extracted from various cell types in pagetic patients has revealed that a huge number of genes may be significantly upregulated or downregulated in PDB, providing novel insights for potential future targeted therapies ().Citation2,Citation154,Citation155 Considering difficulties of performing large-scale proteomic studies in bone cells, genome-wide analyses, such as the genome-wide association study recently published in PDB,Citation55 or genome-wide investigations of copy number alterations or epigenetic modifications, may be considered as innovative and promising ways to identify novel targets or novel pathways for potential future therapies in PDB. Indeed, the recently published genome-wide association study reported a strong genetic association with three common polymorphisms located upstream of the CSF1 gene.Citation55 CSF1 gene encodes macrophage colony-stimulating factor, which is a key cytokine secreted by bone marrow stromal cells and osteoblasts, which induces the expression of RANK in osteoclast precursors, further inducing osteoclast differentiation and osteoclast activity and survival regulation.Citation156 Serum levels of macrophage colony-stimulating factor were reported to be significantly increased in PDB patients who were not currently treated, suggesting that serum measurement of macrophage colony-stimulating factor may be an indicator of disease activity.Citation157 Although CS1 antibody, antisense oligonucleotide, and CSF1 small interfering RNA strategies have demonstrated tumor suppression capabilities in several disease (excluding PDB) and model systems,Citation158,Citation159 it is not yet clear enough how specific is their intervention on osteoclast formation, in bone disorders such as PDB, because other cell lineages derived from hematopoetic precursors use similar signaling pathways.Citation160
Table 2 Genes which showed statistically significant differential gene expression in various cell types from patients affected by Paget’s disease of bone
Conclusion
In conclusion, nitrogen-containing bisphosphonates are currently the treatment of choice for PDB, particularly with the last generation and more powerful bisphosphonates, which have led us to aim for a more stringent definition of biochemical response to therapy. Major advances in the understanding of PDB pathophysiology in recent years could give rise to novel alternative treatment, such as targeted therapies, as a medium-term perspective for the management of PDB and other bone metabolic disorders.
Acknowledgements
LM is supported by a career award from the Fonds de la Recherche en Santé du Québec. The authors thank Mr Thomas Pornin for the preparation of .
Disclosure
LM has served as a speaker for Amgen, Merck, and Novartis. JPB has served as a speaker and/or investigator and/or board member for Abbott, Amgen, Eli Lilly, Merck, Novartis, Pfizer, Roche, sanofi-aventis, Servier, and Warner Chilcott.
References
- RoodmanGDWindleJJPaget disease of boneJ Clin Invest2005115220020815690073
- NaotDBavaUMatthewsBDifferential gene expression in cultured osteoblasts and bone marrow stromal cells from patients with Paget’s disease of boneJ Bone Miner Res200722229830917129176
- VellengaCJPauwelsEKBijvoetOLFrijlinkWBScintigraphic aspects of the recurrence of treated Paget’s disease of boneJ Nucl Med19812265105177229723
- HamadoucheMMathieuMTopouchianVde PinieuxGCourpiedJPTransfer of Paget’s disease from one part of the skeleton to another as a result of autogenous bone-grafting: A case reportJ Bone Joint Surg Am.200284-A112056206112429770
- MeunierPJCoindreJMEdouardCMArlotMEBone histomorphometry in Paget’s disease. Quantitative and dynamic analysis of pagetic and nonpagetic bone tissueArthritis Rheum19802310109511037426075
- EekhoffMEvan der KliftMKroonHMPaget’s disease of bone in The Netherlands: A population-based radiological and biochemical survey – the Rotterdam StudyJ Bone Miner Res200419456657015005843
- RogersJJeffreyDRWattIPaget’s disease in archeological populationJ Bone Miner Res20021761127113412054169
- WaldronHARecalculation of secular trends in Paget’s diseaseJ Bone Miner Res.200419352315049284
- Van StaaTPSelbyPLeufkensHGLylesKSprafkaJMCooperCIncidence and natural history of Paget’s disease of bone in England and WalesJ Bone Miner Res200217346547111878305
- SelbyPLDavieMWRalstonSHStoneMDGuidelines on the management of Paget’s disease of boneBone200231336637312231408
- RousiereMMichouLCornelisFOrcelPPaget’s disease of boneBest Pract Res Clin Rheumatol20031761019104115123049
- SetonMMosesAMBodeRKSchwartzCPaget’s disease of bone: The skeletal distribution, complications and quality of life as perceived by patientsBone201148228128520858558
- RebelAMalkaniKBasleM[Nuclear anomalies in osteoclasts in Paget’s bone disease]Nouv Presse Med197432012991301 French.4843079
- RebelAMalkaniKBasleMBregeonCOsteoclast ultrastructure in Paget’s diseaseCalcif Tissue Res19762187199177153
- GordonMTAndersonDCSharpePTCanine distemper virus localised in bone cells of patients with Paget’s diseaseBone19911231952011910961
- GordonMTMeeAPAndersonDCSharpePTCanine distemper virus transcripts sequenced from pagetic boneBone Miner19921921591741345324
- MeeAPDixonJAHoylandJADaviesMSelbyPLMawerEBDetection of canine distemper virus in 100% of Paget’s disease samples by in situ-reverse transcriptase-polymerase chain reactionBone19982321711759701477
- HoylandJADixonJABerryJLDaviesMSelbyPLMeeAPA comparison of in situ hybridisation, reverse transcriptase-polymerase chain reaction (RT-PCR) and in situ-RT-PCR for the detection of canine distemper virus RNA in Paget’s diseaseJ Virol Methods2003109225325912711070
- ReddySVSingerFRRoodmanGDBone marrow mononuclear cells from patients with Paget’s disease contain measles virus nucleocapsid messenger ribonucleic acid that has mutations in a specific region of the sequenceJ Clin Endocrinol Metab1995807210821117608263
- FriedrichsWEReddySVBruderJMSequence analysis of measles virus nucleocapsid transcripts in patients with Paget’s diseaseJ Bone Miner Res200217114515111771661
- CodyJDSingerFRRoodmanGDGenetic linkage of Paget disease of the bone to chromosome 18qAm J Hum Genet1997615111711229345096
- RalstonSHAfzalMAHelfrichMHMulticenter blinded analysis of RT-PCR detection methods for paramyxoviruses in relation to Paget’s disease of boneJ Bone Miner Res200722456957717227218
- MerlottiDGennariLGalliBCharacteristics and familial aggregation of Paget’s disease of bone in ItalyJ Bone Miner Res20052081356136416007333
- SetonMChoiHKHansenMFSebaldtRJCooperCAnalysis of environmental factors in familial versus sporadic Paget’s disease of bone – the New England Registry for Paget’s Disease of BoneJ Bone Miner Res20031881519152412929942
- Lopez-AbenteGMorales-PigaAElena-IbanezARey-ReyJSCorres-GonzalezJCattle, pets, and Paget’s disease of boneEpidemiology1997832472519115018
- CundyTIs the prevalence of Paget’s disease of bone decreasing?J Bone Miner Res.200621Suppl 2P9P1317229016
- CooperCHarveyNCDennisonEMvan StaaTPUpdate on the epidemiology of Paget’s disease of boneJ Bone Miner Res.200621Suppl 2P3P817229005
- RalstonSHLangstonALReidIRPathogenesis and management of Paget’s disease of boneLancet2008372963315516318620951
- GennariLDi StefanoMMerlottiDPrevalence of Paget’s disease of bone in ItalyJ Bone Miner Res200520101845185016160742
- RendinaDGennariLDe FilippoGEvidence for increased clinical severity of familial and sporadic Paget’s disease of bone in Campania, southern ItalyJ Bone Miner Res200621121828183517002563
- HaddawayMJDavieMWMcCallIWHowdleSEffect of age and gender on the number and distribution of sites in Paget’s disease of boneBr J Radiol20078095553253617646188
- JosseRGHanleyDAKendlerDSte-MarieLGAdachiJDBrownJDiagnosis and treatment of Paget’s disease of boneClin Invest Med2007305E210E22317892763
- MeunierPJSalsonCDelmasPD[Skeletal distribution and biological markers of Paget’s disease]Rev Prat1989391311251128 French.2786643
- KanisJABiochemical and endocrine aspects of Paget’s diseaseKanisJAPathophysiology and Treatment of Paget’s Disease of Bone2nd edLondonMartin Dunitz Ltd1998
- LylesKLSirisESSingerFRMeunierPJA clinical approach to diagnosis and management of Paget’s disease of boneJ Bone Miner Res20011681379138711499860
- ReidIRDavidsonJSWattieDComparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of boneBone200435122423015207761
- AlvarezLGuanabensNPerisPUsefulness of biochemical markers of bone turnover in assessing response to the treatment of Paget’s diseaseBone200129544745211704497
- AlexandersenPPerisPGuanabensNNon-isomerized C-telopeptide fragments are highly sensitive markers for monitoring disease activity and treatment efficacy in Paget’s disease of boneJ Bone Miner Res200520458859515765177
- ShankarSHoskingDJBiochemical assessment of Paget’s disease of boneJ Bone Miner Res.200621Suppl 2P22P2717229003
- MagnussonPDavieMWSharpCACirculating and tissue-derived isoforms of bone alkaline phosphatase in Paget’s disease of boneScand J Clin Lab Invest201070212813520175736
- DelmasPDDemiauxBMalavalLChapuyMCMeunierPJSerum bone GLA-protein is not a sensitive marker of bone turnover in Paget’s disease of boneCalcif Tissue Int198638160613079654
- ReidIRDavidsonJSWattieDComparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of boneBone200435122423015207761
- RenierJCFanelloSRodriguezNAudranMCurrent prevalence of Paget’s disease of bone in a region of France (Anjou)Rev Rhum Engl Ed19956295715758574629
- MeunierPJSalsonCMathieuLSkeletal distribution and biochemical parameters of Paget’s diseaseClin Orthop198721737443103964
- DaroszewskaARalstonSHMechanisms of disease: Genetics of Paget’s disease of bone and related disordersNat Clin Pract Rheumatol20062527027716932700
- Morales-PigaAARey-ReyJSCorres-GonzalezJGarcia-SagredoJMLopez-AbenteGFrequency and characteristics of familial aggregation of Paget’s disease of boneJ Bone Miner Res19951046636707610939
- SirisESOttmanRFlasterEKelseyJLFamilial aggregation of Paget’s disease of boneJ Bone Miner Res1991654955002068956
- LaurinNBrownJPLemainqueAPaget disease of bone: Mapping of two loci at 5q35-qter and 5q31Am J Hum Genet200169352854311473345
- MichouLColletCLaplancheJLOrcelPCornelisFGenetics of Paget’s disease of boneJoint Bone Spine200673324324816574459
- FotinoMHaymovitsAFalkCTEvidence for linkage between HLA and Paget’s diseaseTransplant Proc19779418671868146288
- HughesAEShearmanAMWeberJLGenetic linkage of familial expansile osteolysis to chromosome 18qHum Mol Genet1994323593617911698
- KovachMJWaggonerBLealSMClinical delineation and localization to chromosome 9p13.3-p12 of a unique dominant disorder in four families: Hereditary inclusion body myopathy, Paget disease of bone, and frontotemporal dementiaMol Genet Metab200174445847511749051
- SparksABPetersonSNBellCMutation screening of the TNFRSF11A gene encoding receptor activator of NF kappa B (RANK) in familial and sporadic Paget’s disease of bone and osteosarcomaCalcif Tissue Int200168315115511351498
- WuytsWVan WesenbeeckLMorales-PigaAEvaluation of the role of RANK and OPG genes in Paget’s disease of boneBone200128110410711165949
- AlbaghaOMViscontiMRAlonsoNGenome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget’s disease of boneNat Genet201042652052420436471
- HockingLJHerbertCANichollsRKGenomewide search in familial Paget disease of bone shows evidence of genetic heterogeneity with candidate loci on chromosomes 2q36, 10p13, and 5q35Am J Hum Genet20016951055106111555792
- LaurinNBrownJPMorissetteJRaymondVRecurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of boneAm J Hum Genet20027061582152811992264
- HockingLJLucasGJDaroszewskaADomain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s diseaseHum Mol Genet200211222735273912374763
- LucasGJRichesPLHockingLJIdentification of a major locus for Paget’s disease on chromosome 10p13 in families of British descentJ Bone Miner Res2008231586317907922
- GoodDABusfieldFFletcherBHLinkage of Paget disease of bone to a novel region on human chromosome 18q23Am J Hum Genet200270251752511742440
- GoodDABusfieldFFletcherBHIdentification of SQSTM1 mutations in familial Paget’s disease in Australian pedigreesBone200435127728215207768
- DaroszewskaAHockingLJMcGuiganFESusceptibility to Paget’s disease of bone is influenced by a common polymorphic variant of osteoprotegerinJ Bone Miner Res20041991506151115312251
- BeyensGDaroszewskaAde FreitasFIdentification of sex-specific associations between polymorphisms of the osteoprotegerin gene, TNFRSF11B, and Paget’s disease of boneJ Bone Miner Res20072271062107117388729
- WattsGDWymerJKovachMJInclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing proteinNat Genet200436437738115034582
- LucasGJMehtaSGHockingLJEvaluation of the role of valosin-containing protein in the pathogenesis of familial and sporadic Paget’s disease of boneBone200638228028516199218
- ChungPYBeyensGBoonenSThe majority of the genetic risk for Paget’s disease of bone is explained by genetic variants close to the CSF1, OPTN, TM7SF4, and TNFRSF11A genesHum Genet2010128661562620839008
- MorissetteJLaurinNBrownJPSequestosome 1: Mutation frequencies, haplotypes, and phenotypes in familial Paget’s disease of boneJ Bone Miner Res.200621Suppl 2P38P4417229007
- CundyTBollandMPaget disease of boneTrends Endocrinol Metab200819724625318691901
- ColletCMichouLAudranMPaget’s disease of bone in the French population: Novel SQSTM1 mutations, functional analysis, and genotype-phenotype correlationsJ Bone Miner Res200722231031717129171
- MichouLMorissetteJGagnonERNovel SQSTM1 mutations in patients with Paget’s disease of bone in an unrelated multiethnic American populationBone201148345646021073987
- BeyensGVan HulEVan DriesscheKEvaluation of the role of the SQSTM1 gene in sporadic Belgian patients with Paget’s diseaseCalcif Tissue Int200475214415215164150
- CaveyJRRalstonSHSheppardPWLoss of ubiquitin binding is a unifying mechanism by which mutations of SQSTM1 cause Paget’s disease of boneCalcif Tissue Int200678527127716691492
- NajatDGarnerTHagenTCharacterization of a non-UBA domain missense mutation of sequestosome 1 (SQSTM1) in Paget’s disease of boneJ Bone Miner Res200924463264219049332
- LayfieldRThe molecular pathogenesis of Paget disease of boneExpert Rev Mol Med200792711317903332
- DuranASerranoMLeitgesMThe atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesisDev Cell20046230330914960283
- ChamouxECoutureJBissonMMorissetteJBrownJPRouxSThe p62 P392L mutation linked to Paget’s disease induces activation of human osteoclastsMol Endocrinol200923101668168019589897
- KerscherOFelberbaumRHochstrasserMModification of proteins by ubiquitin and ubiquitin-like proteinsAnnu Rev Cell Dev Biol20062215918016753028
- ReinsteinECiechanoverANarrative review. Protein degradation and human diseases: The ubiquitin connectionAnn Intern Med2006145967668417088581
- SumpterRJrLevineBSelective autophagy and virusesAutophagy201173 [Epub ahead of print].
- ShellySLukinovaNBambinaSBermanACherrySAutophagy is an essential component of Drosophila immunity against vesicular stomatitis virusImmunity200930458859819362021
- SeglenPOBohleyPAutophagy and other vacuolar protein degradation mechanismsExperientia19924821581721740188
- CuervoAMAutophagy: In sickness and in healthTrends Cell Biol2004142707715102438
- HaradaMKumemuraHOmaryMBProteasome inhibition induces inclusion bodies associated with intermediate filaments and fragmentation of the Golgi apparatusExp Cell Res20032881606912878159
- KomatsuMWaguriSChibaTLoss of autophagy in the central nervous system causes neurodegeneration in miceNature2006441709588088416625205
- ZatloukalKFrenchSWStumptnerCFrom Mallory to Mallory-Denk bodies: What, how and why?Exp Cell Res2007313102033204917531973
- RimaBKDuprexWPMolecular mechanisms of measles virus persistenceVirus Res2005111213214715893837
- ReddySVMenaaCSingerFRMeasles virus nucleocapsid transcript expression is not restricted to the osteoclast lineage in patients with Paget’s disease of boneExp Hematol199927101528153210517494
- MottetGCurranJRouxLIntracellular stability of nonreplicating paramyxovirus nucleocapsidsVirology19901761172158685
- GriffinDEMeasles virus-induced suppression of immune responsesImmunol Rev201023617618920636817
- EhrlichLARoodmanGDThe role of immune cells and inflammatory cytokines in Paget’s disease and multiple myelomaImmunol Rev200520825226616313353
- MurabayashiNKurita-TaniguchiMAyataMMatsumotoMOguraHSeyaTSusceptibility of human dendritic cells (DCs) to measles virus (MV) depends on their activation stages in conjunction with the level of CDw150: Role of toll stimulators in DC maturation and MV amplificationMicrobes Infect20024878579412270725
- JinZLiYPittiRCullin 3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signalingCell2009137472173519427028
- GoodeALayfieldRRecent advances in understanding the molecular basis of Paget disease of boneJ Clin Pathol201063319920319858527
- SelbyPLDaviesMMeeAPCanine distemper virus induces human osteoclastogenesis through NF-kappaB and sequestosome 1/P62 activationJ Bone Miner Res200621111750175617002577
- BrandwoodCPHoylandJAHillarbyMCApoptotic gene expression in Paget’s disease: A possible role of Bcl-2J Pathol2003201350451214595764
- KukitaAChenuCMcManusLMMundyGRRoodmanGDAtypical multinucleated cells form in long-term marrow cultures from patients with Paget’s diseaseJ Clin Invest1990854128012862318982
- KuriharaNReddySVArakiNRole of TAFII-17, a VDR binding protein, in the increased osteoclast formation in Paget’s diseaseJ Bone Miner Res20041971154116415176999
- MenaaCReddySVKuriharaNEnhanced RANK ligand expression and responsivity of bone marrow cells in Paget’s disease of boneJ Clin Invest2000105121833183810862799
- RoodmanGDKuriharaNOhsakiYInterleukin 6. A potential autocrine/paracrine factor in Paget’s disease of boneJ Clin Invest199289146521729280
- KuriharaNReddySVMenaaCAndersonDRoodmanGDOsteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotypeJ Clin Invest2000105560761410712432
- KuriharaNHirumaYYamanaKContributions of the measles virus nucleocapsid gene and the SQSTM1/p62(P392L) mutation to Paget’s diseaseCell Metab2011131233421195346
- KuriharaNZhouHReddySVExpression of measles virus nucleocapsid protein in osteoclasts induces Paget’s disease-like bone lesions in miceJ Bone Miner Res200621344645516491293
- RalstonSHPathogenesis of Paget’s disease of boneBone200843581982518672105
- KuriharaNHirumaYZhouHMutation of the sequestosome 1 (p62) gene increases osteoclastogenesis but does not induce Paget diseaseJ Clin Invest2007117113314217187080
- HirumaYKuriharaNSublerMAA SQSTM1/p62 mutation linked to Paget’s disease increases the osteoclastogenic potential of the bone microenvironmentHum Mol Genet200817233708371918765443
- RoodmanGDInsights into the pathogenesis of Paget’s diseaseAnn N Y Acad Sci2010119217618020392234
- LylesKWSirisESSingerFRMeunierPJA clinical approach to diagnosis and management of Paget’s disease of boneJ Bone Miner Res20011681379138711499860
- TakataSHashimotoJNakatsukaKGuidelines for diagnosis and management of Paget’s disease of bone in JapanJ Bone Miner Metab200624535936716937267
- AdamiSBartolozziPBrandiML[Italian guidelines for the diagnosis and treatment of Paget’s disease of bone]Reumatismo2007592153168 Italian.17603696
- WermersRATiegsRDAtkinsonEJAchenbachSJMeltonLJ3rdMorbidity and mortality associated with Paget’s disease of bone: A population-based studyJ Bone Miner Res200823681982518269308
- SingerFRPaget disease: When to treat and when not to treatNat Rev Rheumatol20095948348919652650
- MeunierPJVignonETherapeutic strategy in Paget’s disease of boneBone199917Suppl 5489S491S8573424
- BrownJPChinesAAMyersWREusebioRARitter-HrncirikCHayesCWImprovement of pagetic bone lesions with risedronate treatment: A radiologic studyBone200026326326710709999
- BrownJPHoskingDJSte-MarieLRisedronate, a highly effective, short-term oral treatment for Paget’s disease: A dose-response studyCalcif Tissue Int199964293999914313
- BrownJPMetabolic bone diseases: Treating Paget disease: when matters more than howNat Rev Rheumatol200951266366519946296
- LangstonALCampbellMKFraserWDMacLennanGSSelbyPLRalstonSHRandomized trial of intensive bisphosphonate treatment versus symptomatic management in Paget’s disease of boneJ Bone Miner Res2010251203119580457
- ReidIRCundyTBollandMJGreyAResponse to publication of PRISM trialJ Bone Miner Res20102561463146420499373
- WhitsonHELobaughBLylesKWSevere hypocalcemia following bisphosphonate treatment in a patient with Paget’s disease of boneBone200639495495816769264
- ReidIRHoskingDJBisphosphonates in Paget’s diseaseBone992010 [Epub ahead of print]
- ChambersTJMagnusCJCalcitonin alters behaviour of isolated osteoclastsJ Pathol1982136127397057295
- FleischHRussellRGFrancisMDDiphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivoScience1969165899126212645803538
- RogersMJNew insights into the molecular mechanisms of action of bisphosphonatesCurr Pharm Des20039322643265814529538
- MillerPDBrownJPSirisESHoseyniMSAxelrodDWBekkerPJA randomized, double-blind comparison of risedronate and etidronate in the treatment of Paget’s disease of bone. Paget’s Risedronate/Etidronate Study GroupAm J Med1999106551352010335722
- GrayREYatesAJPrestonCJSmithRRussellRGKanisJADuration of effect of oral diphosphonate therapy in Paget’s disease of boneQ J Med1987642457557672966965
- FraserWDStampTCCreekRASawyerJPPicotCA double-blind, multicentre, placebo-controlled study of tiludronate in Paget’s disease of bonePostgrad Med J1997738624965029307742
- AndersonDCRichardsonPCBrownJKIntravenous pamidronate: Evolution of an effective treatment strategySemin Arthritis Rheum19942342732758009254
- SirisEWeinsteinRSAltmanRComparative study of alendronate versus etidronate for the treatment of Paget’s disease of boneJ Clin Endocrinol Metab19968139619678772558
- ReidIRMillerPLylesKComparison of a single infusion of zoledronic acid with risedronate for Paget’s diseaseN Engl J Med2005353989890816135834
- WalshJPWardLCStewartGOA randomized clinical trial comparing oral alendronate and intravenous pamidronate for the treatment of Paget’s disease of boneBone200434474775415050907
- MerlottiDGennariLMartiniGComparison of different intravenous bisphosphonate regimens for Paget’s disease of boneJ Bone Miner Res200722101510151717605632
- HooperMFaustinoAReidIRRandomized, active-controlled study of once-weekly alendronate 280 mg high dose oral buffered solution for treatment of Paget’s diseaseOsteoporos Int200920114115018536953
- HoskingDLylesKBrownJPLong-term control of bone turnover in Paget’s disease with zoledronic acid and risedronateJ Bone Miner Res200722114214817032148
- ReidILylesKWSuGLong-term efficacy of zoledronic acid compared with risedronate in Paget’s diseaseBone201047Suppl 1OC21
- PapapoulosSEEekhoffMEZwindermanAHAcquired resistance to bisphosphonates in Paget’s disease of boneJ Bone Miner Res.200621Suppl 2P88P9117229015
- PazianasMAbrahamsenBSafety of bisphosphonatesBone1122011 [Epub ahead of print]
- HoskingDJPrediction and assessment of the response of Paget’s disease to bisphosphonate treatmentBone199924Suppl 569S71S10321933
- EbertRZeckSKrugRPulse treatment with zoledronic acid causes sustained commitment of bone marrow derived mesenchymal stem cells for osteogenic differentiationBone200944585886419442618
- GreenJClezardinPThe molecular basis of bisphosphonate activity: A preclinical perspectiveSemin Oncol.201037Suppl 1S3S1120682369
- RomasEClinical applications of RANK-ligand inhibitionIntern Med J200939211011619356186
- CrockettJCMellisDJScottDIHelfrichMHNew knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: Focus on the RANK/RANKL axisOsteoporos Int201122112020458572
- RoodmanGDInterleukin-6: An osteotropic factor?J Bone Miner Res1992754754781615755
- Corral-GudinoLdel Pino-MontesJGarcia-AparicioJAlonso-GarridoMGonzalez-SarmientoRPaget’s disease of bone is not associated with common polymorphisms in interleukin-6, interleukin-8 and tumor necrosis factor alpha genesCytokine201052314615020709566
- NishimotoNMiyasakaNYamamotoKKawaiSTakeuchiTAzumaJLong-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): Evidence of safety and efficacy in a 5-year extension studyAnn Rheum Dis200968101580158419019888
- DayerJMChoyETherapeutic targets in rheumatoid arthritis: The interleukin-6 receptorRheumatology (Oxford)2010491152419854855
- MarshallMJEvansSFSharpCAPowellDEMcCarthyHSDavieMWIncreased circulating Dickkopf-1 in Paget’s disease of boneClin Biochem20094210–1196596919389391
- McCarthyHSMarshallMJDickkopf-1 as a potential therapeutic target in Paget’s disease of boneExpert Opin Ther Targets201014222123020055719
- RoodmanGDStudies in Paget’s disease and their relevance to oncologySemin Oncol.2001284Suppl 11152111544571
- RoodmanGDOsteoblast function in myelomaBone201148113514020601285
- ZhouFLMengSZhangWGPeptide-based immunotherapy for multiple myeloma: Current approachesVaccine201028375939594620619381
- LiggettACrawfordLJWalkerBMorrisTCIrvineAEMethods for measuring proteasome activity: Current limitations and future developmentsLeuk Res201034111403140920674016
- Chanan-KhanAABorrelloILeeKPReeceDEDevelopment of target-specific treatments in multiple myelomaBr J Haematol2010151131520618339
- RoodmanGDNovel targets for myeloma bone diseaseExpert Opin Ther Targets200812111377138718851694
- DealCFuture therapeutic targets in osteoporosisCurr Opin Rheumatol200921438038519461517
- MichouLChamouxECoutureJMorissetteJBrownJPRouxSGene expression profile in osteoclasts from patients with Paget’s disease of boneBone201046359860319925894
- NagyZBGergelyPDonathJBorgulyaGCsanadMPoorGGene expression profiling in Paget’s disease of bone: Upregulation of interferon signaling pathways in pagetic monocytes and lymphocytesJ Bone Miner Res200823225325918197754
- BruzzanitiABaronRMolecular regulation of osteoclast activityRev Endocr Metab Disord200671–212313916951988
- NealeSDSchulzeESmithRAthanasouNAThe influence of serum cytokines and growth factors on osteoclast formation in Paget’s diseaseQ J Med2002954233240
- AharinejadSPaulusPSioudMColony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in miceCancer Res200464155378538415289345
- ChengHClarksonPWGaoDPachecoMWangYNielsenTOTherapeutic antibodies targeting CSF1 impede macrophage recruitment in a xenograft model of tenosynovial giant cell tumorSarcoma2010201017452820981142
- VaananenKMechanism of osteoclast mediated bone resorption – rationale for the design of new therapeuticsAdv Drug Deliv Rev200557795997115876398