Abstract
Diabetes mellitus (DM) affects >350 million people worldwide. With many complications that can reduce the patient’s quality of life, vision loss is one of the most debilitating disorders it can cause. Active research in the field of diabetes includes microvascular complications in diabetic retinopathy (DR). Disturbances in the balance of pro-angiogenesis and anti-angiogenesis factors can lead to the progression of DR. The retinal pigment epithelium (RPE) is the outermost layer of the retina, and it is essential in maintaining the visual function. The RPE produces and secretes growth factors as well as protective agents which maintain structural integrity of the retina. Small natural molecules, such as resveratrol, may influence neurotrophic factors of the retina. The pigment epithelium-derived factor (PEDF) and thrombospondin-1 (TSP-1) are secreted by RPE cells. These two proteins inhibit angiogenesis and inflammation in RPE cells. An alteration of their production contributes to various eye diseases. There is a critical balance between two important factors secreted on opposite sides of the RPE: at the basal side, vascular endothelial growth factor (VEGF; acts on the choroidal endothelium) and, on the apical side, PEDF (acts on neurons and photoreceptors). Resveratrol inhibits VEGF expression in human adult RPE cells and limits the development of proliferative vitreoretinopathy, by attenuating transforming growth factor-β2-induced wound closure and cell migration. Possible new mechanisms could include PEDF and TSP-1 expression alterations under physiological and pathological conditions. Resveratrol is currently of interest due to its capacity to influence the cell’s secretory activity. Some limitations arise from its low bioavailability. Several drug delivery systems are currently tested, promising to improve tissue concentrations. This article reviews biological pathways involved in the pathogenesis of DR that could be influenced by resveratrol. A study of these pathways could identify new potential targets for the reduction of diabetic complications.
Introduction
Diabetes mellitus (DM) affected >350 million people worldwide in 2013, and the number is estimated to increase to >590 million people by 2035.Citation1 Without proper treatment, its complications are serious, starting with ketoacidosis, nonketotic hyperosmolar coma, and continuing with serious vessel damage: microangiopathies (retinopathies, neuropathies, or nephropathies) and macroangiopathies (peripheral vascular diseases, ischemic cardiomyopathy, or cerebrovascular accidents). In the long term, these can be an important cause of mortality. One of the leading causes of blindness in adults aged 20–74 years is diabetic retinopathy (DR).Citation2 In DR, progressive damage to the retina occurs when fragile blood vessels break, causing swelling of the retinal tissue and cell loss.
Current treatments are trying to achieve strict glycemic control. A recent European epidemiological study showed that, despite modern protocols, 39% of young adults diagnosed with diabetes in the last 10 years develop DR, and the need for protection from vision loss is greater than ever.Citation3 In 2012, Yau et alCitation4 studied the global prevalence and risk factors of DR. The conclusion of the study was an overall prevalence of 34.6% DR, 7% proliferative DR (PDR), 6.8% diabetic macular edema (DME), and 10.2% vision-threatening DR. DR was present in nearly all diabetic patients after a certain period, with a higher prevalence in type 1 diabetes.Citation4 Once diagnosed, DR was directly linked to insufficient metabolic control; the strongest predicting risk factors for retinopathy are HbA1c and glycated albumin.Citation5 A study performed in 2010 revealed that approximately one third of 285 million people diagnosed with diabetes showed signs of DR, and the number is expected to rise further.Citation6
Standard care for PDR includes panretinal photocoagulation (PRP) and more recently anti-vascular endothelial growth factor (VEGF) injections.Citation7–Citation9 PRP was initiated as a treatment option in the 1960s and was documented as beneficial over time. It is used to improve the retinal circulation, allowing more oxygen to reach the retina and limiting the formation and release of pro-angiogenic cytokines.Citation10 Laser treatment can reduce the rates of vision loss by up to 50% over 3 years.Citation11 Anti-VEGF injections have been recently used to treat PDR and proved to be a promising potential alternative. A multicenter randomized clinical trial comparing ranibizumab versus PRP recently concluded that ranibizumab has similar effects in improving visual acuity at a 2-year follow-up. Ranibizumab proved superiority over PRP when it came to safety concerns. It showed fewer side effects, such as peripheral visual field loss or contrast sensitivity loss. Although intravitreal ranibizumab of 0.5 mg is more expensive than PRP by almost 60%, its reduced adverse effects may support its future use.Citation7
DME affects ~30% of patients with DR and is a major factor leading to vision loss.Citation12 It can occur at any stage of DR, and the risk increases with the severity of the disease: 3% cases with mild nonproliferative diabetic retinopathy (NPDR), 38% cases with moderate-to-severe NPDR, and 71% cases with PDR.Citation13 Intravitreal VEGF inhibitors are currently the standard care for DME. Following the results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study after 6 months, 2 years, and 3 years, ranibizumab has become a frequently used first-line therapy agent for DME.Citation14–Citation16 Intravitreal steroid implants (such as dexamethasone or fluocinolone acetonide) exert their anti-inflammatory action, with minimum side effects, and have a nonspecific anti-VEGF effect. For this reason, they are currently reimbursed by different health care systems in the management of DME.Citation12 There is a lack of consensus on the management of patients with DME who underwent vitrectomy. For this reason, current studies evaluate the outcome of steroid implants, such as fluocinolone acetonide, on visual acuity and patient compliance, suggesting that further research and real-life data collection might support the use in current practice, as well as in earlier DME development stages.Citation17 Refractory cases of DME to ranibizumab or dexamethasone could be switched to aflibercept. Studies comparing the two agents (ranibizumab and aflibercept) offer controversial results. Evidence suggests that the two agents are equal in terms of efficiency, with aflibercept having the advantage of fewer injection numbers required.Citation18 One case–control study on a 69-year-old man with bilateral DME showed a significant improvement with aflibercept over ranibizumab treatment. A plausible explanation might be the additional capacity of aflibercept to target the placental growth factor-1, a member of the VEGF family.Citation19 Given the fact that aflibercept is well tolerated, has a comfortable dosage regimen, and has a complex mechanism of action, it might be considered for DME first-line treatment.Citation20 Future prospective studies need to focus on a long-term follow-up regarding safety and efficacy when switching from one anti-VEGF agent to another.Citation21
With strong level 1 evidence supporting anti-VEGF injections over PRP for the treatment of DME, new data suggest that it may become a key player in the treatment of DR and PDR.Citation22 Anti-VEGF agents may slow down the development of DME in preproliferative eyes, but data are scarce regarding DR progression after anti-VEGF injections are discontinued. Some important issues need more clarification: cost, prospective studies on other VEGF agents, and new sustained-release devices. While PRP remains the standard care for PDR, combination therapy could be a valid alternative: anti-VEGF initially followed by PRP.Citation7
The Early Treatment Diabetic Retinopathy Study classification covers the full range of retinopathies and is the most complex one.Citation11 In 2002, the Global Diabetic Retinopathy Project Group proposed a version designed to be used for population screeningCitation23 ().Citation24
Table 1 Classification of DR severity
In the past few years, natural polyphenols, found in plants, including vegetables and fruits, have gained interest, and medical nutrition therapy is emerging as a tailored therapeutic approach that could reduce the risk of developing complications in chronic diseases such as type 2 diabetes.
The search for new substances to diminish vision loss due to DM includes natural products, with resveratrol emerging as a promising “player.”Citation25 This polyphenolic phytoalexin has beneficial properties according to several in vitro and in vivo studies. Such benefits include antidiabetic activity,Citation26 anti-inflammatory effect,Citation27 anti-neovascularization protection,Citation28 and prevention of DR.Citation29
Biochemical mechanisms involved in DR
Diabetes, in all its forms, is characterized mainly by hyperglycemia. During its course, the development of micro-vascular pathology is likely. shows the four main mechanisms of hyperglycemia-induced damage that are considered responsible for the occurrence of DR: increased polyol pathway flux, increased advanced glycation end-products (AGEs) formation, increased hexosamine pathway flux, and activation of protein kinase C (PKC). Each of these pathways leads to an abnormal function and production of the cells, resulting in early apoptosis, progressive capillary occlusion, extracellular matrix overproduction, and deposition of plasma proteins.Citation30,Citation31 These pathways to DR are associated with an overproduction of reactive oxygen species (ROS), and they are discussed next.
Figure 1 The main mechanisms of hyperglycemia-induced damage considered responsible for the occurrence of diabetic retinopathy: increased polyol pathway flux, increased AGEs formation, increased hexosamine pathway flux, and activation of PKC.
Abbreviations: AGEs, advanced glycation end-products; PKC, protein kinase C; ROS, reactive oxygen species.
Increased polyol pathway flux
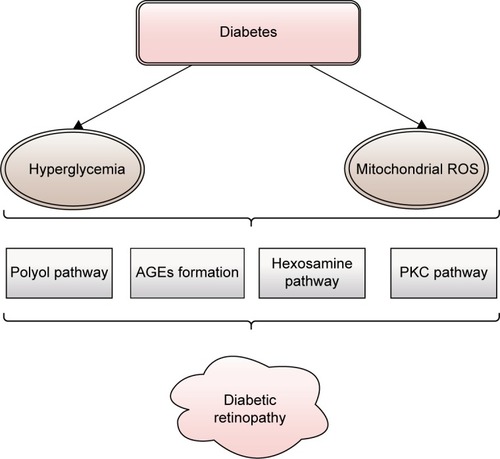
This two-step metabolic pathway is an important contributor to DR. The key enzyme of the polyol pathway is aldose reductase (AR), a cytosolic, rate-limiting, monomeric oxidoreductase. The second enzyme involved is sorbitol dehydrogenase (SDH). Under euglycemic conditions, AR has a low affinity for glucose, but a high capacity of conversion, reducing it to sorbitol (with nicotinamide adenine dinucleotide phosphate – reduced [NADPH] as a cofactor) at a low level. SDH has a high affinity, but a low capacity to oxidize sorbitol to fructose (using nicotinamide adenine dinucleotide – oxidized [NAD+] as a cofactor) independent of its concentration within physiological values (). In diabetic patients, the polyol pathway activity increases in the retina, causing local accumulation of sorbitol and osmotic damage. Other factors that contribute to further damage are a decrease in cytosolic NADPH and an increase in cytosolic nicotinamide adenine dinucleotide – reduced (NADH)/NAD+ under hyperglycemic conditions.Citation31
Figure 2 Schematic presentation of stages of polyol pathway: (I) glucose reduction to sorbitol by AR (using NADPH as a cofactor) followed by (II) sorbitol oxidation to fructose by SDH (using NAD+ as a cofactor).
Abbreviations: AR, aldose reductase; NAD+, nicotinamide adenine dinucleotide – oxidized; NADH, nicotinamide adenine dinucleotide – reduced; NADP+, nicotinamide adenine dinucleotide phosphate – oxidized; NADPH, nicotinamide adenine dinucleotide phosphate – reduced; SDH, sorbitol dehydrogenase.
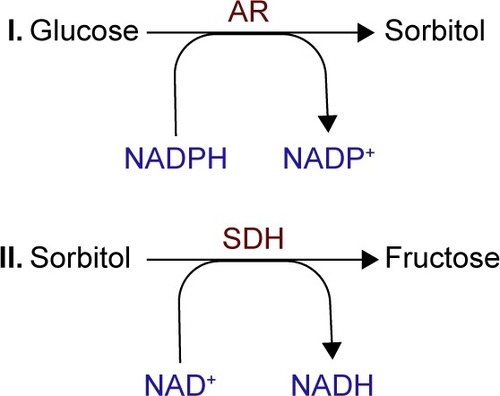
The alteration in the NADH (NADPH)/NAD+ (NADP+) ratios reduces the activity of glutathione reductase, lessening the cell’s ability to respond to ROS accumulation, while the increase in NADH/NAD+ mimics hypoxia in the tissue ().Citation31,Citation32
Figure 3 Polyol pathway changes induced in NADH (NADPH)/NAD+ (NADP+) ratios with the decrease of GR, leading to ROS accumulation and tissue impairments.
Abbreviations: GR, glutathione reductase; NAD+, nicotinamide adenine dinucleotide – oxidized; NADH, nicotinamide adenine dinucleotide – reduced; NADP+, nicotinamide adenine dinucleotide phosphate – oxidized; NADPH, nicotin-amide adenine dinucleotide phosphate – reduced; ROS, reactive oxygen species.
Increased AGEs formation
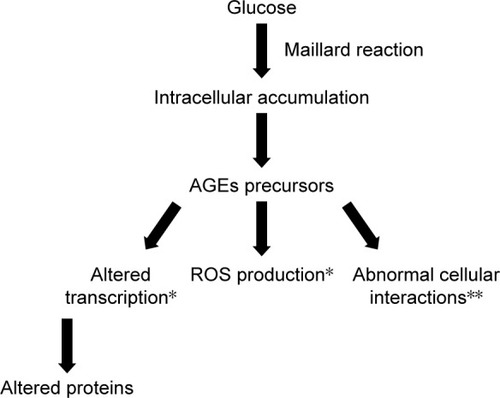
Prolonged hyperglycemia amplifies certain physiological nonenzymatic processes, resulting in the formation of a complex and irreversible group of compounds termed AGEs.Citation33 Glucose derivatives enter a condensation reaction (Maillard reaction) with the amine residues of proteins, nucleic acids, or lipids and follow a series of chemical rearrangements, leading to structural and functional degradation that in turn leads to mural pericyte loss and vascular lesions.Citation33 Markers of AGEs formation and accumulation are found in high concentrations in diabetic patients in retinal vessels, in vitreous tissues, and in other tissues affected by hyperglycemia.Citation34,Citation35 There are three general mechanisms through which AGEs target cells: modification of intracellular proteins resulting in an altered function, modification of matrix components resulting in an abnormal interaction between each other and with cellular protein receptors (integrins), and modification of plasma proteins, which bind to specific AGEs receptors (receptor for AGE [RAGE]) inducing an increased production of ROS ().Citation30 RAGE ligation activates transcription factor nuclear factor-κB (NF-κB), which in turn induces abnormal gene expression, as well as NADPH oxidase that contributes to ROS formation and pericyte apoptosis.Citation36–Citation38
Figure 4 Schematic presentation of AGEs formation and the pathways through which AGEs target cells: altered function of intracellular proteins; abnormal interaction between matrix components and protein receptors (integrins); increased production of ROS due to abnormal interaction between plasma proteins and specific AGE receptors.
Abbreviations: AGEs, advanced glycation end-products; ROS, reactive oxygen species.
The polyol and the increased AGEs formation pathways intertwine. The fructose produced through the polyol pathway undergoes further transformations, generating glycation agents that can produce AGEs.Citation39 These factors increase VEGF gene transcription, mediating capillaries hyperpermeability and intercellular adhesion molecule (ICAM) production and influencing retinal capillary leukocyte adherence.Citation40
Increased hexosamine pathway flux
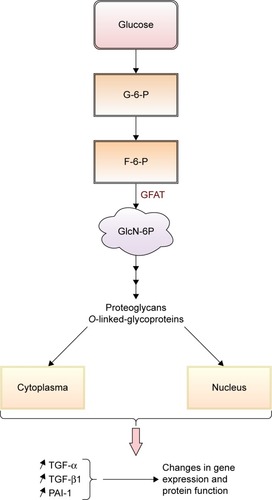
This hyperglycemia-induced pathway impacts the development of diabetic complications. In its glycolytic pathway, glucose is normally converted to glucose-6-phosphate and then isomerized to fructose-6-phosphate (F-6-P). In hyper-glycemic conditions, F-6-P is diverted from glycolysis to N-acetylglucosamine-6-phosphate by glutamine–fructose-6 -phosphate aminotransferase (GFAT). The conversion continues with end-products such as proteoglycans and O-linked glycoproteins (cytoplasmic and nuclear proteins).Citation41 Inhibition of GFAT blocks the increased transcription of transforming growth factor-alpha, transforming growth factor-beta 1 (TGF-β1), inducers of cell proliferation and differentiation, and plasminogen activator inhibitor-1 (PAI-1), involved in cell differentiation, growth, and apoptosis.Citation31,Citation41 Through the hexosamine pathway, hyperglycemia induces changes in gene expression and protein function, that contribute to the pathogenesis of DR ().
Figure 5 Schematic presentation of the contribution of increased hexosamine pathway flux in pathogenesis of DR.
Abbreviations: DR, diabetic retinopathy; F-6-P, fructose-6-phosphate; G-6-P, glucose-6-phosphate; GFAT, glutamine–fructose-6-phosphate aminotransferase; GlcN-6P, N-acetylglucosamine-6-phosphate; PAI-1, plasminogen activator inhibitor-1; TGF-α, transforming growth factor-alpha; TGF-β1, transforming growth factor-beta 1.
Activation of PKC
PKC is a family of multifunctional serine/threonine kinases divided into three groups: classical (PKC-α, PKC-β1, PKC-β2, and PKC-γ), novel (PKC-δ, PKC-ε, PKC-η/l, and PKC-θ), and atypical (PKCζ and PKCλ/ι).Citation42 Studies showed that intracellular hyperglycemia increases de novo diacylglycerol synthesis, a lipid second messenger that activates the classical PKC isoforms.Citation43 Hyperglycemia can also activate PKC isoforms through the ligation of AGEs receptorsCitation44 and increase flux through the polyol pathway.Citation45 Oxidants such as H2O2 can activate PKCs through a mechanism unrelated to lipid second messengersCitation46 through mitochondrial super-oxide induced by hyperglycemia.Citation47 PKC activation alters the bioavailability of nitric oxide, affects VEGF expression (by decreasing the expression of prostacyclin and increasing the expression of thromboxane),Citation48 and directly increases albumin and other macromolecules permeability through barriers formed by endothelial cells.Citation49,Citation50 It has also been linked to mitogen-activated protein kinase activation, a factor that increases gene expression ().Citation43,Citation51
Figure 6 Schematic presentation of the contribution of PKC activation to the development of diabetic retinopathy.
Abbreviations: DAG, diacylglycerol; MAPK, mitogen-activated protein kinase; NO, nitric oxide; PKC, protein kinase C; VEGF, vascular endothelial growth factors.
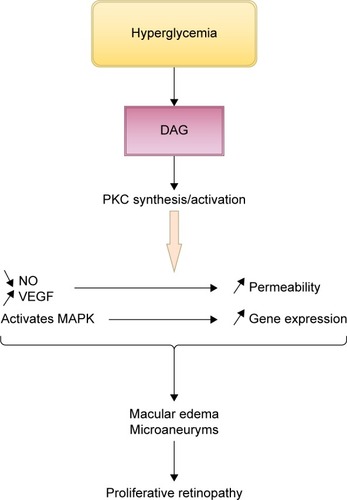
In previous studies, it has been stated that PKC activation is responsible for major changes resulting in diabetes complications: vascular permeability, increased blood flow, extracellular matrix protein accumulation, membrane hypertrophy, leukocyte adhesion, angiogenesis, and apoptosis.Citation52–Citation54
Hyperglycemia-induced pathological changes in the retina
These four biochemical pathways linked to an altered glucose metabolism disturb the balance of pro-angiogenesis and antiangiogenesis factors involved in the progression of DR. An increase in the polyol pathway flux correlates with tissue hypoxia, which in turn triggers VEGF production.Citation55 A recent in vitro study also showed an increase in VEGF-C secretion by AGEs, followed by the increased effect of VEGF-A, underlining the role of AGEs in the onset of retinal neovascularization.Citation56
The hexosamine pathway increases TGF-β1 synthesis through the GFAT enzyme. Inhibition of this pathway lowers TGF-β1 synthesis and bioactivity associated with the development of DR.Citation57,Citation58 Overactivity of PKC also alters VEGF production causing retinal vascular dysfunctions, while in vivo studies showed an association between platelet-derived growth factor (PDGF) resistance and the increase of retinal PKC-δ in diabetic mice. Inhibition of the survival signaling pathway of PDGF causes retinal cell apoptosis.Citation54
For this reason, new pharmacological treatments are based on understanding the molecular mechanisms that occur and the factors involved in the process. The retinal pigment epithelium (RPE) is the outermost layer of the retina, and it has several functions, making it essential in assuring our visual function: transporting nutrients and metabolism products through the blood–retinal barrier, light absorption, secretion, and immunity. This monolayer of pigmented cells is well documented in producing and secreting certain types of growth factors, as well as protective agents, which maintain structural integrity. RPE cells secrete proteins at two opposite poles: at the apical cell side or at the basal side.Citation59 The angiogenic/antiangiogenic factor ratio balances the cellular redox status (or cellular oxidative status), with >55 differentially secreted proteins in hyperglycemic conditions.Citation60 lists some of them.
Table 2 Proteins secreted from RPE cells
New potential targets
Research in the field of DR has focused mainly on pro- angiogenic compounds, while protective factors have received less attention. In healthy adults, endogenous antiangiogenic factors, such as thrombospondin-1 (TSP-1), pigment epithelium-derived factor (PEDF), and angiostatin,Citation61–Citation63 maintain the ocular vasculature under control. These proteins modulate cellular proliferation, migration, differentiation, and apoptosis and influence the oxidative state and phagocytic activity of RPE cells.
TSP-1 induced angiogenesis in vivo at low concentrations,Citation64,Citation65 whereas in higher concentrations, inhibition occurred. This homotrimeric matricellular glycoprotein is produced by various cell types. At retinal level, it supports RPE cell structure and inhibits vascular endothelial cell adhesion.Citation66 TSP-1 production in the eye is dramatically reduced in diabetes.Citation75
PEDF is found in the human eye from early embryonic stages,Citation58 and different studies confirm its presence in RPE cells.Citation67–Citation69 This homotrimeric matricellular glycoprotein proved to be a more potent inhibitor of endothelial cell migration caused by angiogenic inducers compared with TSP-1 and angiostatin;Citation70 one of its mechanisms includes the inhibition of NADPH oxidase activity induced by AGEs.Citation71,Citation72
Sheibani et al demonstrated that high glucose levels affect TSP-1 production by endothelial cells in vitro with the appearance of retinal vasculopathies.Citation73 An in vivo study performed on Akita/+ male mice deficient in TSP-1 demonstrated an increase in pathological vascular changes; in addition, Akita/+ PEDF-deficient mice also showed an increase in acellular capillaries.Citation74 Other previous studies measured the levels of TSP-1 and PEDF from vitreous samples of diabetic and nondiabetic patients. The diabetic group had lower levels of TSP-1 and a higher molecular weight PEDF isoform.Citation75
PEDF has been showed to inhibit the development of DR, proving to be a good candidate for the treatment of DR. However, practical pharmaceutical applications are limited due to its structure. Finding a natural compound which could modulate its expression might be a solution to rebalance the oxidant status of the cell.
Identification of new mechanisms of action for natural antiangiogenic compounds is very important and the normal step to take in case of resistance to long-term use of angiogenic inhibitors.
Natural therapeutic agents
It is important, when considering a new therapeutic strategy, to target a mechanism independent of VEGF since present anti-VEGF drugs have shown similar efficacy and only an independent mechanism of action could improve outcomes, rather than an additional suppression of this pathway.
Resveratrol, or 3,5,4′-trihydroxystilbene, is a phytoalexin produced by plants as a response to stress, fungal infections, injuries, or ultraviolet radiation. This polyphenol belongs to the class of stilbenes and has been isolated for the first time from Veratrum grandiflorum. It has made the subject of >50,000 scientific articles over the past decade only, and its ability to target intracellular molecules and processes is being intensively studied. Polygonum cuspidatum, Vitis vinifera, different Vaccinium species, and Arachis hypogaea are among the known sources of resveratrol.Citation76
Resveratrol modulates various pathways, including PKC,Citation77 rebalances the cellular oxidative status,Citation78,Citation79 and is proved to be a powerful activator of sirtuin 1 (SIRT-1), an angiogenesis key regulator.Citation80 In vitro and in vivo studies showed multiple mechanisms of action of resveratrol, with a preponderant targeting of angiogenic factors: it reduces VEGF accumulation via the activation of AMPK,Citation81 suppresses TGF-β2-induced cell migration in ARPE-19 cells,Citation82 and inhibits PDGF-induced RPE cell migration,Citation83 slowing the process of degradation of the blood–retina barrier. A double-blind, randomized, placebo-controlled study on 44 healthy subjects revealed that a daily intake of 400 mg of resveratrol for a month reduced endothelial activation and vascular inflammation quantified by the reduction in the expression of interleukin-8 and cell adhesion molecules (ICAM-1 and vascular cell adhesion molecule-1).Citation84 In addition, daily administration of 8 mg of resveratrol for 6 months to patients undergoing primary cardiovascular disease prevention, followed by an increase to 16 mg of resveratrol for another 6 months, significantly reduced the levels of C-reactive protein, tumor necrosis factor-α, and PAI-1, improving the inflammatory status.Citation85
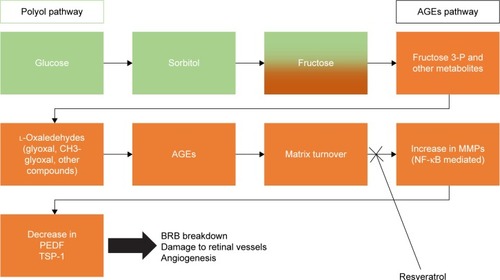
Published literature documents the inhibitory effect of resveratrol on pro-angiogenic protein molecules. Research on the influence of resveratrol on antiangiogenic cytokines is scarce. One study was performed on human retinal pigment epithelial cells and failed to reveal a positive influence on PEDF secretion (enzyme-linked immunosorbent assay).Citation86 Another study explored SIRT-1 activation in Müller glial cells and found that resveratrol enhances antiangiogenic factors by increasing gene expression of PEDF and TSP-1 by 1.4-fold and 1.5-fold, respectively. Respiratory syncytial virus upregulation of PEDF and TSP-1 may have anti-choroidal neovascularization properties.Citation87 A high-glucose environment decreases SIRT-1 activity and promotes matrix metalloproteinase-9 (MMP-9) activation through NF-κB pathway.Citation88 MMPs contribute to the development of tissue injury and inflammation at various sites, including the retina.Citation89 A study using a mouse model of retinopathy investigated MMP effects on PEDF proteolysis and found that it abolishes PEDF protective activity and retinal survival.Citation90 Knowing the neuroprotective role of resveratrol against ischemia, its interaction with MMPs was studied, and it revealed an inhibitory activity by interaction with MMP active site residues.Citation91 A possible pathway to investigate could be the enhancement of PEDF and TSP-1 by resveratrol through the inhibition of MMPs, bearing in mind that PEDF expression increases TSP-1 productionCitation92 and promotes retinal survival. illustrates possible mechanisms of action for resveratrol in the development of DR.
Figure 7 Schematic presentation of a possible mechanism of action for resveratrol in the development of diabetic retinopathy.
Delivery systems overview on oral pharmaceutical forms
Resveratrol is a chemically instable compound with poor water solubility, being well absorbed (~75%), but with a low bioavailability (<1%),Citation93 which limits its activity and biological effects. Following oral administration, the biological half-life is short (8–14 minutes), and in vitro evidence revealed undetected levels in targeted tissues.Citation94
Short-term studies reported minor adverse effects, most of them being gastrointestinal side effects.Citation95 Still, the large number of in vitro effects of free resveratrol remains controversial, since it is rapidly metabolized, achieving a negligible concentration in the bloodstream.
Lately, numerous formulations have been investigated to increase the solubility and the bioavailability of resveratrol or to obtain targeted/prolonged delivery.Citation96–Citation98 Those formulations contain different forms of resveratrol varying from pro-drug administration to nanodelivery systems.Citation94 Among the large variety of nanoformulations reported, liposomal preparations are biocompatible because the addition of organic solvents such as DMSO in the preparation step is excluded.Citation100 Encapsulation of the active substance in liposomes prevents the inactivation through cis–trans isomerization and leads to an improved chemical stability of resveratrol.Citation100,Citation102 Several studies showed a targeted action for liposomes, resulting in an enhanced delivery with superior efficacy compared with free compounds.Citation100,Citation103,Citation104
Moreover, combined delivery systems such as liposomes loaded with resveratrol encapsulated in alginate, alginate–sucrose, and alginate–chitosan microbeadsCitation98 and liposomes loaded with resveratrol–cyclodextrin inclusion complexesCitation100 were reported, as having an improved drug release profile. Nanoemulsions have been developed to enhance the solubility and bioavailability of lipophilic compounds or to protect the antioxidant properties of resveratrol.Citation101,Citation112,Citation113 According to Sessa et al, nanoemulsions sustain the controlled release of the encapsulated resveratrol,Citation101 along with the increase of its half-life.Citation114 Recently, self-emulsifying drug delivery systems have been documented to increase the solubility and bioavailability of resveratrolCitation96 and to enhance the intestinal permeation.Citation115
Other carriers, such as nanoparticles, were extensively studied, and different systems for the delivery of resveratrol have been designed. Solid nanoparticles were prepared using protein molecules such as zein,Citation99 gliadin,Citation105 β-lactoglobulin,Citation106 gelatin,Citation110 or other biodegradable polymers such as poly(dl-lactide-co-glycolide)Citation107 and polyethylene glycol–polylactic acid.Citation108,Citation109 Encapsulation of the drug in nanoparticles protects it against degradation, increases bioavailability, and ensures sustained release. Solid lipid nanoparticles and nanostructured lipid carriers have been proposed as alternative carriers for targeted delivery of resveratrol, showing advantages such as stability and high entrapment efficiency over classical nanoemulsions and liposomes.Citation97,Citation111
To date, a large number of nanodelivery systems have been studied, but more research is needed to transfer in vivo all potential beneficial effects of resveratrol. An ideal delivery system for resveratrol should increase solubility, stability, and bioavailability, should prolong release with targeted delivery to improve its therapeutic efficiency, and should reduce the doses administered.
Summary and future directions
There is an increasing interest toward natural compounds, especially those presenting antioxidant, anti-inflammatory, or immunomodulatory properties. Resveratrol is of high interest for its many mechanisms of action that can provide researchers with new data in molecular biology. DR is a priority eye disease on the VISION 2020 list, for prevention and treatment. Despite laser photocoagulation as the standard care, intravitreal drugs as alternative options, and vitreoretinal surgery for severe cases of DR, this vision-threatening condition remains a continuous clinical challenge.
This review aimed at underlining the complexity of the pathogenesis of diabetic microvascular complications, with four main mechanisms being directly responsible for the oxidant/antioxidant cellular imbalance and tissue damage. Each pathway contains intermediate products produced through other reactions involved in this pathological process. The retinal secretome is sensitive to glycemic fluctuations, and because of the accumulation of deleterious and highly reactive products, proteins secreted in the retina are overproduced or inhibited depending on their overall actions.
The need for targeting new mechanisms in the development of DR is more evident than ever. The retina produces pro-angiogenic factors as well as protective molecules in its attempt to balance the cellular media and to assure the tissue survival. This review underlines the importance of further studying natural protective agents and how they can influence or even rise the levels of “good” proteins in specific tissues that can protect our eyes from diabetic complications.
Acknowledgments
This research was financially supported by the University of Medicine and Pharmacy, Cluj-Napoca, Romania (Grant number 7690/73/15.04.2016). The work of Rugină Dumitriţa was supported by Executive Agency for Higher Education, Research, Development and Innovation Funding (PN-II-RU-TE-2014-4-1135, 14/01.10.2015). The authors thank Royal College of Ophthalmologists for allowing the use of data from Diabetic Retinopathy Guidelines.
Disclosure
The authors report no conflicts of interest in this work.
References
- KharroubiATDarwishHMDiabetes mellitus: the epidemic of the centuryWorld J Diabetes20156685086726131326
- CheungNMitchellPWongTYDiabetic retinopathyLancet2010376973512413620580421
- HenricssonMNyströmLBlohméGThe incidence of retinopathy 10 years after diagnosis in young adult people with diabetes. Results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS)Diabetes Care200326234935412547861
- YauJWRogersSLKawasakiRGlobal prevalence and major risk factors of diabetic retinopathyDiabetes Care201235355656422301125
- NathanDMMcGeePSteffesMWLachinJMDCCT/EDIC Research GroupRelationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC studyDiabetes201463128229023990364
- LeeRWongTYSabanayagamCEpidemiology of diabetic retinopathy, diabetic macular edema and related vision lossEye Vis (Lond)201521726605370
- LiXZarbinMABhagatNAnti-vascular endothelial growth factor injections: the new standard of care in proliferative diabetic retinopathy?Dev Ophthalmol20176013114228427072
- BlinderKJDugelPUChenSAnti-VEGF treatment of diabetic macular edema in clinical practice: effectiveness and patterns of use (ECHO Study Report 1)Clin Ophthalmol20171139340128260851
- StittAWLoisNMedinaRJAdamsonPCurtisTMAdvances in our understanding of diabetic retinopathyClin Sci (Lond)2013125111723485060
- KinshuckDProliferative retinopathy Available from: http://www.diabeticretinopathy.org.uk/proliferative.html#lAccessed January 14, 2018
- Early Treatment Diabetic Retinopathy Study Research GroupETDRS Report No. 7: Early Treatment Diabetic Retinopathy Study design and baseline patient characteristicsOphthalmology1991987417562062510
- DugelPUHillenkampJSivaprasadSBaseline visual acuity strongly predicts visual acuity gain in patients with diabetic macular edema following anti-vascular endothelial growth factor treatment across trialsClin Ophthalmol2016101103111027366049
- Diabetic Retinopathy with Diabetic Macular Edema Available from: https://www.genentech-forum.com/content/dam/gene/managedcare/forum/pdfs/Disease-Information/diabetic-retinopathy-with-diabetic-macular-edema-disease-state-overview.pdfAccessed January 14, 2018
- NguyenQDShahSMHeierJSPrimary end point (six months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) studyOphthalmology2009116112175.e12781.e119700194
- NguyenQDShahSMKhwajaAATwo-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) studyOphthalmology2010117112146215120855114
- DoDVNguyenQDKhwajaAARanibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatmentJAMA Ophthalmol2013131213914523544200
- MeirelesAGoldsmithCEl-GhrablyIEfficacy of 0.2 μg/day fluocinolone acetonide implant (ILUVIEN) in eyes with diabetic macular edema and prior vitrectomyEye (Lond)201731568469028085139
- FoudaSMBahgatAMIntravitreal aflibercept versus intravitreal ranibizumab for the treatment of diabetic macular edemaClin Ophthalmol20171156757128356711
- VandekerckhoveKRAflibercept versus ranibizumab for treating persistent diabetic macular oedemaInt Ophthalmol201535460360925989873
- KorobelnikJFWolfSAn overview of intravitreal aflibercept in diabetic macular oedemaEur Ophthal Rev2015913741
- HerbautAFajnkuchenFQu-KnafoLNghiem-BuffetSBodaghiBGiocanti-AureganASwitching to aflibercept in diabetic macular edema not responding to ranibizumab and/or intravitreal dexamethasone implantJ Ophthal20172017803501328900543
- StewartMWA review of ranibizumab for the treatment of diabetic retinopathyOphtalmol Ther2017613347
- WilkinsonCPFerrisFLKleinREProposed international clinical diabetic retinopathy and diabetic macular edema disease severity scalesOphthalmology200311091677168213129861
- The Royal College of OphthalmologistsDiabetic retinopathy guidelines Available from: https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2013-SCI-301-FINAL-DR-GUIDELINES-DEC-2012-updated-July-2013.pdfAccessed May 13, 2017
- NanjanMJBetzJResveratrol for the management of diabetes and its downstream pathologiesEur Endocrinol2014101313529872461
- SharmaSMisraCSArumugamSAntidiabetic activity of resveratrol, a known SIRT1 activator in a genetic model for type-2 diabetesPhytother Res2011251677320623590
- KubotaSOzawaYKuriharaTRoles of AMP-activated protein kinase in diabetes-induced retinal inflammationInvest Ophthalmol Vis Sci201152129142914822058332
- HuaJGuerinKIChenJResveratrol Inhibits Pathologic Retinal Neovascularization in Vldlr−/− miceInvest Ophthalmol Vis Sci20115252809281621282584
- SoufiFGMohammad-NejadDAhmadiehHResveratrol improves diabetic retinopathy possibly through oxidative stress – nuclear factor κB – apoptosis pathwayPharmacol Rep20126461505151423406761
- BrownleeMBiochemistry and molecular cell biology of diabetic complicationsNature2001414686581382011742414
- SafiSZQvistRKumarSBatumalaieKIsmailISMolecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targetsBiomed Res Int2014201480126925105142
- FreyTAntonettiDAAlterations to the blood–retinal barrier in diabetes: cytokines and reactive oxygen speciesAntioxid Redox Signal20111551271128421294655
- SharmaYSaxenaSMishraASaxenaANatuSMAdvanced glycation end products and diabetic retinopathyJ Ocul Biol Dis Infor201253–4636924596941
- GohSYCooperMEClinical review: the role of advanced glycation end products in progression and complications of diabetesJ Clin Endocrinol Metab20089341143115218182449
- StittAWAdvanced glycation: an important pathological event in diabetic and age related ocular diseaseBr J Ophthalmol200185674675311371498
- IbrahimASEl-RemessyABMatragoonSRetinal microglial activation and inflammation induced by amadoriglycated albumin in a rat model of diabetesDiabetes20116041122113321317295
- YamagishiSMatsuiTNakamuraKUedaSNodaYImaizumiTPigment epithelium-derived factor (PEDF): its potential therapeutic implication in diabetic vascular complicationsCurr Drug Targets20089111025102918991613
- YamagishiSMatsuiTAdvanced glycation end products (AGEs), oxidative stress and diabetic retinopathyCurr Pharm Biotechnol201112336236820939798
- MathebulaSDPolyol pathway: a possible mechanism of diabetes complications in the eyeAfr Vision Eye Health201574115
- StittAWAGEs and diabetic retinopathyInvest Ophthalmol Vis Sci201051104867487420876889
- GiaccoFBrownleeMOxidative stress and diabetic complicationsCirc Res201010791058107021030723
- Protein kinase C signaling interactive pathway Available from: https://www.cellsignal.com/contents/science-cst-pathways-ca-camp-and-lipid-signaling/protein-kinase-c-signaling-interactive-pathway/pathways-kinase-cAccessed July 20, 2017
- GeraldesPKingGLActivation of protein kinase C isoforms & its impact on diabetic complicationsCirc Res201010681319133120431074
- PortillaDDaiGPetersJMGonzalezFJCrewMDProiaADEtomoxir-induced PPARalpha-modulated enzymes protect during acute renal failureAm J Physiol Renal Physiol20002784F667F67510751229
- KamiyaHNakamuraJHamadaYPolyol pathway and protein kinase C activity of rat Schwannoma cellsDiabetes Metab Res Rev200319213113912673781
- LiMLinYFPalchikGAMatsunagaSWangDChenBPThe catalytic subunit of DNA-dependent protein kinase is required for cellular resistance to oxidative stress independent of DNA double-strand break repairFree Radic Biol Med20147627828525224041
- NishikawaTEdelsteinDDuXLNormalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damageNature2000404677978779010783895
- DingYVaziriNDCoulsonRKamannaVSRohDDEffects of simulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expressionAm J Physiol Endocrinol Metab20002791E11E1710893317
- AndusKLRaubTLChapter 10. Mechanisms of increased endothelial permeabilityBiological Barriers to Protein DeliveryNew YorkPlenum Press1993254256
- SukritiSTauseefMYazbeckPMehtaDMechanisms regulating endothelial permeabilityPulm Circ20144453555125610592
- RosseCLinchMKermorgantSCameronAJBoeckelerKParkerPJPKC and the control of localized signal dynamicsNat Rev Mol Cell Biol201011210311220094051
- PKC-DRS2 GroupAielloLPDavisMDEffect of ruboxistaurin on visual loss in patients with diabetic retinopathyOphthalmology2006113122221223016989901
- Das EvcimenNKingGLThe role of protein kinase C activation and the vascular complications of diabetesPharmacol Res200755649851017574431
- GeraldesPKingGLActivation of protein kinase C isoforms and its impact on diabetic complicationsCirc Res201010681319133120431074
- RamakrishnanSAnandVRoySVascular endothelial growth factor signaling in hypoxia and inflammationJ Neuroimmune Pharmacol20149214216024610033
- PudduASanguinetiRDuranteANicolòMVivianiGLVascular endothelial growth factor-C secretion is increased by advanced glycation end-products: possible implication in ocular neovascularizationMol Vis2012182509251723112566
- KitadaMZhangZMimaAKingGLMolecular mechanisms of diabetic vascular complicationsJ Diabetes Investig2010137789
- WeightCFriessUBrodbeckKHaringHUSchleicherEDGlutamine:fructose-6-phosphate aminotransferase enzyme activity is necessary for the induction of TGF-β1 and fibronectin expression in mesangial cellsDiabetologia200346685285512802498
- StraussOThe retinal pigment epithelium in visual functionPhysiol Rev200585384588115987797
- ChenYHChouHCLinSTChenYWLoYWChanHLEffect of high glucose on secreted proteome in cultured retinal pigmented epithelium cells: its possible relevance to clinical diabetic retinopathyJ Proteomics20127711112822813881
- PonnalaguMSubramaniMJayadevCShettyRDasDRetinal pigment epithelium-secretome: a diabetic retinopathy perspectiveCytokine20179512613528282610
- QaziYMaddulaSAmbatiBKMediators of ocular angiogenesisJ Genet200988449551520090210
- NybergPXieLKalluriREndogenous inhibitors of angiogenesisCancer Res200565103967397915899784
- LawlerPRLawlerJMolecular basis for the regulation of angiogenesis by thrombospondin-1 and -2Cold Spring Harb Perspect Med201225a00662722553494
- KazerounianSYeeKOLawlerJThrombospondins in cancerCell Mol Life Sci200865570071218193162
- IsenbergJSRidnourLAPerruccioEMEspeyMGWinkDARobertsDDThrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent mannerProc Natl Acad Sci U S A200510237131411314616150726
- KarakousisPCJohnSKBehlingKCLocalization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissuesMol Vis2001715416311438800
- KozulinPNatoliRBumsted O’BrienKMMadiganMCProvisJMThe cellular expression of antiangiogenic factors in fetal primate maculaInvest Ophthalmol Vis Sci20105184298430620357200
- BecerraSPFarissRNWuYQMontuengaLMWongPPfefferBAPigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: apical secretion and distributionExp Eye Res200478222323414729355
- JohnstonEKFrancisMKKnepperJERecombinant pigment epithelium-derived factor PEDF binds vascular endothelial growth factor receptors 1 and 2In Vitro Cell Dev Biol Anim201551773073825948043
- YamagishiSNakamuraKMatsuiTPigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expressionJ Biol Chem200628129202132022016707486
- YamagishiSMatsuiTNakamuraKPigment-epithelium-derived factor suppresses expression of receptor for advanced glycation end products in the eye of diabetic ratsOphthalmic Res2007392929717284935
- SheibaniNSorensonCMCorneliusLAFrazierWAThrombospondin-1, a natural inhibitor of angiogenesis, is present in vitreous and aqueous humor and is modulated by hyperglycemiaBiochem Biophys Res Commun2000267125726110623607
- WangSGottliebJLSorensonCMSheibaniNModulation of thrombospondin 1 and pigmented epithelium-derived factor levels in vitreous fluid of patients with diabetesArch Ophthalmol2009127450751319365032
- SorensonCMWangSGendronRParadisHSheibaniNThrombos-pondin-1 deficiency exacerbates the pathogenesis of diabetic retinopathyJ Diabetes Metab2013Suppl 12
- PinteaAMRuginăDOResveratrol and the human retinaPreedyVHandbook of Nutrition, Diet and the EyeLondonAcademic Press2014481491
- PanySMajhiADasJPKC activation by resveratrol derivatives with unsaturated aliphatic chainPLoS One2012712e5288823285216
- SpanierGXuHXiaNResveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4)J Physiol Pharmacol200960Suppl 4111116
- MokniMElkahouiSLimamFAmriMAouaniEEffect of resveratrol on antioxidant enzyme activities in the brain of healthy ratNeurochem Res200732698198717401679
- ZhangHHeSSpeeCIshikawaKHintonDRSIRT1 mediated inhibition of VEGF/VEGFR2 signaling by resveratrol and its relevance to choroidal neovascularizationCytokine201576254955226174951
- NagaiNKubotaSTsubotaKOzawaYResveratrol prevents the development of choroidal neovascularization by modulating AMP-activated protein kinase in macrophages and other cell typesJ Nutr Biochem201425111218122525091551
- ChenCLChenYHTaiMCLiangCMLuDWChenJTResveratrol inhibits transforming growth factor-β2-induced epithelial-to-mesenchymal transition in human retinal pigment epithelial cells by suppressing the Smad pathwayDrug Des Devel Ther201711163173
- ChanCMChangHHWangVCHuangCLHungCFInhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and MAPK pathwaysPLoS One201382e5681923457620
- AgarwalBCampenMJChannellMMResveratrol for primary prevention of atherosclerosis: clinical trial evidence for improved gene expression in vascular endotheliumInt J Cardiol2013166124624823098852
- Tome-CarneiroJGonzalvezMLarrosaMOne-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular diseaseAm J Cardiol2012110335636322520621
- NagineniCNRajuRNagineniKKResveratrol suppresses expression of VEGF by human retinal pigment epithelial cells: potential nutraceutical for age-related macular degenerationAging Dis2014528810024729934
- IshidaTYoshidaTShinoharaKPotential role of sirtuin 1 in Müller glial cells in mice choroidal neovascularizationPLoS One2017129e018377528886036
- KowluruRASantosJMZhongQSirt1, a Negative regulator of matrix metalloproteinase-9 in diabetic retinopathyInvest Ophthalmol Vis Sci20145595653566024894401
- TuckerBKlassenHYangLChenDFYoungMJElevated MMP expression in the MRL mouse retina creates a permissive environment for retinal regenerationInvest Ophthalmol Vis Sci20084941686169518385092
- NotariLMillerAMartínezAPigment epithelium-derived factor is a substrate for matrix metalloproteinase type 2 and type 9: implications for downregulation in hypoxiaInvest Ophthalmol Vis Sci20054682736274716043845
- PandeyAKBhattacharyaPShuklaSCPaulSPatnaikRResveratrol inhibits matrix metalloproteinases to attenuate neuronal damage in cerebral ischemia: a molecular docking study exploring possible neuroprotectionNeural Regen Res201510456857526170816
- NeliusTMartinez-MarinDHirschJPigment epithelium-derived factor expression prolongs survival and enhances the cyto-toxicity of low-dose chemotherapy in castration-refractory prostate cancerCell Death Dis20145e121024810046
- CottardCHNivet-AntoineVBeaudeuxJLReview of recent data on the metabolism, biological effects, and toxicity of resveratrol in humansMol Nutr Food Res201458172123740855
- SinghGPaiRSRecent advances of resveratrol in nanostructured based delivery systems and in the management of HIV/AIDSJ Control Release201419417818825217813
- BrownVAPatelKRViskadurakiMRepeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axisCancer Res201070229003901120935227
- BalataGFEssaEAShamardlHAZaidanSHAbourehabMASelf-emulsifying drug delivery systems as a tool to improve solubility and bioavailability of resveratrolDrug Des Devel Ther201610117128
- NevesARLúcioMMartinsSLimaJLReisSNovel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailabilityInt J Nanomedicine2013817718723326193
- BalančBTrifkovićKĐorđevićVNovel resveratrol delivery systems based on alginate-sucrose and alginate-chitosan microbeads containing liposomesFood Hydrocoll201661832842
- HuangXDaiYCaiJResveratrol encapsulation in core-shell biopolymer nanoparticles: impact on antioxidant and anticancer activitiesFood Hydrocoll201764157165
- SooEThakurSQuZJambhrunkarSParekhHSPopatAEnhancing delivery and cytotoxicity of resveratrol through a dual nanoencapsulation approachJ Colloid Interface Sci201646236837426479200
- SessaMBalestrieriMLFerrariGBioavailability of encapsulated resveratrol into nanoemulsion-based delivery systemsFood Chem2014147425024206683
- CoimbraMIsacchiBvan BlooisLImproving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomesInt J Pharm2011416243344221291975
- LuXYHuSJinYQiuLYApplication of liposome encapsulation technique to improve anti-carcinoma effect of resveratrolDrug Dev Ind Pharm201238331432221851312
- WangXXLiYBYaoHJThe use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cellsBiomaterials201132245673568721550109
- JoyeIJDavidov-PardoGLudescherRDMcClementsDJFluorescence quenching study of resveratrol binding to zein and gliadin: towards a more rational approach to resveratrol encapsulation using water-insoluble proteinsFood Chem201518526126725952867
- KimJHParkEYHaHKResveratrol-loaded Nanoparticles induce antioxidant activity against oxidative stressAsian-Australas J Anim Sci201629228829826732454
- SinghGPaiRSOptimized PLGA nanoparticle platform for orally dosed transresveratrol with enhanced bioavailability potentialExpert Opin Drug Deliv201411564765924661109
- JungKHLeeJHParkJWResveratrol-loaded polymeric nano-particles suppress glucose metabolism and tumor growth in vitro and in vivoInt J Pharm2015478125125725445992
- da Rocha LindnerGKhalilNMMainardesRMResveratrol-loaded polymeric nanoparticles: validation of an HPLC-PDA method to determine the drug entrapment and evaluation of its antioxidant activitySci World J20132013506083
- KarthikeyanSPrasadNRGanamaniABalamuruganEAnticancer activity of resveratrol-loaded gelatin nanoparticles on NCI-H460 non-small cell lung cancer cellsBiomed Prev Nutr2013316473
- ChenJWeiNLopez-GarciaMDevelopment and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applicationsEur J Pharm Biopharm201711728629128411056
- SessaMTsaoRLiuRFerrariGDonsìFEvaluation of the stability and antioxidant activity of nanoencapsulated resveratrol during in vitro digestionJ Agric Food Chem20115923123521236022026647
- KumarRKaurKUppalSMehtaSKUltrasound processed nano-emulsion: a comparative approach between resveratrol and resveratrol cyclodextrin inclusion complex to study its binding interactions, antioxidant activity and UV light stabilityUltrason Sonochem20173747848928427660
- PengHLXiongHLiJHVanillin cross-linked chitosan micro-spheres for controlled release of resveratrolFood Chem201012112328
- MamadouGCharrueauCDairouJLimas NzouziNEtoBPonchelGIncreased intestinal permeation and modulation of presystemic metabolism of resveratrol formulated into self-emulsifying drug delivery systemsInt J Pharm20175211–215015528216465
