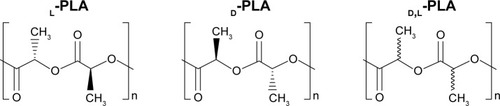
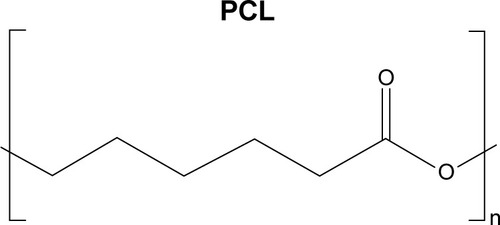
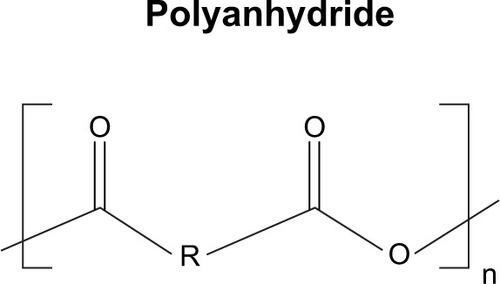
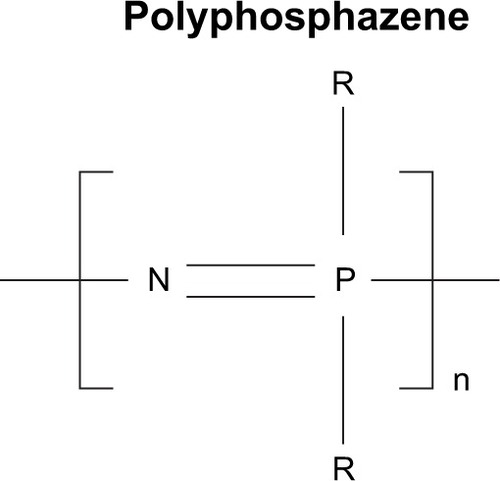
Abstract
In the last half-century, the development of biodegradable polymeric materials for biomedical applications has advanced significantly. Biodegradable polymeric materials are favored in the development of therapeutic devices, including temporary implants and three-dimensional scaffolds for tissue engineering. Further advancements have occurred in the utilization of biodegradable polymeric materials for pharmacological applications such as delivery vehicles for controlled/sustained drug release. These applications require particular physicochemical, biological, and degradation properties of the materials to deliver effective therapy. As a result, a wide range of natural or synthetic polymers able to undergo hydrolytic or enzymatic degradation is being studied for biomedical applications. This review outlines the current development of biodegradable natural and synthetic polymeric materials for various biomedical applications, including tissue engineering, temporary implants, wound healing, and drug delivery.
Introduction
A biomaterial can be defined as a material intended to interface with biological systems in order to evaluate, treat, augment, or replace any tissue, organ, or function of the body.Citation1 The global market for implantable biomaterials was worth nearly $75.1 billion in 2013. This market is expected to grow at a compound annual growth rate (CAGR) of 6.7% between 2014 and 2019, resulting in a $79.1 billion global market in 2014 and a $109.5 billion global market in 2019.Citation2 Although biomedical applications of natural enzymatically degradable polymers date back thousands of years, the application of synthetic biodegradable polymers began only in the second half of the 1960s.Citation3 Considering their advantages over biostable materials in terms of long-term biocompatibility along with the technical and ethical issues accompanying revision surgeries, investigations into the application of biodegradable biomaterials rather than permanent prosthetic devices for assisting in tissue repair and regeneration has vigorously increased recently.Citation4–Citation6 As a result, polymeric biomaterials are quickly replacing other material classes, such as metals, alloys, and ceramics, for use as biomaterials due to their versatility.Citation3–Citation5 Within the global implantable biomaterial market, the polymeric biomaterials sector is expected to show the highest growth, at a CAGR of 22.1%, because of its promising potential in a wide range of biomedical applications.Citation2 With this in mind, the aim of this review is to highlight the most often studied polymeric biomaterials and underscore their immense potential in the areas of drug design, development, and therapy.
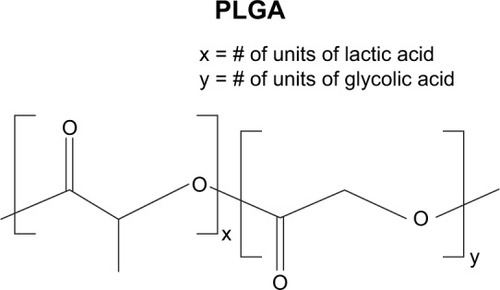
Figure 6 Structure of poly(lactic acid-co-glycolic acid) (PLGA). X=number of units of lactic acid and Y=number of units of glycolic acid.
A critical requirement for a biomaterial is biocompatibility – the ability of a material to function with an appropriate host response in a specific application.Citation1 Many biological and physicochemical characteristics of an implant material govern the host tissue response to the material. For instance, molecular weight, solubility, hydrophilicity/hydrophobicity, surface energy, material chemistry, mechanism of degradation and/or erosion, lubricity, and shape and structure of the implant can all influence the material’s biocompatibility.Citation7 Importantly, a biodegradable biomaterial requires excellent biocompatibility over time because the physicochemical, mechanical, and biological properties of a biodegradable biomaterial will differ with time and, thus, the resulting degradation products can possess varying levels of tissue compatibility as compared with the initial parent material. An ideal biodegradable biomaterial should have degradation products that are nontoxic and easily metabolized and cleared from the body.
In addition to biocompatibility, several other important properties must be considered when choosing a biodegradable biomaterial. First, the degradation time of the biomate-rial should coincide with the regeneration and/or healing process to ensure proper remodeling of the tissue. Second, the biomaterial must maintain suitable permeability and processability for its intended application. Finally, the mechanical properties of the biomaterial should be sufficient to promote regeneration during the patient’s everyday activities, and any change in mechanical properties due to degradation should preserve compatibility with the healing or regeneration process.
Given the complexity of the human body and the scope of applications that polymeric biomaterials are currently utilized for, no single polymeric system can be considered the ideal biomaterial for all medical applications. Thus, recent advances in biodegradable biomaterial synthesis have been directed toward developing and synthesizing polymers with properties tailored for specific biomedical applications. Moreover, current developments incorporating multifunctional and combinatorial approaches in biomaterial design have accelerated the innovation of novel biodegradable biomaterials. Another hotspot in biomaterials research is the development of therapeutic devices, including temporary prostheses, three-dimensional (3D) porous scaffolds for tissue engineering, and delivery vehicles for pharmacological applications. Most recently, 3D bioprinting has also been acknowledged, and preliminary data collection for biomate-rial use as potential bio-ink for printing of 3D scaffolds have begun. Because biodegradable biomaterials exhibit a variety of biological and physicochemical properties and, therefore, can replicate the properties of different tissues, these materials are assessed for use as 1) large implants, including bone screws, bone plates, and contraceptive reservoirs; 2) small implants, in the form of sutures and staples; 3) plain membranes for guided tissue regeneration; and 4) porous structures or multifilament meshes for tissue engineering.Citation8 Moreover, by properly engineering the structure and degradation parameters, these biodegradable materials can be used to generate micro- or nanoscale drug-delivery vehicles for controlled drug delivery in an erosive or diffusive manner, or as a combination of both.Citation9
Because of the interest aroused in the areas demanding biodegradable biomaterials, including regenerative medicine, tissue engineering, controlled drug delivery, gene therapy, and nanotechnology, there has been a robust expansion of biomedical applications of synthetic biodegradable polymers and analogous natural polymers, and our review will focus on exploring the most current development of these polymers for biomedical applications in these fields. Although many other reviews have focused on the topic of biodegradable biomaterials for medical use, to our best knowledge, there has not been a review published within the past 5 years that covers as much breadth as our review does about the development of the most commonly investigated biodegradable polymeric biomaterials in the fields of drug delivery, tissue engineering, and wound application.
Natural biodegradable polymeric biomaterials
Biodegradable biomaterials can be roughly divided into two categories – natural and synthetic – based on their source and whether they are composed of naturally occurring extracellular matrix (ECM). Natural biodegradable polymeric bio-materials generally include proteins (collagen, fibrin, silk, etc.), and polysaccharides (starch, alginate, chitin/chitosan, hyaluronic acid derivatives, etc.).Citation10–Citation12 Furthermore, a family of native polyesters – polyhydroxyalkanoates (PHA) – has been recognized as natural biodegradable biomaterials and, more recently, sundew adhesives (natural polysaccharide-based hydrogels) and ivy nanoparticles (macromolecular compositions of nanospherical arabinogalactan proteins) have garnered more attention for their ability to create effective nanocomposite adhesives and for their potential use as nano-carriers in drug delivery, respectively.Citation13,Citation14
Collagen
As the most prevalent protein in the human body, collagen offers physical support to tissues by inhabiting the intercellular space, acting not only as native structural support for organizing cells within connective tissues, but also as a mobile, dynamic, and flexible substance essential to cellular behaviors and tissue function.Citation15 Generally, collagen is a rod-type polymer approximately 300-nm long with a molecular weight of approximately 300 kDa. Free amino acids in the body are synthesized into subunit chains of collagen, which then undergo transcription, translation, and post-translational modification processes in suitable cells such as osteoblasts and fibroblasts.Citation13 More than 22 different types of collagen have been identified in the human body, with types I–IV being the most common, and type I collagen being the single greatest abundant protein present in mammals.Citation15 Collagen undergoes enzymatic degradation within the body by a diverse set of enzymes, such as matrix metalloproteinases and collagenases, to yield its corresponding amino acids. Due to its enzymatic degradability; unique mechanical, biological, and physicochemical properties; nontoxicity; and high tensile strength, collagen has been widely studied for biomedical applications.Citation16,Citation17
Collagen plays a critical role in preserving the biological and structural integrity of the ECM and is highly dynamic, undergoing continuous remodeling for proper physiologic functions. For most soft and hard connective tissues (eg, blood vessels, cornea, skin, tendon, cartilage, and bone), collagen fibrils and their networks function through their highly organized 3D structure. Tissue regeneration attempts to repair both the structural integrity and the intricate remod-eling process of the native ECM, particularly restoring the delicate collagen networks under which normal physiologic regeneration occurs; thus, recent efforts have been focused on replacing native collagen-based ECM by developing novel biomaterials that imitate its intricate fibrillar architecture and function as cell scaffolding. Animal-derived and recombinant collagens, especially type I collagen, are recognized as one of the most valuable biomaterials available and are now extensively used for tissue engineering, drug delivery, and cosmetic surgery. For example, a composite of fibrillar collagen, hydroxyapatite, and tricalcium phosphate (Collagraft®, Angiotech Pharmaceuticals) has been approved by the United States Food and Drug Administration (FDA) as a biodegradable synthetic bone graft substitute.Citation18
The main sources of collagen presently utilized for biomedical applications are bovine or porcine skin and bovine or equine Achilles tendons. They are utilized in either their native fibrillar form or after denaturation in various fabricated forms, such as sponges, sheets, plugs, and pellets. Collagen-based materials have been successfully used for skin repair.Citation19 For example, Promogran® (Systagenix) – a spongy collagen matrix containing oxidized cellulose – is available in the USA and Europe for treatment of diabetic and ulcer wounds.Citation20 Similarly, an FDA-approved bilayer skin substitute (Integra® Dermal Regeneration Template, Integra LifeSciences) composed of a dermal layer of cross-linked bovine collagen and glycosaminoglycans (GAGs) as well as an epidermal layer of polysiloxane, which deposits ECM components, is in the market for full-thickness or deep partial thickness thermal injury. Moreover, these skin substitutes constructed from cell-seeded collagens have been widely commercialized (eg, Apligraf®, Organogenesis, Inc.) and (OrCel™, Ortec International Inc.). Interestingly, type I collagen sponges have also been used to engineer patellar tendons in rabbits under different culture conditions.Citation21–Citation23 Using the collagen sponge combined with bone marrow-derived mesenchymal stem cells, Juncosa-Melvin et al demonstrated that the engineered tendon tissue attained almost 75% of the mechanical properties of native tendon.Citation22 In other studies, collagen has also been successfully used as a scaffold in nerve and bladder engineering.Citation24–Citation26 Additionally, a suture-free, 3D-collagen matrix graft – DuraGen® (Integra LifeSciences) – has been developed for spinal dural repair and regeneration and is currently approved by the FDA.Citation27
Collagen is, furthermore, a key initiator of the coagulation cascade and, therefore, it has been successfully developed as a hemostatic agent due to its high thrombogenicity. Multiple collagen-based hemostats are currently available or undergoing clinical trials for a variety of surgical indications; these include Sulzer-Spine® Tech’s sealants composed of bovine collagen and bovine thrombin for cardiovascular and spinal surgical procedures; Floseal® (Baxter Healthcare), a high-viscosity gel hemostatic agent composed of collagen-derived particles and topical bovine-derived thrombin; and CoStasis® Surgical Hemostat (Cohesion Technologies), which consists of bovine thrombin and bovine microfibrillar collagen combined with autologous plasma.
With regard to drug delivery, collagen has been notably studied for the delivery of low-molecular-weight drugs, proteins, genes, and plasmids. Currently, a few collagen-based gentamicin-delivery vehicles are available in the global market (eg, Sulmycin®-Implant and Collatamp®-G, Innocoll Pharmaceuticals Ltd). These delivery systems permit a sustained local delivery of antibiotics with limited systemic exposure. Moreover, another product, Septocoll® (Biomet), achieved prolonged collagen delivery by incorporating two gentamicin salts possessing different solubility, and has been approved for infection prevention.Citation28 More recently, a new biodegradable, collagen-based chlorhexidine chip has been shown to provide a longer, more sustained release of chlorhexidine in the confines of the periodontal pocket as compared to simple subgingival irrigation of chlorhexidine. The constant outflow of the gingival crevicular fluid from the periodontal pocket (up to 40 times an hour) renders subgingival irrigation of chlorhexidine useless in delivering significant antimicrobial benefits, reduction in probing depths, and clinical attachment level gain to manage chronic periodontitis; however, the use of a collagen-based release vehicle allows for a controlled delivery of chlorhexidine to achieve the intended pharmaceutical effects.Citation29 Furthermore, current clinical trials are investigating cross-linked, absorbable collagen sponges as protein-carrier vehicles. Prolonged release of bioactive proteins, such as recombinant human bone morphogenic protein-2 (rhBMP-2), have been reported when utilized in conjunction with collagen matrices due to desirable interactions of the collagen matrix with the protein.Citation30 This combination product is approved by the FDA for simultaneous use with a titanium interbody spine fusion cage for anterior lumbar spinal fusion (InFUSE® Bone Graft/LT-CAGE® Lumbar Tapered Fusion Device, Medtronic Spinal and Biologics), and a similar product InductOs® (Medtronic Spinal and Biologics) is approved in Europe for the treatment of acute tibial fractures in adult patients. Additionally, a recent study reported that the delivery of rhBMP-2 by an absorbable collagen sponge stimulated bone reconstruction in advanced alveolar ridge defects.Citation31 Aside from its use as a protein-delivery vehicle, collagen has been demonstrated to retain gene vector/plasmid DNA whereas simultaneously protecting it from enzymatic or immunological reactions of the body, which underscores its potential in gene and plasmid DNA delivery.Citation32
One disadvantage of these collagen-based biomaterials is their mild immunogenicity due to the antigenic sites in the central helix and the composition of the terminal region, which significantly limits their clinical application.Citation33 The immune response varies depending on the processing techniques, site of implantation, and species from which the collagen has been isolated.Citation34 Other disadvantages of animal-derived collagen include the varying physicochemical and degradation properties, the high cost of pure collagen, and the allogeneic or xenogeneic sources, which increase the risk of infectious disease transmission. In response to these limitations, present investigations have directed focus toward recombinant systems that can produce human-based collagen.Citation35,Citation36 Several potentially useful systems already exist for large-scale production and purification of recombinant collagen, such as yeasts, transgenic animals, and, most recently, Escherichia coli.Citation37,Citation38 Because the amino acid sequence of recombinant collagen can be directly modified, it is possible to make controlled, targeted collagen products for specific applications, thereby diversifying and increasing the potential of collagen-based products.Citation39 However, recombinant collagen is unable to undergo natural posttranslational modification and, thus, may lack critical biological activities of native tissue.Citation33 This lack of posttranslational modification means that, despite its flexibility in creating countless different amino acid sequences for different targeted applications, recombinant collagens may not be suitable for many biomedical applications, especially those that require stability and strength such as heart valves. Furthermore, the commercially available recombinant collagens are still expensive to produce and, thus far, only limited amounts are available.Citation39
Collagen as a biomaterial has already seen significant use in several specific applications in skin repair, hemostatic agents, and drug delivery, but its slight immunogenicity, high cost, and varying physicochemical and degradation properties prevent further expansion of collagen-based biomaterials. Although recombinant collagens have the potential to catapult collagen-based biomaterials for widespread use, significant limitations such as their lack of posttranslational modification will need to be continually studied for them to have any noticeable impact for use as a biomaterial.
Gelatin
Gelatin is a natural biopolymer derived from collagen via controlled alkaline, acid, or enzymatic hydrolysis.Citation40 As a result of its biological origin, it has excellent biodegradability and biocompatibility and because it is widely available, gelatin is a relatively low-cost polymer.Citation40 Gelatin has been used in medical and pharmaceutical fields as a matrix for implants, and as stabilizers in vaccines such as measles, mumps, and rubella.Citation41 Moreover, gelatin is water permeable and soluble in water and has multifunctional properties as a drug-delivery carrier.Citation42 Gelatin’s mechanical properties, swelling behavior, thermal properties, and many other physiochemical properties can be dependent upon the collagen source, extraction method, amount of thermal denatured employed, and the degree of cross-linking, thereby making gelatin a very versatile polymer.Citation43 Furthermore, its ability to produce a thermoreversible gel makes it a very good candidate as a targeted drug-delivery carrier and, as a result, gelatin can be utilized to develop specific drug-release profiles, allowing for a broad range of applications in drug delivery.Citation43
Gelatin is a versatile biopolymer that traditionally enabled the design of several different drug carrier systems, such as microparticles, nanoparticles, fibers, and hydrogels.Citation44 Each of these different systems has certain properties that make them particularly suitable for drug delivery.Citation44 For example, gelatin microparticles are popular for serving as vehicles for cell amplification and delivery of large bioactive molecules, and gelatin nanoparticles are better for drug delivery to the brain or in intravenous delivery.Citation44 Recently, a novel and relatively simple method was discovered for producing gelatin microparticles which allow for very high protein- and drug-loading efficiencies.Citation45 In the experiment, bovine serum albumin (BSA) was added at a 0.1%–0.4% gelatin concentration, resulting in a gelatin solution.Citation45 The solution was freeze-dried and the resulting spongy membranes were hardened with liquid nitrogen and subsequently ground to a powder, producing BSA-loaded microparticles in random forms and shapes.Citation45 These microparticles were then incorporated into porous scaffolds constructed of polylactide (PLLA) and poly-caprolactone (PCL).Citation45 The in vitro release profile of the protein from the particles alone and from the particle-incorporated scaffolds demonstrated up to 90% protein-loading efficiency, showing that BSA-loaded microparticles in conjunction with particle-incorporated scaffolds for growth factor release may be possible for application in bone tissue engineering.Citation45 Another study studied the in vitro efficacy and toxicity of incorporating polyenes into electrospun gelatin fiber mats as a topical antifungal application.Citation46 The research group found that polyene-loaded antifungal gelatin mats displayed better antifungal activity than using traditional electrospun gelatin fiber mats.Citation46 Furthermore, they found that polyenes stabilized the triple helical conformation of gelatin whereas gelatin decreased the hemolytic activity of polyenes, making polyene antifungal-loaded gelatin fiber mats a possibility in managing superficial skin infections in the future.Citation46 Cohen et al studied novel tissue adhesives based on gelatin, with alginate as a polymeric additive, loaded with bupivacaine or ibuprofen for pain management.Citation47 They found that the release of drugs from the adhesive matrix was primarily controlled by the gelatin/alginate bioadhesive’s characteristics, such as swelling and hydrophilic group concentrations that can be adjusted by changing the ratio of gelatin to alginate.Citation47 Moreover, they found that the hydrophilicity and electrical interactions between bupivacaine and ibuprofen with the adhesive components had some effect on the release profile of the drug, and that incorporating bupivacaine improved the bonding strength of the adhesive due to its inert nature whereas ibuprofen decreased the bonding strength due to its reactive nature with the alginate/gelatin adhesive.Citation47 Overall, the study shows that this loading bupivacaine in a gelatin/ alginate bioadhesive can potentially be used for wound-closing applications.Citation47
The primary advantages of gelatin are its biodegradability, availability, and cheap cost.Citation44 Porcine skin-derived gelatin is the most popular source, followed by bovine skin, and bone.Citation48 However, religious issues may arise, as porcine-derived products and bovine-derived products are forbidden in Judaism and Hinduism, respectively.Citation48 In addition, there are health concerns over transmission of pathogenic vectors such as prions.Citation48 Recombinant gelatins, however, can be utilized to overcome the disadvantages with animal tissue-derived materials.Citation49 Currently, the primary use of gelatin is in the food, pharmaceutical, and photographic industries.Citation49 In the biomedical field, gelatin is used in drug delivery and in some wound dressing and tissue-regeneration applications, but its use is heavily limited by its poor mechanical properties.Citation49 These mechanical properties can be strengthened through physical cross-linking as well as chemical cross-linking due to a large number of functional side groups that gelatin possesses; however, agents used to stabilize cross-linked gelatin are often somewhat toxic to the human body.Citation49 Research into improving gelatin’s mechanical properties will need to be investigated in the future for gelatin to have a promising future as a primary biomaterial; but, until then, the most likely incorporation of gelatin will be as a composite with other natural or synthetic biomaterials, or as a carrier for drug delivery.
Fibrin
Fibrin is a 360-kD fibrinogen-derivative biopolymer involved in the natural blood-clotting process, enhancing cell adhesion and proliferation.Citation50 In addition to possessing excellent biodegradability and biocompatibility, fibrin exhibits high elastic and viscous properties; stiffens in response to shear, tension, or compression; and has excellent deformability.Citation51 One of the first developed fibrin-based products was fibrin glue (fibrin sealant). Tissucol/Tisseel™ (Baxter Healthcare) and Beriplast HS/Beriplast P™ (CSL Behring) are the first-generation products of fibrin sealants marketed in Europe. Today, an expansive variety of products possessing different compositions and adhesive properties are available on the market. These products are broadly used for hemostasis and tissue-sealing applications in various surgical procedures, including neurosurgery and plastic and reconstructive surgery.Citation52–Citation54
Furthermore, fibrin has been utilized as a scaffold for the regeneration of numerous tissues, such as adipose tissue, bone, cardiac tissue, cartilage, muscle tissue, nervous tissue, ocular tissue, respiratory tissue, skin, tendons and ligaments, and vascular tissue as well as a carrier vehicle for bioactive molecules (drugs, antibiotics, or chemotherapy agents) due to its injectability and biodegradability.Citation55–Citation60 For instance, several studies have demonstrated that clinical application of fibrin significantly improved the healing of intrabony defects in chronic periodontitis.Citation61,Citation62 Further evidence suggests that proteins interact differently with fibrin clots and, as a result, several cross-linking techniques are presently being studied to control the release profile of bioactive molecules from the fibrin matrix.Citation63 Additionally, fibrin matrices have been utilized as excellent cell-carrier vehicles. One successful example obtained by mixing keratinocytes with fibrin is Bioseed® (DCM Shriram Limited), a fibrin-based product used to treat chronic cutaneous wounds.
Fibrin is natural, highly available, implantable, inexpensive, easy to use, and has low fibrinogen concentrations. Due to its porous morphology, a fibrin scaffold system is adequate for cell attachment, proliferation, differentiation,Citation58,Citation64 and as a release system of growth factors such as vascular endothelial growth factor and basic fibroblast growth factor (bFGF).Citation60,Citation65 Fibrin-immobilized growth factors have been shown to be continuously released for several days in a controlled manner, making it optimal for numerous tissue-engineering purposes.Citation50 Thus, autologous fibrin could prevent the complications of techniques derived from the use of current commercial available fibrin products and should be further investigated. Fibrin-based scaffolds do have some limitations, such as weak mechanical strength and quick degradation rates; however, these properties have been shown to be improved by incorporating stronger natural and synthetic polymers, utilizing various cross-linking methods, and utilizing micro/nanospheres.Citation50 Next-generation scaffolds will likely involve specific cell lines, combined with biomolecules and growth factors to accelerate and increase cell proliferation and differentiation on immobilized scaffolds for a variety of tissues, and fibrin-based drug-delivery carriers and tissue-engineered scaffolds, including the relatively recent scaffold development of fibrin microspheres, nanospheres, microfibers, microtubes, and porous sheets, all of which will play a big role in regenerative medicine.Citation50
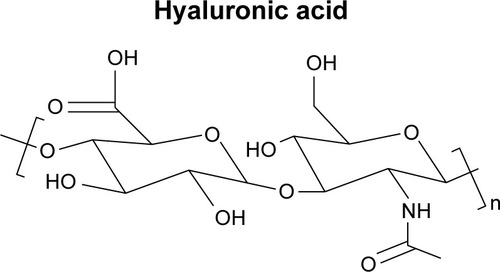
Hyaluronic acid (HA)
HA is an essential component of the ECM and its structural and biological properties mediate cellular signaling, morphogenesis, matrix organization, and wound repair.Citation66,Citation67 In 1943, HA was first isolated from the vitreous humor by Meyer and Palmer.Citation68 HA is a member of the GAG family, which involves linear polysaccharides consisting of alternating units of N-acetyl-d-glucosamine and glucuronic acid ranging in size from 5,000 to 20,000,000 Daltons in vivo, and is present in almost every tissue in vertebrates. Native sources of HA include rooster combs, bovine vitreous humor, and synovial fluids.Citation69 HA degradation occurs in the body through free radicals, such as nitric oxide and matrix metalloproteinases found in the ECM, and then undergoes endocytosis. Further digestion of HA by lysosomal enzymes results in mono-and disaccharides, which are then converted into ammonia, carbon dioxide, and water.Citation69
Recently, HA has become recognized as an important building block for the creation of new biomaterials for use in cell therapy, 3D cell culture, and tissue engineering.Citation70–Citation72 As HA is secreted at the early stage of wound healing, it has been extensively researched for wound-dressing applications.Citation73,Citation74 HA can be recognized by receptors on a variety of cells associated with tissue repair, and thus presents the capability to stimulate angiogenesis and to regulate injury-induced inflammation as a free radical scavenger.Citation75 Moreover, HA promotes epithelial and mesenchymal cell migration and differentiation, making it vital for tissue repair.Citation75 These properties combined with its immunoneutral potency make HA an ideal biomaterial for tissue engineering.Citation75 Moreover, its aqueous solubility allows for modification of HA into various porous and 3D structures for drug delivery. For example, HYAFF®11 (Anika Therapeutics, Inc.), an HA-based product, is currently utilized as a carrier vehicle for a variety of growth factors, morphogens, and stem cells.Citation76 In a comparative study, Hunt et al reported an improved healing response to rhBMP-2 delivered by HYAFF®11 than that delivered by an absorbable collagen sponge.Citation76 A more recent study indicated that HA-based materials maintain the potential to replace collagen-based materials as injectable soft tissue fillers.Citation77 With regard to tissue engineering, HA has been successfully incorporated into multiple complex systems. For example, HA-modified poly(D,L-lactic acid-co-glycolic acid) (PLGA) scaffolds were successful in inducing cartilage tissue formation in terms of type II collagen expression and tissue morphological characteristics.Citation78 Additionally, high-molecular-weight viscous HA solutions (eg, AMVISC® and AMVISC® PLUS, Bausch & Lomb) currently function as vitreous humor substitutes and as protection for sensitive eye tissue during glaucoma surgery, cataract extraction, and corneal transplantation. Viscous HA solutions (eg, SYNVISC®, SanofiBiosurgery; and ORTHOVISC®, Anika Therapeutics, Inc.) are also clinically applied as synovial fluid substitutes to reduce pain and enhance joint mobility in patients with osteoarthritis.Citation79
Moreover, HA derivatives, such as HA esters and cross-linked HA gels, have been thoroughly researched for wound dressing applications. Reports suggest that these chemical modifications significantly reduce the degradation of HA.Citation69,Citation80 For example, in the absence of enzymatic activity, benzyl HA esters undergo hydrolytic degradation via ester bonds, and the degradation time varies from 1 to 2 weeks to 2–3 months depending on the degree of esterification.Citation69,Citation80 Together, these studies underscore the potential of HA in combined systems for an array of biomedical applications, including tissue engineering, drug delivery, wound healing, and temporary implants.
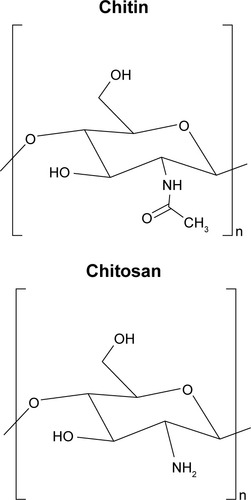
Chitin/chitosan
Chitosan is another naturally derived biodegradable polysaccharide commonly used in tissue engineering.Citation81 Chitosan is a derivative of chitin – the second most abundant natural polymer commonly found in the exoskeletons of crustacean and insects as well as the cells walls of fungi.Citation81 Chitin is partially deacetylated to form chitosan, which is composed of glucosamine and N-acetyl glucosamine linked in a β(1–4) manner.Citation82 The molecular weight and the degree of deacetylation, which are essential in assessing the characteristics of chitosan, are reliant on the source and production process.
Chitin and chitosan are biodegraded by human enzymes, such as lysozymes, which disrupt the linkage between acetylated units and degrades chitin/chitosan to oligosaccharides.Citation83 The degradability of chitin/chitosan-based materials is essential for scaffold construction because it can influence cell behavior and tissue formation of the engineered construct.Citation84 However, the low mechanical resistance of chitosan makes it disadvantageous to be used as a supporting material in tissue engineering.Citation85 To create a better mechanical profile, cross-linking agents are used with functional reactive groups to allow for bridges to be made between polymeric chains, optimizing the resistance and elasticity of chitosan membranes.Citation85 Further, chitin/chitosan have the ability to chelate with the Ca2+ or Mg2+ present in the cell wall of bacteria, thus destroying the entity of bacterial cell wall, and to react with the anionic phosphate groups of phospholipids found on the bacterial cell membrane by using their NH3+ amino group, thereby leading to changes in the cell membrane permeability and eventual release of the bacteria’s cellular contents.Citation84,Citation86 These functional capabilities make chitin/chitosan-based materials exhibit a bactericidal effect on both Gram-negative and Gram-positive bacteria, giving them a broad medical utility in tissue engineering and biomedical applications.Citation84,Citation86
Specifically, chitin/chitosan-based materials have demonstrated potential with regard to connective, nerve, adipose, and vascular tissue-engineering applications.Citation87–Citation89 For example, a silk fibroin (SF)/chitin-based scaffold was used to repair a musculofascial defect in the abdominal wall, displaying continuous integration with adjacent native tissue and mechanical strength similar to native tissue.Citation88 Similarly, chitosan/hydroxyapatite-based scaffolds loaded with bFGF for periodontal tissue regeneration promoted vigorous proliferation and migration in periodontal ligamental cells and cementoblasts.Citation87 A more recent study produced nanosized chitosan/hydroxyapatite-based scaffolds via thermally induced phase separation and lyophilization techniques and found that this combination demonstrated greater compressed mechanical properties as compared to the pure chitosan scaffold – a property critical for the success of scaffolds to be used as tissue-engineering scaffolds.Citation90 Further tests will need to be conducted, but it is expected that this scaffold will allow cell attachment and tissue growth in vivo as well. Taken together, these results highlight the potential of chitin/chitosan-based scaffolds providing a cell-favorable microenvironment for connective tissue healing.
Though chitosan has been widely used for bone tissue-engineering applications due to its various favorable properties as mentioned earlier, it has a lower tensile strength and modulus range than those of natural bones.Citation91,Citation92 In an effort to reinforce the mechanical properties of chitosan, Tamburaci et al combined diatomite (diatomaceous earth), a natural silica material, with chitosan to produce a scaffold for bone tissue regeneration; they found that diatomite-reinforced chitosan composite membranes improved the surface area, roughness, swelling properties, and protein adsorption capacities of traditional chitosan membranes, while showing no cytotoxic effect in Saos-2 osteosarcoma cell line with excellent biocompatibility.Citation93 Interestingly, Tamburaci et al also found that diatomite incorporation into chitosan scaffolds increased the proliferation and alkaline phosphatase activity of Saos-2 cells remarkably.Citation93 Overall, diatomite-reinforced chitosan scaffolds showed improved properties while maintaining the high biological activity typical of traditional chitosan scaffolds, illustrating the potential of chitosan for bone tissue-engineering applications.
Moreover, glutaraldehyde-cross-linked collagen-chitosan hydrogels were successfully applied to adipose tissue engineering. A study confirmed the in vitro viability of pre-adipocytes (PAs) on glutaraldehyde-cross-linked collagen– chitosan hydrogel scaffolds. Subsequently, a rat subcutaneous pocket assay was used to evaluate PA-seeded scaffolds in vivo which displayed excellent biocompatibility, formed adipose tissue, and induced vascularization.Citation89 Additionally, chitin/chitosan-based materials have found success as biomaterials used in cartilage repair due to their biocompatibility and structural similarity with GAGs found in cartilage.Citation12,Citation94,Citation95 Recently, Lee’s group synthesized an injectable hydrogel consisting of methacrylated glycol chitosan and HA by photo-cross-linking with a riboflavin photo-initiator under visible light.Citation12 This resulted in the formation of a cross-linked chitosan network in which high-molecular-weight HA was entrapped, forming a semi-interpenetrating network that provided a more chondrogenic-favorable microenvironment.Citation12 Likewise, these photopolymerizable hydrogels exhibit robust capacity as cell carrier vehicles and further emphasize the advantage of chitin/chitosan-based materials for tissue engineering.
Furthermore, chitin/chitosan-based materials have been incorporated successfully into cutaneous wound management. A number of studies have reported the use of chitin/ chitosan scaffolds and membranes to treat patients with deep burns.Citation96,Citation97 Recently, novel α-chitin/silver nanoparticles (AgNPs) and β-chitin/AgNP composite scaffolds were tested for wound-healing applications.Citation96,Citation97 These chitin/AgNPs composite scaffolds were found to possess excellent antibacterial activity against Staphylococcus aureus and E. coli, combined with good blood clotting ability.Citation96,Citation97 In addition, β-chitin/AgNP composite scaffolds functioned as promising matrices capable of providing good cell attachment apart from their antibacterial activity, which suggests that these composite scaffolds are ideal for wound-healing applications.Citation97
Finally, chitin/chitosan-based materials have presented excellent potential in drug delivery systems. For example, in one study, when carboxymethyl chitin nanoparticles were cross-linked with FeCl3 and CaCl2, they were shown to be nontoxic to mouse L929 cells while showing significant antibacterial activity against Staphylococcus strains.Citation98 Studies indicating that chitin/chitosan-based materials may be used for controlled drug delivery in managing HIV are more exciting. In one study that focused on anti-transferrin and anti-bradykinin B2 antibody-conjugated chitosan nanoparticles, the investigators found that chitosan nanoparticles presented the potential to effectively penetrate across the blood–brain barrier and thus enhance the drug delivery in the brain to inhibit HIV replication in the neural system.Citation99 Another study showed that using chitosan nanoparticles to encapsulate conventional antiretroviral drugs targeting HIV led to a more efficient control of the viral proliferation in target T cells.Citation100 Greater cell targeting efficiency was achieved, mostly due to the fact that chitosan nanoparticles are mildly immunogenic, making them more visible to the immune system, allowing for more efficient uptake by phagocytes.Citation100 A separate investigation studied the effects of poly(lactic acid) (PLA)/chitosan nanoparticles loaded with lamivudine (a type 1 and type 2 HIV selective inhibitor) in mouse L929 fibroblast cells.Citation101 The in vitro drug-release studies showed that the drug release rate from PLA/chitosan nanoparticles decreased when the pH of the medium changed from alkaline to acidic and further decreased from acidic to neutral, which could be a result of the repulsion between H+ ions and cationic groups present in the polymeric nanoparticles.Citation101 Because it is ideal that the drug encapsulated in the delivery system will be protected in the stomach environment at acidic pH and then provide sustained release in the intestines (neutral pH), these findings suggest that the chitosan-based nanoparticles exhibit excellent potential as a carrier system for HIV-controlled drug delivery.
Besides HIV management, chitin/chitosan-based materials can be used in cancer therapy as drug-delivery vehicles. For example, a pH-responsive magnetic nanocomposite was wrapped in chitosan for targeted and controlled drug delivery, and the yield product was found to be nontoxic and exhibited a high antitumor activity while maintaining its excellent pH sensitivity at pH <6.0.Citation102
In addition to improving the targeting and efficiency of cancer and HIV drug-delivery vehicles, chitin/chitosan-based materials have been utilized in various other delivery systems. In a very recent study, Di et al developed an ultrasound-triggered insulin-delivery system which allows for pulsatile insulin release that can provide both long-term, sustained and fast on-demand responses.Citation103 This system incorporated insulin-loaded PLGA nanocapsules encapsulated within chitosan microgels and, upon ultrasound treatment, the stored insulin can be rapidly released to regulate blood glucose levels.Citation103 The research group found that in a mouse model of type 1 diabetes, a 30-second ultrasound administration could effectively achieve glycemic control for 1 week and concluded that this delivery system may potentially be used to release other therapeutics in a noninvasive and convenient manner.Citation103 Moreover, Liang et alCitation104 and Jing et alCitation105 have shown that chitosan derivatives as a delivery system can also enhance oral tablet absorption of bioactive compounds and potentially allow protein and certain peptide drugs to be orally administrable. In addition, hydroxyethyl chitosan – a derivative of chitosan – has shown great potential as a drug-delivery material for the treatment of glaucoma and other ocular diseases due to its great water-solubility and excellent biocompatibility.Citation106 Further investigations must be carried out to assess any side-effect or instability of chitosan-based materials; however, there seems to be substantial potential for chitosan-derived drug-controlled-release systems.
Chitin/chitosan-based materials are widely studied for several different tissue-engineering applications, regenerative medicine, wound healing, and drug delivery for good reason. They have excellent biodegradability and biocompatibility and has been known to have antiulcer, anti-acid, hypocholesterolemic action, wound-healing, antitumor, and hemostatic properties.Citation107–Citation110 Although chitin/chitosan-based materials tend to lack mechanical properties, they possess functional reactive side groups that can be cross-linked to make bridges between polymeric chains, optimizing the resistance and elasticity of these materials.Citation85 Due to their unique combination of physical and chemical properties, chitin/chitosan can be molded relatively easily into porous scaffolds, and their cationic nature allows them to form polyelectrolyte complexes with many types of anionic GAGs, making them capable of modulating the activity of a variety of growth factors and cytokines for tissue-engineering purposes.Citation85 An important property of chitin/chitosan is its mucoadhesive nature and its ability to open epithelial tight junctions, making them well-suited for drug delivery across nasal, intestinal, ocular, buccal, and pulmonary systems.Citation85 However, some challenges do exist. Chitosan is insoluble in most organic solvents, making delivery of hydrophobic drugs difficult; moreover, various methodologies to adapt the solubilization of chitosan, such as alkylation, acetylation, and carboxymethylation, all come with certain drawbacks and limitations.Citation111
Overall, chitin/chitosan is a polymer that will have very important applications in both the industrial and biomedical fields of the future. Its unique chemical properties have recently allowed it to be studied as part of a biological functionalization of microelectromechanical systems, which will enable it to perform functions such as biorecognition, enzymatic catalysis, and controlled drug release, all of which is critical to the advancement of drug-delivery and scaffold technology.Citation85
Starch
Starch is the primary energy reserve polysaccharide in plants, and it is present in the form of granules composed of amylose and amylopectin.Citation112 Amylose is a linear polymer composed of glucose monomers linked through α-D-(1–4) glycosidic linkages, whereas amylopectin molecules are huge, branched polymers of glucose known to be one of the highest molecular-weight natural polymers.Citation113 Different plants have slightly different granule sizes, amylose/amylopectin ratio, mineral contents, and amount of phosphorous and phospholipid contents that lead to varying starch properties.Citation112 The specific characterization of starch is particularly important due to different swelling, solubility, gelatinization, mechanical behavior, enzymatic digestibility, rheological characteristics, and surface characteristics, which affect the way it needs to be processed to convert it to a more usable form such as hydrogels, pastes, and nanoparticles.Citation114 Generally, the native starch isolated from different plants tends to have limited shear resistance, thermal resistance, thermal decomposition, and a high tendency toward retrogradation.Citation112 These limitations have been overcome by combining starch with more stable, synthetic, thermoplastic polymers, or utilizing physical treatment of starch, such as heat or moisture, or utilizing chemical modifications, introducing certain functional groups to remarkably alter its physicochemical properties, making starch vastly more useful in tissue engineering, drug delivery, and delivery of biologically active compounds.Citation112
Among its tissue-engineering applications, starch is most famous for its usefulness in generating scaffolds for bone regeneration due to its bone-bonding behavior when reinforced with hydroxyapatite, good mechanical properties, non-cytotoxic and biocompatible nature, excellent substrate for cell adhesion, and thermoplastic behavior when combined with thermoplastic polymers.Citation115 Lately, further improvements have been made to these starch-based scaffolds. In one recent study, Mahdieh et al synthesized a nanocomposite biomaterial consisting of a blend of thermoplastic starch and ethylene vinyl alcohol as the polymer matrix, and incorporated nano-structured forsterite and vitamin E as the ceramic reinforcing phase and thermal stabilizer, respectively.Citation116 What they found was that nanofosterite, a newly developed bioceramic, resulted in improved biological and mechanical properties, thereby reducing the degradation rate of the scaffold whereas stimulating bone cell proliferation in comparison to the traditional starch–ethylene vinyl alcohol matrix.Citation116
In particular, starch and PCL blends (SPCL) have garnered much attention from many research groups for application as a tissue-engineering construct.Citation117 PCL improves the processability of starch, reduces its high stiffness, and can overcome the high moisture sensitivity of starch, which is one of the greatest weakness of starch as a biomaterial.Citation118 On the other hand, starch improves the biodegradability of PCL and, as the cheapest biomaterial on the planet, starch can substantially lower the high cost of the final product.Citation119 Through the appropriate blending of starch and PCL, SPCL can overcome limitations of both PCL and starch, whereas additionally allowing for control over mechanical and deg-radative properties by adjusting the component ratio – a significant advantage that allows it to conform to the numerous differing tissue-regenerative rates.Citation120 SPCL is particularly effective in bone engineering because one of the greatest biological specifications of SPCL composites is their ability to enhance and stimulate osteoblast cell proliferation.Citation117 In fact, several different research groups have recently experimented with SPCL for this purpose.Citation117 Carvalho et al studied the bone regeneration potential of undifferentiated human adipose-derived stromal/stem cells loaded in SPCL scaffolds for the regeneration of critical-sized mice calvarial defect.Citation121 They found that SPCL was a suitable scaffold for bone tissue engineering, allowing for new tissue formation in the calvarial defect to be approximately 20% of the defect size after 4 weeks and 43% at 8 weeks.Citation121 Link et al did a similar study of critical-sized cranial defects in male Fisher rats and found that the SPCL fiber mesh proved to be an effective osteoconductive material for bone regeneration.Citation122 Requicha et al studied the in vivo behavior of a novel, double-layered SPCL scaffold functionalized with silanol groups (SPCL-Si) in a mandibular rodent model and compared the results to a commercial collagen membrane.Citation123 They found that SPCL-Si scaffolds induced significantly higher new bone formation when compared to the commercial collagen membrane.Citation123
Starch has been proposed as a possible drug-delivery system.Citation124 In its hydrogel form, it can efficiently entrap drugs in interstitial spaces, thus protecting them from undesirable conditions in the human body.Citation125 In addition, starch hydrogels are resistant to gastric juices, allowing for potential oral drug-delivery systems, and they can be modified to be degraded in very specific portions of the gastrointestinal tract, thus allowing for site-specific delivery.Citation126 Moreover, physical modification of starch by retrogradation leads to the development of high levels of type three resistant starch – a very thermally stable, low solubility form of starch that makes it suitable for colon-specific delivery systems.Citation124 However, in practice, studies on starch as a possible drug-delivery system have been very limited. Most of the research on starch as part of a drug-delivery system is theoretical, and scientists are still exploring the possible consequences of physical modification of starch on the mechanical and structural properties of hydrogels. To date, there is no conclusive data on this subject.
As the cheapest, most bioavailable, natural polymer, starch is an interesting renewable resource that may have many different biomedical applications.Citation119 Starch is entirely biodegradable, noncytotoxic, biocompatible, and exhibits a high Young’s modulus with low levels of elongation at break.Citation117 It is relatively easy to modify chemically, and it has the ability to replace some more expensive, synthetic polymers in the fabrication of composite biopolymers.Citation127 When starch is combined with PCL, SPCL exhibits additional properties, such as better processability, increased mechanical properties, controllable degradability, and enhanced osteoblastic cell proliferation whereas still being relatively cheap to produce.Citation117 As the study by Requicha et al showed recently, functionalized SPCL-Si polymers induced much higher rates of bone formation as compared to the traditional (30:70) SPCL blend, further showing the potential of starch-based biopolymers as scaffolds.Citation123 However, research outside of bone tissue engineering has largely been limited, and the potential for drug delivery, while theoretically studied, has been relatively unexplored as compared to many other natural polymers. Further research needs to be undertaken to exploit the abundance of starch for use in more than just bone tissue engineering.
Alginate
Alginate is a polysaccharide derived from the cell wall of brown seaweed and extracellularly in some bacteria. It is an anionic polymer that is biocompatible, nontoxic, and non-inflammatory – as long as it undergoes multi-purification steps – but it is primarily known for its mild gelation conditions, low cost, and relatively simple modifications in making alginate derivatives with new properties.Citation128 In particular, alginate hydrogels have been prepared by various chemical or physical cross-linking methods for diverse applications in wound healing, delivery of bioactive agents, and tissue engineering.Citation129 The primary drawbacks of alginate are its generalized lack of strong mechanical properties, poor cell adhesion, and its lack of degradability in mammals.Citation128 However, by combining alginate with other biomaterials such as agarose and chitosan, and by partially oxidizing alginate with molecules like sodium periodate, scientists have managed to enhance its mechanical properties and degradability, conferring significant promise on alginate-based biomaterials.Citation129
Lately, there has been a surge of research in the use of alginate for regeneration and engineering of various tissues and organs in the body. One study attempted to create a tissue-engineered skin substitute by developing a fish collagen/alginate (FCA) sponge scaffold that was then functionalized by combining different molecular-weight chitooligosaccharides (COS) with the help of a cross-linking agent.Citation130 What they found was that the excellent biological and functional properties of collagen, along with the controllable porosity of sodium alginate, helped create a matrix for the cellular growth in skin tissue regeneration.Citation130 The addition of COS to FCA, creating FCA/COS1, resulted in a scaffold with improved cell adhesion and proliferation, ECM compatibility, improved porosity and water uptake, and overall superior physical, mechanical, and biologic properties that could potentially be a candidate for skin tissue-engineering application.Citation130 Another study blended alginate with poly(ethylene oxide) (PEO), and the resulting product was then modified by acidification of carboxylate groups via trifluoroacetic acid (TFA) to produce poly(alginic acid).Citation131 Whereas electrospun scaffolds of sodium alginate are often limited in use for tissue engineering due to high solubility and uncontrollable degradation dynamics, the poly(alginic acid) exhibited an enhanced stability in the aqueous environment and controllable degradability by changing the duration of the TFA–alginate reaction, making it attractive in the production of biomedical devices for tissue engineering.Citation131
Alginate – due to its favorable gelation conditions, biocompatibility, and relatively simple modifications – has also been studied for its use in bone regeneration and myocardial tissue regeneration.Citation132 One study showed that utilizing alginate as a dispersing agent in hydroxyapatite/chitosan composites helps to create more uniform pore structures than using just hydroxyapatite/chitosan composites.Citation133 The increased pore morphology contributed to an increase in the elastic modulus and compressive strength of the scaffold, thus substantially improving osteoblastic differentiation for bone regeneration.Citation133 In a separate study, a novel nano-biocomposite scaffold combining chitosan, gelatin, alginate, and hydroxyapatite was shown to have mechanical and biological properties mimicking natural bone.Citation134 The found that they could take advantage of alginate’s anionic nature, coupled with its excellent cross-linking abilities – especially in the presence of multivalent cations – to produce an incredibly stable nanocomposite scaffold with a prolonged degradation time necessary for the formation of neotissue and the ECM.Citation134 When it comes to tissue engineering, treatment of cardiac tissue is of notable interest, especially in the case of myocardial infarctions (MIs) where large portions of functional tissue are often lost. In a study of a rat model of acute MI, Kim et al attempted an immediate post-MI local injection of alginate–chitosan hydrogel into the peri-infarct zone and assessed the results 8 weeks later.Citation135 What they found was that this treatment promoted greater angiogenesis, increased recruitment of endogenous repair at the infarct zone via recruitment of cardiac stem cells, prevented cell apoptosis, induced cardiomyocyte cell re-entry, and, most importantly, prevented deterioration of cardiac function.Citation135 The hydrogel-injected animals demonstrated marked improvements after extensive MI and, given the simplicity of manufacturing and the entirely natural makeup of such a hydrogel, alginate-based biomaterials for myocardial regeneration is likely to play a huge role in cardiovascular repair in the future.Citation135 Furthermore, sodium alginate has one of the largest applications in the field of wound healing, due in part to not only its excellent bioresorbable and biocompatible nature, but also because of its ease of gelation and its physical cross-linking abilities, which is often favored over chemical cross-linking due to the ease with which they can be performed.Citation132,Citation136 One study formulated freeze-dried wafers combining a 75/25 sodium alginate-to-gelatin ratio and loaded it with silver sulfadiazine (a metal antimicrobial) for application on infected wounds.Citation137 Sodium alginate gel was used as the main biomaterial primarily because it allows exchange between ions in the wound exudate and the dressing, thereby creating a moist environment that promotes healing.Citation137 The gelatin is mixed in to prevent hydrated alginate from losing cation-crosslinkers over time, allowing the wound dressing to release the silver sulfadiazine over a 7-hour period, and severely reducing the bacterial bioburden compared to traditional wound dressings.Citation137 Another study found that combining the use of alginate and deoxyribonucleic acid (DNA)-based gels resulted in a sustained release of bioactive factors such as outgrowth endothelial cells, as well as neuropeptides and growth factors for treatment of diabetic foot ulcers, leading to a substantially better healing outcome than the delivery of these bioactive factors alone.Citation138 Moreover, in yet a different study, scientists found that integrating hyaluronic acid in an ionically cross-linked alginate matrix hydrogel promoted significant gap closure on dermal wound injuries compared to using either biomaterial alone.Citation139 The addition of hyaluronic acid significantly improved the mechanical properties necessary for a wound dressing whereas only very minimally affecting alginate gelation time, providing an effective and easy way to improve excisional wound injuries in a clinical setting. That is not to say that alginate-derived wound dressings have been entirely successful.Citation139 In one study, a 500-block randomized prospective study was done to test the effects of a silver-eluting alginate dressing to reduce lower-extremity vascular surgery wound complications compared to a standard dry surgical dressing. They found no significant difference in using this silver alginate dressing in reducing postoperative wound complications.Citation140 However, it is clear from these novel studies and others that alginate-based wound dressings will continue to play a significant role in the future as the relative ease of modifiability of alginate properties will allow researchers to explore a limitless number of possibilities and solutions to promote better healing.
Alginate is a widely utilized biomaterial, especially in regenerative medicine and in tissue engineering, due to its biocompatibility, mild and physical gelation process, chemical and physical cross-linking abilities, non-thrombogenic nature, and the resemblance of its hydrogel matrix texture to that of the ECM.Citation132 Moreover, alginate happens to be easily modified into any form, such as microspheres, sponges, foams, elastomers, fibers, and hydrogels, thereby broadening the scope of application of alginate-based biomaterials, and it can be combined with other natural biomaterials to create and enhance new and existing properties.Citation141 Due to the abundance of algae in water bodies, alginate is one of the most prevalent natural biomaterials in the world, making it relatively a low-cost and feasible biomaterial to use.Citation142 However, better control of polymer properties and development of its tissue-interactive forms are necessary for breakthroughs in many tissue-engineering applications.Citation132 The introduction of cell-interactive features to alginate biomaterials will become crucial in the future to properly produce replacement tissues and even organs.Citation132 The type of adhesion ligands and spatial organizations in the hydrogel of alginates are key for proper functioning of regenerated tissues, and although arginylglycylaspartic acid (RGD) peptides have been used mostly to date as a cell adhesion ligand, multiple ligands in combination – along with solubility factors – will be necessary to further our application of alginate-based biomaterials for regenerative purposes.Citation129 For wound-healing applications, alginate-based gels will need to play a more active role, incorporating one or more bioactive agents to facilitate wound healing as compared to the rather passive process they play in current clinical applications.Citation129 The future of alginate-based wound dressings hinges upon establishing more control over the delivery of one or more drugs, as well as their duration and sequence of release while considering external environmental changes.Citation129 Furthering our understanding of the fundamentals of alginate properties will help researchers take advantage of the remarkable properties and bioavailability of alginate and utilize genetic engineering techniques to control the bacterial synthesis of alginate with new and improved properties, thus revolutionizing the use of this material.Citation129
Silk
Silk fibers are natural biopolymers derived primarily from the silkworm Bombyx mori.Citation143 The silk fiber consists of two parallel SF proteins, held together by a layer of silk sericin protein glue on the surface.Citation144 Until recently, silk sericin has been deemed to be immunologically incompatible with the human body and has, therefore, been largely neglected as a biopolymer.Citation145 However, SF has been used as a biomedical suture material for centuries.Citation143 It is a semi-crystalline structure that has an incredible combination of mechanical properties, possessing very high tensile strength, coupled with excellent elasticity and flexibility.Citation143 In fact, its strength-to-density ratio is up to ten times higher than that of steel.Citation146 SF’s unique mechanical properties, tunable biodegradability, diverse side-chain chemistries, and the fact that genetic engineering techniques can be used to tailor the protein, allows it to have a variety of novel properties, functions, and applications in the biomedical field.Citation143 Furthermore, SF derived from other species in the order Lepidoptera often have other unique properties, increasing their potential for biomedical use.Citation145 In addition, some spiders, such as Antheraea mylitta, have been found to produce silks that possess better cell adhesion and a more highly ordered crystalline structure, leading to increased mechanical strength and lower solubility in acidic solvents.Citation145 Additionally, because SF is easily processed into gels, films, nanoparticles, membranes, nanofibers, scaffolds, and foam-like forms, they can be adapted to mimic a tremendous diversity of tissues in the human body.Citation143 As a result, SF has lately been studied for use in several different applications, including almost all fields of tissue engineering, wound repair, drug delivery, and even as a possible bio-ink for 3D bioprinting.Citation147
SF’s unique combination of elasticity, strength, and potential self-healing modifications (via cross-linking), along with its biocompatibility, tunable biodegradation, anti-bacterial, and other mechanical properties, makes it an attractive material to include as a part of a composite scaffold for tissue engineering.Citation148 Several different studies in the past few years have explored SF-based biomaterials for tissue engineering. In one study, Shao et al designed a nanostructured composite scaffold with the core consisting of hydroxyapatite and SF.Citation149 The composite fibers were fabricated by electrospinning – a technique used to generate nanoscale polymeric fibers using electrical energy – to create a nanofiber composite scaffold, and then subsequently compared to nanofibers of pure SF.Citation149 They found that the composite scaffold demonstrated a 90-fold and 2-fold increase in initial modulus and breaking stress, respectively.Citation149 Osteoblast-like cells were cultivated on the composite, and Shao et al found that the composite scaffold demonstrated increased biocompatibility, better cell adhesion, and proliferation as well as functionally promoted alkaline phosphatase and biomineralization.Citation149 The group concluded that the nanostructured composite scaffold consisting of a hydroxyapatite and SF core has excellent biomimetic and mechanical properties and has potential as a biocompatible scaffold for bone tissue engineering.Citation149 In another study, Tian et al utilized the same coaxial electrospinning technique to fabricate a nanofiber scaffold (p-PS/N) consisting of nerve growth factor, SF, and PLA.Citation150 After 11 days, the PC12 cells (a model for neuronal differentiation) that were cultured on these scaffolds showed elongated neurites with lengths up to 95 µm, leading the research group to conclude that p-PS/N scaffolds were able to support the attachment and differentiation of PC12 cells for nerve tissue engineering.Citation150 Another research group independently developed a 3D porous SF scaffold derived from the non-mulberry muga silkworm of Antheraea assamensis to examine its ability to support cartilage tissue engineering.Citation151 They found that the SF scaffold could generate enhanced sulfated glycosaminoglycans and type II collagen, and demonstrated in vivo biocompatibility after 8 weeks of implantation in a subcutaneous model of rat, suggesting that these non-mulberry SF scaffold may be suitable for chondrocyte-based cartilage repair.Citation151 Moreover, in a separate study, that same research group developed a 3D-blended scaffold consisting of SF and human hair-derived keratin to investigate their ability to promote enhanced fibroblast cell adhesion and proliferation.Citation152 They found that the scaffold demonstrated high porosity and interconnected pores, with excellent thermal, degradation, and mechanical properties.Citation152 In addition, they found increased expression of collagen type I in cultured cells, demonstrating functional fibroblast proliferation, and the research group concluded that blended biomaterials, specifically SF and human hair keratin-blended scaffolds, may have a promising future as a dermal substitute for skin tissue engineering.Citation152 In an attempt to engender enthesis (functional repair of tendons and ligaments), another research group, Tellado et al, engineered a complex scaffold consisting of biphasic SF scaffolds with integrated anisotropic and isotropic pore alignment, similar to what is found in native tendons/ligaments and bone/ cartilage, respectively.Citation153 The scaffolds were functionalized with heparin, and human primary adipose-derived mesenchymal stem cells were cultured on the scaffold and assessed for their ability to deliver transforming growth factor β2 (TGF-β2) and growth/differentiation factor 5 (GDF5).Citation153 This research group found that heparin functionalization increased the amount of TGF-β2 and GDF5 attached to the scaffold, leading to enhanced expression of cartilage and collagen II protein contents and enthesis, demonstrating that growth factor-loaded biphasic SF scaffolds may be useful in tendon/ligament repair.Citation153
In addition to its numerous tissue-engineering applications, SF has recently been explored for its use as a biomaterial for skin repair due to its excellent hemostatic properties, low inflammatory potential, and permeability to oxygen and water vapor.Citation154 Preliminary studies have even shown that SF film and sponge-based dressings promote wound healing and enhance skin regeneration compared to traditional hydrocolloids.Citation154,Citation155 Currently to date, there are only three SF-based medical products approved for clinical use in the world: SeriScaffold (Allergan Medical, Inc.) from the US FDA, TymPaSil (CG Bio Inc.) from the Ministry of Food and Drug Safety of South Korea and Sidaiyi (Suzhou Soho Biomaterial Science and Technology Co., Ltd) from the China Food and Drug Administration.Citation156 Of these three, only Sidaiyi is indicated for skin wound healing, but none of these three products are widely utilized in clinical practice.Citation156 A very recent clinical study done in 2017 by Zhang et al focused on developing a translational SF film for clinical application.Citation156 They conducted a single-blind, parallel, controlled clinical trial on 71 patients for treating donor-site wounds.Citation156 Compared to the positive control Sidaiyi, the SF film demonstrated substantially faster wound healing, taking approximately 9.86 days on average to complete wound healing, compared to 11.35 days for Sidaiyi.Citation156 Further, 100% of the patients treated with the SF film healed by Day 14, whereas 88.6% of patients treated with Sidaiyi healed by Day 19.Citation156 Only one inflammatory reaction case and zero adverse reaction cases was noticed in the SF film group, whereas three inflammatory reaction cases and four adverse cases were recorded in the Sidaiyi group.Citation156 The SF film group had cleaner wounds compared to the exudate-prone wounds of the Sidaiyi group, due to its better fluid handling capacity and gas permeability.Citation156 This translational study, taken together with its previously successful studies on rabbit and porcine wound models, demonstrates that this newly developed SF film may indeed be safer and more effective than the Sidaiyi material currently available for clinical use in China for the treatment of skin repair and regeneration.Citation156 To date, many other studies have recently shown positive in vitro results with electrospun nanofiber SF dressings, or composite SF dressings, as a way to enhance the bulk properties of SF.Citation157–Citation161 Based on all these studies, it is clear that SF potentially has a very good outlook for the future.Citation156
Besides the properties already mentioned, SF has many other unique and standout properties that make it excellent for use as a drug carrier.Citation148 It can allow for loading of even the most sensitive of drugs, such as proteins and nucleic acids, due to its mild, all-aqueous processing conditions.Citation148 Moreover, SF has a diverse range of amino acids with several functional groups that can simplify the attachment of different types of biomolecules or antibodies, giving it a wide degree of functionalization.Citation162 Finally, SF naturally has an intrinsic respond to pH changes, making it easy to control drug-release kinetics, and the mechanism of elimination from the body can easily be done by degradation via proteolytic enzymes in the body, leaving no likely side effects.Citation163 Recently, most research groups have been investigating the mechanistic component of incorporating SF nanoparticulate for protein delivery, small-molecules delivery, and even anticancer delivery.Citation164–Citation166 These studies have been investigating properties of SF such as electrostatic interactions for loading efficiency, different drug-delivery mediums for controlling drug-release kinetics, and encapsulation efficiency, as well as compatibility, degradability, and drug retention.Citation163 However, SF’s potential role in drug delivery has yet to be established due to the relative infancy of SF nanoparticulation, and further intensive examinations regarding SF properties and how to best exploit and improve those properties for drug delivery remains to be seen.Citation163
Tissue-engineering techniques at present, while having improved vastly from years before, still fail to capture the complexity of the 3D anatomy and functionality of human tissues and, as a result, very few engineered constructs reach human clinical trials.Citation147 3D bioprinting offers an untapped potential to capture the complexity of human tissues, and it has been touted as the future of tissue-regeneration strategies.Citation167 One of the most crucial aspects of 3D printing is bio-ink design, which not only provides the 3D architecture but also acts as the first point of contact for cells to synthesize regulatory proteins and cytokines appropriate for the tissue it is mimicking.Citation168 Silk has become one of the most popular choices for bio-ink preparations. The ability to physically cross-link its protein polymer chains via inter- and intramo-lecular β-sheet semi-crystalline structure formation allows it to be stabilized after printing without the need for any chemical or photochemical reactions or additives.Citation169 In addition, silk is a very strong and robust material as previously mentioned, and its inherent spinnability, cytocompatibility, and controllable degradability make silk a very strong candidate for future bio-ink preparations.Citation147 Of course, studies are still in its infancy stages and, so far, reported literature on silk 3D bioprinting has demonstrated cell viability after printing to be anywhere from 45% to 98%.Citation170 However, of the limited available hydrogel bio-inks currently tested, optimized blends of B. Mori silk–gelatin bio-inks has shown the most potential for 3D bioprinting of functional tissue equivalents.Citation147 Significant scientific and regulatory challenges still remain before 3D bioprinting technology can hit com-mercialization levels, and more experimental studies need to be done on optimizing silk as a potential bio-ink.Citation147
SF-based biomaterials have immense potential as one of the preeminent natural biopolymers studied today. Its ease of structural modification, controllable degradability, high tensile strength, elasticity and flexibility, potential to introduce physical cross-links, hemostatic and self-healing attributes, and its ability to be processed into numerous different forms, such as sponges, films, and hydrogels, make SF a polymer with various biomedical applications.Citation143 However, unlike starch or more bioavailable polymers, silk is produced by only a select number of species, and only in B. Mori are they available in appreciable amounts.Citation143 Although spider silk has been shown to display impressive toughness, stiffness, strength, and extensibility, it is impractical to obtain any large quantities of silk from any spider.Citation145 One study has pointed to the transgenic expression of spider silk in plants (eg, tobacco and potato) or utilizing mammalian epithelial cells as a way to garner a more substantive production of silk, but so far, no solutions are in place.Citation171,Citation172 Besides sustainability, the key to developing new silk-incorporated biomaterials and to advance our current technology in tissue engineering is to work on surface modifications or compositing with other synthetic polymers and to figure out how to control crystallization in SF when it is thermally treated or mechanically stretched.Citation145 With novel cross-linking methods, SF-based materials can be designed to self-heal, which will result in new applications for tissue engineering and wound healing.Citation145 Silk sericin – the neglected protein in silk fibers – also needs to be studied in more depth. While it has long been deemed biologically incompatible with the human body, new studies show that silk sericin is only immunogenic when associated in conjunction with SF.Citation145 When used by itself, or combined with other biopolymers, sericin has been shown to have attractive bioactive properties, with its antioxidant character, moisturizing ability, and mitogenic effect on mammalian cells.Citation173 Its promotion of keratinocytes and fibroblasts have led to the development of sericin-based biomaterials for skin tissue repair, and its ability to be cross-linked with genipin can allow for it to be used in bone, dermal, and neural tissue engineering.Citation174 Furthermore, sericin may be utilized for drug delivery because it can help facilitate the fabrication of nano-and microparticles, hydrogels, and conjugated molecules, through its chemical reactivity and pH-responsiveness, thus improving the bioactivity of drugs.Citation173 Currently, methods of purifying silk sericin protein have been mostly met with unpredictable results in size, composition, and biological activity, but future technological advancements and recovery methods may lead silk sericin to be an important biomaterial in the field of tissue engineering and drug delivery.Citation144
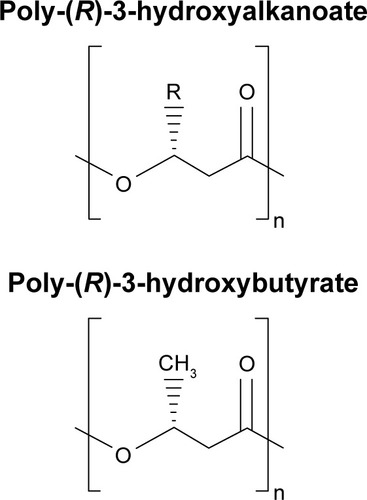
Polyhydroxyalkanoates (PHA)
PHA is a class of natural, biodegradable polyesters synthesized by microorganisms as intracellular carbon- and energy-storage compounds in uneven growth conditions.Citation175 They have exceptional biodegradability and biocompatibility, and produce nontoxic degradation products, making them excellent for use in biomedical applications such as drug delivery, tissue engineering, and substitute for implantable devices,Citation176 including sutures, repair patches slings, orthopedic pins, scaffold, stents, and adhesion barriers.Citation177 However, unmodified PHAs – despite their exceptional abilities mentioned above – have some important limitations, such as the presence of large crystals causing poor mechanical properties,Citation178 poor thermal stability, high hydrophobicity, and slow degradation rate, rendering them unfavorable to be used for many biomedical applications.Citation179 The intrinsic hydrophobicity of PHAs impedes their utilization in biomedical applications,Citation177 as many biomedical appliances require better hydrophilicity.Citation179 Moreover, PHAs are lacking in chemical functionalities, and the polyesters are frequently incompatible when combined with drugs.Citation177 Although biodegradable, PHAs are extremely stable when unmodified, thus diminishing their therapeutic functions in other areas.Citation177 Meanwhile, uncommon PHAs possessing functional side groups including hydroxyl- and/or carboxyl groups, methylated-branches, and other hydrophilic derivatives have been produced by a few organisms to broaden the applicability of PHAs.Citation180 Therefore, it is crucial that PHAs need to be modified to fix these properties while maintaining their exceptional characteristics to make them capable for use in biomedical applications.
A very important character of PHAs is that they can be developed in a way to have various physicochemical behav-iors in properties such as amphiphilicity, crystallinity, and mechanical properties by simply choosing an appropriate production strain, cultivation conditions, and carbon sources.Citation181,Citation182 Because PHAs are generated by over 300 different types of Gram-positive and Gram-negative bacteria, there are numerous ways to produce different properties of PHAs, making PHA the largest group of biopolymers and, therefore, one of the most promising in biomedical applications.Citation176 In addition to the biosynthesis of PHA using different bacteria, there are several alternative approaches that have been developed, making PHA a vastly accessible biopolymer. Blending one PHA with another PHA, blending PHAs with other biodegradable polymers and polyesters, and chemical modifications of PHAs such as grafting and copolymerization and, recently, electrospinning,Citation183 all allow easy, and more importantly, precise modulation of the PHA structure, leading to predictable functionalities.Citation179 Whereas blending has been shown to produce properties in PHAs that fall within extremes, grafting and copolymerization have led to a development of a variety of PHAs with different characteristics that may be used for several biomedical applications.Citation179 With the advantages gained from the development of metabolic engineering, PHAs with various combinations of monomers with differing proportions can be also produced from inexpensive substrates, such as glucose and fatty acids.Citation175
PHAs have been widely studied for a vast field of biomaterial applications. For example, one study compared the various properties of different PHA scaffolds and their fabrication techniques for use in bone tissue engineering and posited that composite scaffolds via mixing different PHA materials may produce even better scaffolds.Citation184 Another study specifically identified poly-3-hydroxyoctanoate – a type of PHA generated by Pseudomonas mendocina – as a PHA biomaterial that successfully showed great, distinctive properties for cardiac tissue engineering.Citation185 A different study studied solution-cast films and nonwoven electrospun membranes prepared from poly(3-hydroxybutyrate-co-4-hydroxybutyrate) solutions as experimental wound dressings and found that they accelerated wound vascularization and the healing process.Citation186 Another study assessed a different variation of PHA – a composite scaffold consisting of PHA/ ceramic composites – which showed greater bioactivity and bone regenerating potential both in vitro and in vivo.Citation187
A couple of studies found that, compared to either poly(3-hydroxybutyrate) (PHB) or poly(ethylene glycol) (PEG), a novel alternating block copolymer based on PHB and PEG (PHB-alt-PEG) had enhanced tunable mechanical properties and improved processability while remaining non-cytotoxic and thereby overcoming significant limitations mentioned previously of single unmodified PHAs.Citation188 Furthermore, Loh et al found that, by adjusting the 3-hydroxybutyrate (3HB) to ethylene glycol ratio in this synthesized thermogelling PHB-alt-PEG copolymer or the concentration of the copolymer in the hydrogel itself, the drug-release rates of multi-block PHB-alt-PEG can be easily controlled.Citation189 Further study has shown that the PHB-alt-PEG system can also be used as a long-term drug-delivery system for a mouse model of hepatocellular carcinoma by changing the concentration of the gel, showing significant potential for further development in anticancer applications.Citation190
In addition, within the last few years, discoveries regarding more therapeutic applications of PHA monomers have emerged, including the treatment of epilepsy and neurode-generative disorders. A study showed that increasing the blood concentration of 3HB – the monomer of PHB – was useful in controlling seizures: the mean latency to the onset of seizure was significantly prolonged in 3HB-treated mice, suggesting that the ketone body 3HB maintains potential as an anticonvulsant for patients with epilepsy.Citation191 Another study investigating murine L929 fibroblasts, human umbilical vein endothelial cells, and rabbit articular cartilages demonstrated that 3HB had a stimulatory effect on cell-cycle progression that was mediated by a signaling pathway dependent upon increases in [Ca2+].Citation192 Similarly, Maalouf et al found that 3HB significantly decreased the mitochondrial production of ROS and the accompanying excitotoxic changes by increasing nicotinamide adenine dinucleotide (NADH) oxidation in the mitochondrial respiratory chain, but did not affect levels of the endogenous antioxidant glutathione. Therefore, 3HB reduced glutamate-induced free radical formation by improving the NAD+/NADH ratio and increasing mitochondrial respiration in neocortical neurons.Citation193 In the same study, 3HB in combination with acetoacetate reduced neuronal cell death and prohibited changes in neuronal membrane properties. Together, these studies indicate that the ability to modify and design PHAs with a wide range of accessible properties shows great promise for PHAs as potential therapeutic biomaterials.
Clearly, extensive research has already been done on PHAs and their potential for success, but there has only been one FDA-approved PHA for biomedical use – poly(4-hydroxybutyrate) – which has very high elasticity for use as an absorbable suture.Citation194 Because each individually modified PHA needs to undergo extensive tests to prove that biocompatibility and biodegradability are still as exceptional as it is in its unmodified form, more tests need to be done for PHA use to be expanded. This is particularly true of composite PHAs, which seem to have nearly unlimited potential in creating diverse biomaterials with even better properties for their specific purpose. Furthermore, despite their enormous potential, commercial uptake of PHAs is limited due to the inconsistent polymer properties and high production costs of the raw polymer.Citation195 Moreover, sustainability is another significant issue. One recent article advocated that because substrates for PHA production can be found in carbon-rich waste located mostly in economically poor countries, integration of biopolymer production into these areas will provide both sustainable PHA production and a more extensive labor market for these countries.Citation196 However, in order for such sustainable practice to happen, policymakers, scientists, and the heads of the relevant industrial branches will need to work together.
Sundew adhesives
Sundew adhesive is a natural polysaccharide-based hydrogel which was discovered when scientists began to investigate a carnivorous plant known for its peculiar lifestyle.Citation197 This plant, the Sundew Drosera, uniquely utilizes two different glands: the sessile gland, which secretes digestive enzymes, and the stalked gland, which produces a sticky exudate to attract and then restrict prey from escaping.Citation197 This sticky exudate produced by the latter gland has, in particular, garnered the interest of many researchers due to not only its biocompatibility, biodegradability, and eco-friendly nature, but also because of its antibiotic characteristics, its unique properties of enhancing cell adhesion and differentiation, and for its extremely high elasticity.Citation198 In fact, its elasticity has been shown in studies to be so remarkable that it can be drawn into threads of approximately 1 m in length.Citation198 Upon further investigation, scientists have discovered the chemical structure of the exudate produced by Drosera to be a bio-adhesive formed by xylose, mannose, galactose, glucuronic acid, and ester sulfate in the ratio of 1:6:6:6:1.Citation199
Studies done on the Sundew have mostly returned with positive findings. For example, Zhang et al found that drying the adhesive produced by the sundew plant allowed neuron-like cells to attach and grow on the nanofibers made by the dried adhesive, showing the potential of the sundew adhesive for tissue engineering.Citation199 In more recent studies, fibrous scaffolds obtained from the Sundew adhesive have been found to increase adhesion of numerous types of cells, including fibroblast and smooth muscle cells. It was found that nano-networks within the sundew adhesives exhibit viscoelastic behaviors that allow for, among other things, more powerful adhesion of multiple mammalian cells.Citation197
However, despite the promise Sundew adhesives have shown, some concerns have been raised that may limit the potential of the sundew adhesives. One study found that the sundew adhesives are highly susceptible to temperature variation and that adhesion strength was substantially diminished at temperatures between −20°C and −80°C.Citation197 However, a much more important issue that may overshadow the sundew adhesives’ therapeutic potential is the ability to collect sufficient quantities of the hydrogel.Citation200 However, Sun et al were able to develop a sundew-inspired adhesive hydrogel mimicking the native sundew adhesives with superior adhesive strength, nanostructure, and resistance to shearing compared to other hydrogels in vitro, whereas also demonstrating superior wound-healing capabilities when paired with mouse adipose-derived stem cells in vivo.Citation200 Despite these findings, much more study needs to be done to see if there are more efficient methods of collecting the natural Sundew adhesive, and to assess the safety and efficacy of sundew-inspired adhesive hydrogels.
Ivy nanoparticles
Ivy, which belongs to the genus Hedera, is known for the unique ability to affix itself to and grow upwards on surfaces such as rocks, trees, and fences, to name just a few.Citation201 To do this, ivy has been found to use adhering disks generated by the stem of the Ivy plant.Citation201 These adhering disks consist of four to seven tendrils or “fingers” which generate a substance called ivy nanoparticles (INPs).Citation201 These INPs are entirely organic and are formed by a group of macromolecules composed of arabinogalactan proteins, and it is the INPs that are primarily responsible for the immense amount of force the Ivy can generate.Citation202 In fact, it has been shown that an ivy disc weighing just 0.5 mg can produce approximately 0.9 kg pull-off force, which is 1.8 million times larger than the weight of the adhering disc itself.Citation203 This incredible adhesive strength – combined with their excellent aqueous solubility, low intrinsic viscosity, biocompatibility, and biodegradability – demonstrate the immense potential of INPs in the field of tissue engineering.Citation14
In one recent study investigating the potential of INPs in drug delivery, INP-conjugated doxorubicin exhibited a stronger cytotoxic effect against multiple cancer cell lines in vitro and in vivo.Citation14 In that same study, INPs were also found to allow stronger adhesion of smooth muscle cells in the collagen scaffolds once they were embedded with INPs, showing that not only can INPs be used as drug carriers for cancer therapy, but also as nano-fillers to enrich scaffolds.Citation14 In addition, INPs demonstrate unique optical properties that exhibit strong Ultraviolet (UV) absorbance and scattering, potentially allowing them to play an important role as a sunscreen protective agent.Citation204 Currently, most sunscreens today utilize metal oxide nanoparticles as fillers; however, environmental concerns, as well as safety concerns of using these nanoparticles on skin, make using traditional sunscreens less than ideal. When compared to TiO2 nanoparticles, which are the metal oxide nanoparticles used frequently in traditional sunscreens today, the ivy nanoparticles block more UV light, are less toxic to mammalian cells, and are more easily biodegradable, showing that ivy nanoparticles may potentially be used as a better, safer sunscreen agent.Citation205
Considering their excellent physical profile, it is likely there are far more applications for these INPs. Unfortunately, application of INPs is severely limited due to the obscurity they have been in.Citation202 In fact, the structure of INPs has only recently been identified. More studies need to be done to verify their safety and efficacy, as well as to discover a sustainable way to produce these nanoparticles – whether they are naturally derived or synthetically mimicked.Citation202
Synthetic biodegradable polymers
Although natural polymers have demonstrated the potential advantage of supporting cell function and adhesion, there are a few limitations and concerns with regard to their use. For example, it is difficult to control the mechanical properties and degradation rates of natural polymers, and there exists the potential for a natural polymer to elicit an immune response or carry microbes or viruses.Citation16,Citation206 In contrast, synthetic polymers can be modified to possess a much wider range of mechanical and chemical properties than natural polymers. Although synthetic polymers can avoid problems with immunogenicity, biocompatibility poses a new challenge.Citation207 Thus, degradable synthetic polymers are currently being studied extensively in order to avoid the potential long-term effects associated with non-degradable polymers, such as scarring and inflammation.Citation207,Citation208 Meanwhile, synthetic polymers can be produced under controlled conditions and thus exhibit predictable and reproducible mechanical and physical properties such as tensile strength, elastic modulus, and degradation rate.Citation207 A further advantage of synthetic polymers is the control of material impurities. Lastly, pure synthetic polymers with well-defined and simple structures have lower possible risks with regard to toxicity, immunogenicity, and infections.
Saturated aliphatic polyesters
Saturated aliphatic polyesters, such as poly(glycolic acid) (PGA), PLA, and PLGA copolymers, are the most often used biodegradable synthetic polymers for 3D scaffolds in tissue engineering.Citation207–Citation211 The chemical properties of these polymers allow hydrolytic degradation through de-esterification. For example, PLA and PGA can be processed easily, and their degradation rates and physical and mechanical properties are adjustable over a wide range by using various molecular weights, structure, composition, and copolymers.Citation207,Citation208,Citation211 Moreover, the body contains highly regulated mechanisms for completely removing monomeric components of glycolic and lactic acids when these polyesters degrade: glycolic acid is converted to metabolites or eliminated by other mechanisms, whereas lactic acid can be cleared through the tricarboxylic acid cycle.Citation212 Due to their biodegradability and biocompatibility, PGA and PLA have been approved by the FDA for use in medical devices, such as degradable sutures and other implantable devices.Citation213
PGA
PGA is a hydrophilic and highly crystalline polymer with a relatively fast degradation rate. It degrades rapidly in aqueous solutions or in vivo, and loses its mechanical integrity between 2 and 4 weeks – depending on the molecular weight and the degradation conditions.Citation212,Citation214 Moreover, PGA has great flexibility in the tuning of its material properties and physical parameters such as pore size and tortuosity, which are important for developing scaffold-based tissue-engineering constructs.Citation212,Citation214 Thus, PGA can be used not only in tissue engineering, but also in wastewater treatment, food products, and other biomedical applications such as drug delivery or biological glues.Citation215–Citation219 Furthermore, previous studies have successfully developed PGA sheets combined with fibrin glue to treat open soft tissue wounds during oral surgery.Citation220,Citation221 Similarly, PGA sheets with fibrin glue were efficient at prohibiting postoperative bleeding, reducing postoperative pain, and enhancing epithelialization during the reconstruction of bone surfaces following tumor resection in the oral cavity.Citation222 However, the relatively fast degradation rate – along with the acidic degradation products and low solubility – confer inherent disadvantages and, consequently, limit the biomedical applications for PGA.Citation223 Thus, ongoing research continues with regard to several PGA-based copolymers in order to bypass these obstacles.
PLA
PLA is another widely used biodegradable scaffolding material in biomedical applications.Citation224 Although similar in structure to PGA, PLA exhibits different chemical, physical, and mechanical properties due to the presence of an extra methyl group in its repeating units. For example, it can take months to years to lose the mechanical integrity of a PLA scaffold,Citation224 which makes PLA a more suitable biomaterial for load-bearing applications, such as orthopedic fixation devices. To date, multiple PLA-based orthopedic products are available in the market, including the Phantom Soft Thread Soft Tissue Fixation Screw® (DePuy), Phantom Suture Anchor® (DePuy), Full Thread Bio Interference Screw® (Arthrex), BioScrew® (Conmed), Bio-Anchor® (Conmed), Meniscal Stinger® (Linvatec), and the Clearfix Meniscal Dart® (Inno-vasive Devices). A novel promising biomedical engineering application for PLA involves photoluminescent graphene quantum dots (GQDs) with a large surface area and superior mechanical flexibility, which possess interesting optical and electronic properties.Citation225 A recent study reported that the multifunctional nanocomposite of PLA and PEG-grafted GQDs (f-GQDs) is biocompatible with low cytotoxicity, which makes it suitable for simultaneous intracellular microRNAs (miRNAs) imaging analysis as well as gene delivery.Citation225 These results underscore the potential of the extremely versatile multifunctional nanocomposite – f-GQDs – in biomedical applications of intracellular molecular analysis and clinical gene therapeutics.
Three forms of PLA exist: L-PLA, D-PLA, and a racemic mixture of D,L-PLA. Recently, the stereoisomer D,L-PLA has been significantly explored as a biomedical coating for orthopedic material due to its high mechanical stability and excellent biocompatibility.Citation207,Citation226,Citation227 Furthermore, D,L-PLA of low molecular weight can be combined with drugs such as growth factors, antibiotics, or thrombin inhibitors to establish a locally acting drug-delivery system.Citation9 Thus, recent efforts have shifted focus toward applying D,L-PLA as a scaffold material for tissue engineering due to these highly desirable features.
PLGA
PLGA with varying lactide/glycolide ratios can be synthesized to achieve intermediate degradation rates between PLA and PGA. Generally, the copolymer PLGA is favored in comparison with its constituent homopolymers for the development of bone substitute constructs because PLGA offers greater control of degradation properties by varying the ratio between its monomers.Citation228 For example, PLGA possesses a wide range of degradation rates dictated by the composition of chains, hydrophobic/hydrophilic balance, and crystallinity.Citation228 However, despite biocompatibility, the clinical application of pure PLGA for bone regeneration is hindered by low osteoinductivity and suboptimal mechanical properties for load-bearing applications. Thus, PLGA is typically used in conjunction with other materials, such as ceramics or bioactive glass, and is routinely modified in order to render it more biomimetic, thereby improving its ability to enhance bone regeneration.Citation229 By implanting AgNP/PLGA composite grafts into grossly infected critical-sized bone segmental defects, our group demonstrated that AgNP/PLGA composite grafts possess significant antibacterial properties and osteoconductivity in vivo.Citation209 In a follow-up experiment, we unexpectedly found that AgNP/PLGA-coated stainless steel alloy materials not only exhibited strong antibacterial activity but also presented significant osteoinductivity that was not observed in their individual components alone.Citation210 Similarly, Shi et al reported that PLGA/hydroxyapatite microsphere composites with the bisphosphonate-based osteoporosis-preventing drug alendronate significantly inhibited the growth of macrophages, which have been identified as precursors of osteoclasts and as being potentially responsible for osteoporosis, while improving osteoblast proliferation and upregulating the expression of a key osteogenic enzyme – alkaline phosphatase.Citation230 Together, these findings require more research into the osteoconduc-tive and osteoinductive properties of PLGA-based systems but, nevertheless, offer promising therapeutic material for orthopedic surgery.
Poly(ε-caprolactone) (PCL)
PCL is another saturated aliphatic biodegradable polyester used in the development of tissue-engineering scaffolds and other biomedical applications.Citation231–Citation233 PCL is a semi-crystalline polymer with a melting temperature of 55°C–60°C and a very low glass-transition temperature of approximately −54°C and, thus, it tends to maintain a rubbery state and high material permeability under physiological conditions.Citation231–Citation233 It can be degraded by microorganism, hydrolytic, enzymatic, or intracellular mechanisms under physiological conditions; however, PCL’s slow degradation rate of 2–4 years, compared to PLA, PGA, and PLGA, along with its hydrophobicity makes it less attractive for general tissue-regeneration applications and more attractive for long-term implants and drug-delivery systems.Citation234,Citation235 A recent study demonstrated that gravity-spun collagen-coated PCL fibers enhanced proliferation rates of human osteoblast cells.Citation234 Accordingly, these findings underscore the potential of gravity-spun PCL fibers as a delivery platform for ECM proteins to enhance cell adherence and proliferation for tissue repair. Similarly, PCL has been used to effectively entrap antibiotic drugs and, subsequently, was incorporated into a drug-delivery system for enhancing bone ingrowth and regeneration in the treatment of bone defects.Citation236 Moreover, ongoing research continues regarding micro- and nanoscale drug-delivery vehicle systems centered on PCL.Citation237 Additionally, a solid, freeform, fabrication-based injection molding process has been developed for the fabrication of PCL, and the resulting PCL–calcium phosphate scaffolds display in vitro cytocompatibility and suitable mechanical properties for hard tissue repair.Citation238 Further, Chiari et al have reported the viability of using a composite matrix composed of PCL and HA as a possible meniscus substitute.Citation239 Overall, PCL is a useful biodegradable biomaterial deserving further investigation as a porous scaffold for bone tissue engineering and as a drug-delivery vehicle.
Polyanhydrides
PLA, PGA, and PLGA possess immense utility for drug delivery, but they do have limitations, including a tendency for a non-uniform release profile with certain drugs. Thus, in response to these problems, polyanhydrides were created. Polyanhydrides were initially designed for drug-delivery applications due to their hydrophobicity and ability to undergo degradation through surface erosion rather than bulk degradation, which allows for a constant release profile for certain drugs and is especially important in the case of extremely potent drugs.Citation240 Polyanhydrides are biocompatible and degradable in vivo into nontoxic diacid byproducts, which are eliminated from the body as metabolites.Citation240 For these reasons, drugs can be well protected when implanted in such polymers due to the fact that almost no water penetrates before the polymer erodes.Citation241 Therefore, in 1996, polyanhydrides were approved as a drug-delivery vehicle by the FDA following comprehensive drug release and biocompatibility evaluations.Citation242
Generally, polyanhydrides can be easily synthesized from widely available, low-cost sources and have been modified to possess desirable characteristics.Citation243 Poly[(carboxyphenoxy propane)-(sebacic acid)] is the most widely investigated FDA-approved polyanhydride. It is typically utilized as a localized delivery vehicle, specifically for the controlled delivery of the chemotherapeutic agent bis-chloroethyl nitrosourea during brain cancer treatment (Gliadel®, Arbor Pharmaceuticals, LLC).Citation244 Moreover, the results of previous animal studies were validated in a recent human trial, verifying the promising potential of this polymer drug-delivery system.Citation244 Clearly, future exploration of polyanhydrides in drug-delivery systems is warranted.
Polyurethane (PUR)
Owing to their toughness, durability, biocompatibility, and biostability, PURs are a favorable choice for medical devices and, since the 1960s, they have been utilized typically as biostable and inert materials in heart valves, vascular grafts, catheters, and prostheses.Citation245,Citation246 However, in the late 1990s, interest regarding the design of biodegradable PURs for tissue engineering and drug delivery surged due to their relative sensitivity to biodegradation as well as the desire to further understand the biological mechanisms for in vivo biodegradation.Citation246 Biodegradable PURs possess convincing potential as scaffolds for tissue regeneration.Citation245,Citation246 They exhibit a broad range of versatility in terms of modifiable mechanical properties, biological properties, physical properties, biodegradability, and blood and tissue compatibility due to their segmented-block structural character.
PURs are normally synthesized through a polycondensation reaction of diisocyanates with alcohols/amines, whereas the synthesis of biodegradable PURs allows for the incorporation of hydrolyzable segments into their backbone.Citation247 Nevertheless, the toxicity of common diisocyanates such as toluene diisocyanate and 4,4′-methylenediphenyl diiso-cyanate has led to the consideration of other biocompatible aliphatic diisocyanates for developing a new generation of biodegradable PURs. Recently, the development of biocompatible aliphatic diisocyanates and amino acid-derived diisocyanates possessing reduced toxicity, such as lysine diisocyanate and 1,4-diisocyanatobutane, has allowed for novel opportunities to synthesize biocompatible and biodegradable PURs that can enhance cell proliferation and adhesion without adverse effects.Citation247 Moreover, a biodegradable elastic PUR – Degrapol® (Abmedica) – is currently being used to develop a highly porous scaffold for tissue- engineering applications.Citation248 It is also worth noting that a unique, injectable, two-component lysine–diisocyanate-based PUR system – PolyNova® (PolyNovo Biomaterials Pvt. Ltd) – has been developed for orthopedic applications.Citation249 PolyNova® polymerizes at physiological temperature in situ and, thus, arthroscopic administration in its liquid form results in suitable mechanical support and comparable or superior bonding strength relative to standard bone cement. Additionally, it supports favorable cell adhesion and proliferation.Citation249 Together, the research indicates the promising potential for PURs in various biomedical applications, such as porous scaffolds for tissue engineering and drug delivery.Citation250
Polyphosphazenes
Polyphosphazenes are a relatively newer class of inorganic– organic hybrid polymers composed of an inorganic backbone of repeating phosphorus and nitrogen atoms with alternating single and double bonds. To date, polyphosphazenes have been investigated as potential biodegradable biomaterials due to their synthetic flexibility, unparalleled functionality, and adaptability for numerous applications.
The phosphorous–nitrogen backbone of polyphosphazenes confers extraordinary flexibility, whereas their side groups determine the different properties of these polymers. Considering these features, polymers possessing highly controlled properties including solubility, hydrophobicity/ hydrophilicity, extent of crystallinity, and appropriate thermal transitions can be designed and developed through modification of their side groups.Citation251 Furthermore, the degradation profiles of the polymers can be controlled by modifying their side groups to achieve appropriate degradation profiles ranging from a few hours to years.Citation251 Laurencin et al studied different poly[(amino acid ester) phosphazenes] and revealed that polyphosphazenes showed the fastest degradation after modification with glycine ethyl ester.Citation252 In contrast with polyesters, the poly[(amino acid ester) phosphazenes] degrade into neutral and nontoxic products such as ammonia, phosphates, and the corresponding ester side groups.Citation252 Taking advantage of this unique property, a recent study combined polyphosphazenes with PLGA to form self-neutralizing blend systems.Citation253 In addition, polyphosphazenes are suitable for drug delivery with their distinct ability to undergo both surface and bulk erosion while maintaining controllable rates and modes of degradation.Citation254
With regard to biocompatibility and toxicity, the majority of the poly[(amino acid ester) phosphazenes] implanted subcutaneously elicited minimal to mild tissue responses.Citation251,Citation255–Citation257 Furthermore, several of the poly[(amino acid ester) phosp-hazenes] have demonstrated significant osteoconductivity and have been explored as matrices for bone tissue engineering.Citation251,Citation255 Recently, by utilizing the advantageous interactions between polyphosphazene side groups and calcium phosphate ceramics, a polyphosphazene-self setting calcium phosphate composite cement system was created.Citation258 However, as a result of the flexible backbone, many of the poly[(amino acid ester) phosphazenes] are soft elastomeric polymers and, thus, present limitations as a biomaterial used for load-bearing applications. Additionally, a recent study found success in using a glycine-based photo-polymerizable polyphosphazene as a scaffold for adipose tissue regeneration.Citation259 Preliminary results demonstrated the non-cytotoxic nature of the polymers and their degradation products and the cell adhesion and proliferation of adipose-derived stem cells.Citation259 Meanwhile, another report suggests that blends with PLGA may benefit the application of polyphosphazenes in osteogenesis. The initial hydrolytic degradation of PLGA generates a porous structure with some residual strength, which would then degrade over a much longer period of time. Therefore, fine-tuning the system could potentially yield biomaterials with tissue-engineering properties superior to those of PLGA.Citation260 However, their utility as scaffolds in tissue engineering is still under investigation and warrants further studies.
In truth, with regard to on-the-market applications and clinical studies, polyphosphazenes are severely lacking compared to the more established polymers for drug-delivery applications. However, the many promising in vitro and in vivo studies spanning a broad range of therapies highlights the potential of polyphosphazenes in this area.Citation251–Citation260 Furthermore, the inherent high functionality of the phosphorus–nitrogen backbone along with the intrinsic, tunable, biodegradability of polyphosphazenesCitation251 underscores their immense potential as a group of biomaterials for drug delivery and other biomedical applications.
Conclusion
Due to the presence of a wide diversity of biomaterials, both natural and synthetic, varying quality of material formulation, and a general lack of comparative studies of different biomaterials for specific biomedical applications, it is impossible to conclude which polymer is the most ideal. In general, natural biomaterials have greater inherent biocompatibility compared to synthetic biomaterials, but they are also mechanically inferior, as their mechanical, structural, and chemical properties cannot be altered in the same way as synthetic biomaterials.
However, instead of trying to categorize specific polymers for specific biomedical applications, it seems that the future of biopolymer application is to utilize different combinations of polymers to develop hybrid polymers that have a better specificity profile for specific biomedical applications – be it for tissue engineering, drug delivery, or wound healing. Indeed, numerous recent studies regarding polymers for biomedical use have addressed combining different biomaterials, via techniques like blending, grafting, and chemical cross-linking reactions, and the results have been mostly positive. With techniques like electrospinning – which allows for the creation of various different forms of nanoscale polymers, such as nanotubes, nanofibers, and nanospheres – combined with the nearly limitless potential of generating numerous different combinations of natural and synthetic biomaterials, there exists a very good chance that more effective biomaterial-based materials can be fabricated with the proper biocompatibility, degradation, and physicochemical properties for a specific biomedical application. Numerous challenges still exist today across most or all biomaterials, such as the feasibility of mass production at a relatively low cost as well as overcoming certain physiochemical limitations of specific biomaterials; however, with the advent of cloning and genetic engineering techniques to express both native and synthetic biomaterials in a variety of host systems, there remains a bright future for biopolymeric use in medicine.
Currently, in the field of tissue engineering, temporary, artificial composite scaffolds are being researched and developed for cells to adhere to, differentiate, and form new tissue. However, there is an increased interest in developing 3D scaffolds that can not only support tissue regeneration, but also act as a biomatrix that can support cues and signals to promote functional tissue connections.Citation145 One increasingly popular way to generate such a scaffold is to utilize 3D bioprinting techniques, incorporating polymeric biomaterials as the bio-ink. This technology will allow us to have more control in the scaffold properties that were traditionally very difficult or impossible to control, such as cell distribution, fluid flow, and porosity. These resulting 3D-engineered constructs will allow us to mimic the properties of tissue in the human body better than any other traditionally engineered approach, making it more desirable for tissue regeneration. The ultimate goal of 3D bioprinting is to eventually be able to develop patient-specific tissues and organs, and although several technical, scientific, regulatory, and even ethical challenges still exist, there has been an exponential increase in research and interest into making such a technology a reality.
Drug-delivery systems traditionally are either orally administered or injected, but problems exist in using such methods for protein and nucleic acid delivery. Furthermore, traditional drug-delivery systems still face problems with drug side effects, efficacy, and patient compliance. Recently, the use of nanotechnology has ushered in a novel strategy for drug delivery. Utilizing nanotechnology has led to the development of numerous novel carriers that are capable of releasing not only a wider range of molecules, proteins, peptides, and nucleic acids, but also allows for a more specific targeted delivery with controlled release. Nanoparticles based on biodegradable and biocompatible polymers, in particular, have recently shown potential in cancer therapy and as sustained drug-delivery vehicles, prolonging drug half-life, improving solubility, and reducing immunogenicity. Furthermore, they have shown the ability to simultaneously co-deliver multiple drugs, making patient compliance more likely whereas also enhancing potential synergistic effects of certain drugs and suppressing drug resistance. These biodegradable and biocompatible polymeric-based nanoparticulation can also be applied in wound-healing applications via delivery of bioactive agents. Current clinical wound-healing applications play a much more passive role, and development of this technology will help with better, more efficient wound healing. Nanosized particles for drug delivery clearly demonstrate significant advantages over traditional drug-delivery systems, and the key to future development of drug-delivery systems is the increased understanding and application of nanotechnology.
Disclosure
The authors report no conflicts of interest in this work.
References
- PiskinEBiodegradable polymers as biomaterialsJ Biomater Sci Polym Ed1995697757957772566
- TaylorPGlobal Markets For Implantable Biomaterials [market report on the Internet]MassachusettsBCC Research20151 [cited March 1, 2016] Available from: http://www.bccresearch.com/market-research/advanced-materials/implantable-biomaterials-markets-report-avm118a.htmlAccessed February, 2018
- BarbucciRIntegrated Biomaterials ScienceNew YorkKluwer Academic/Plenum Publishers2002
- LangerRVacantiJPTissue engineeringScience (New York, N. Y.)19932605110920926
- VacantiJPLangerRTissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantationLancet1999354Suppl 1S32S34
- DvirTTimkoBPKohaneDSLangerRNanotechnological strategies for engineering complex tissuesNat Nanotechnol201161132221151110
- KohaneDSLangerRPolymeric Biomaterials in Tissue EngineeringPediatr Res200863548749118427292
- VertMAliphatic Polyesters: Great Degradable Polymers That Cannot Do EverythingBiomacromolecules20056253854615762610
- GollwitzerHIbrahimKMeyerHMittelmeierWBuschRStembergerAAntibacterial poly(D,L-lactic acid) coating of medical implants using a biodegradable drug delivery technologyJ Antimicrob Chemother200351358559112615858
- FreymanTMYannasIVYokooRGibsonLJFibroblast contraction of a collagen–GAG matrixBiomaterials200122212883289111561894
- MarijnissenWJCMvan OschGJVMAignerJAlginate as a chondrocyte-delivery substance in combination with a non-woven scaffold for cartilage tissue engineeringBiomaterials20022361511151711833491
- ParkHChoiBHuJLeeMInjectable chitosan hyaluronic acid hydrogels for cartilage tissue engineeringActa Biomater2013914779478622935326
- LenaghanSCSerpersuKXiaLHeWZhangMA naturally occurring nanomaterial from the Sundew (Drosera) for tissue engineeringBioinspir Biomim20116404600922064887
- HuangYWangYJWangYExploring naturally occurring ivy nanoparticles as an alternative biomaterialActa Biomater20152526828326219859
- GelseKPöschlEAignerTStructureC-function, and biosynthesisAdvanced Drug Delivery Reviews200355121531154614623400
- LeeCHSinglaALeeYBiomedical applications of collagenInt J Pharm20012211–212211397563
- ChattopadhyaySRainesRTReview collagen-based biomaterials for wound healingBiopolymers2014101882183324633807
- KretlowJDYoungSKloudaLWongMMikosAGInjectable Bio-materials for Regenerating Complex Craniofacial TissuesAdv Mater (Deerfield Beach, Fla)20092132–3333683393
- PomahačBSvensjöTYaoFBrownHErikssonETissue Engineering of SkinCrit Rev Oral Biol Med1998933333449715370
- VinFTeotLMeaumeSThe healing properties of Promogran in venous leg ulcersJ Wound Care200211933534112430368
- AwadHABoivinGPDresslerMRSmithFNLYoungRGButlerDLRepair of patellar tendon injuries using a cell–collagen compositeJ Orthop Res200321342043112706014
- Juncosa-MelvinNShearnJTBoivinGPEffects of Mechanical Stimulation on the Biomechanics and Histology of Stem Cell–Collagen Sponge Constructs for Rabbit Patellar Tendon RepairTissue Eng20061282291230016968169
- Juncosa-MelvinNMatlinKSHoldcraftRWNirmalanandhanVSButlerDLMechanical Stimulation Increases Collagen Type I and Collagen Type III Gene Expression of Stem Cell–Collagen Sponge Constructs for Patellar Tendon RepairTissue Eng20071361219122617518715
- AtalaATissue engineering for bladder substitutionWorld J Urol200018536437011131316
- LiuSPeulvePJinOAxonal regrowth through collagen tubes bridging the spinal cord to nerve rootsJ Neurosci Res19974944254329285519
- BozkurtAClaeysKGSchradingSClinical and biometrical 12-month follow-up in patients after reconstruction of the sural nerve biopsy defect by the collagen-based nerve guide NeuromaixEur J Med Res20172213428938917
- NarotamPKJoséSNathooNTaylonCVoraYMatrixCCollagen Matrix (DuraGen) in Dural Repair: Analysis of a New Modified TechniqueSpine20042924286828669
- GruessnerUClemensMPahlplatzPVSperlingPWitteJRosenHRImprovement of perineal wound healing by local administration of gentamicin-impregnated collagen fleeces after abdominoperineal excision of rectal cancerAm J Surg2001182550250911754859
- JohnPLazarusFGeorgeJPSelvamAPrabhujiMLAdjunctive Effects of A Piscean Collagen-Based Controlled-Release Chlorhexi-dine Chip in the Treatment of Chronic Periodontitis: A Clinical and Microbiological StudyJ Clin Diagn Res201595ZC70ZC74
- GeigerMRhLFriessWCollagen sponges for bone regeneration with rhBMP-2Adv Drug Deliv Rev200355121613162914623404
- JovanovicSAHuntDRBernardGWSpiekermannHWozneyJMWikesjöUMEBone reconstruction following implantation of rhBMP-2 and guided bone regeneration in canine alveolar ridge defectsClin Oral Implants Res200718222423017348887
- SanoAMaedaMNagaharaSAtelocollagen for protein and gene deliveryAdv Drug Deliv Rev200355121651167714623406
- KoideTDesigned triple-helical peptides as tools for collagen biochemistry and matrix engineeringPhilosophical Trans Royal Society B Biol Sci2007362148412811291
- BadylakSFGilbertTWImmune response to biologic scaffold materialsSemin Immunol200820210911618083531
- AnBKaplanDLBrodskyBEngineered recombinant bacterial collagen as an alternative collagen-based biomaterial for tissue engineeringFront Chem201424025003103
- SmitNWTen SandeJNParviziMRecombinant human collagen-based microspheres mitigate cardiac conduction slowing induced by adipose tissue-derived stromal cellsPLoS One2017128e018348128837600
- TangYYangXHangBEfficient Production of Hydroxylated Human-Like Collagen Via the Co-Expression of Three Key Genes in Escherichia coli Origami (DE3)Appl Biochem Biotechnol201617871458147026712247
- PengYYHowellLStoichevskaVWerkmeisterJADumsdayGJRamshawJAMTowards scalable production of a collagen-like protein from Streptococcus pyogenes for biomedical applicationsMicrob Cell Fact201211114614623126526
- BrodskyBRamshawJABioengineered CollagensParryDADSquireJMFibrous Proteins: Structures and MechanismsChamSpringer International Publishing2017601629
- Ghasemi-MobarakehLPrabhakaranMPMorshedMNasr- EsfahaniM-HRamakrishnaSPolyEElectrospun poly(ɛ-caprolactone)/ gelatin nanofibrous scaffolds for nerve tissue engineeringBiomaterials200829344532453918757094
- BurkeCJHsuTAFormulationVDBstability, and delivery of live attenuated vaccines for human useCrit Rev Ther Drug Carrier Syst199916118310099898
- GouYMiaoDZhouMWangLZhouHSuGBio-Inspired Protein-Based Nanoformulations for Cancer TheranosticsFront Pharmacol2018942129755355
- SaddlerJMHorseyPJThe new generation gelatins. A review of their history, manufacture and propertiesAnaesthesia198742999810043314576
- FooxMZilbermanMDrug delivery from gelatin-based systemsExpert Opin Drug Deliv20151291547156325943722
- OzkizilcikATuzlakogluKA new method for the production of gelatin microparticles for controlled protein release from porous polymeric scaffoldsJ Tissue Eng Regen Med20148324224722499408
- LakshminarayananRSridharRLohXJInteraction of gelatin with polyenes modulates antifungal activity and biocompatibility of electrospun fiber matsInt J Nanomedicine201492439245824920895
- CohenBShefy-PelegAZilbermanMNovel gelatin/alginate soft tissue adhesives loaded with drugs for pain management: structure and propertiesJ Biomater Sci Polym Ed201425322424024156311
- KarimAABhatRFish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatinsFood Hydrocoll2009233563576
- BigiACojazziGPanzavoltaSRoveriNRubiniKStabilization of gelatin films by crosslinking with genipinBiomaterials200223244827483212361622
- RajangamTSsaAFibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applicationsInt J Nanomedicine201383641366224106425
- AhmedTAEDareEVHinckeMFibrin: A Versatile Scaffold for Tissue Engineering ApplicationsTissue Eng Part B Rev200814219921518544016
- KjaergardHKVeladaJLPedersenJHFleronHHollingsbeeDAComparative Kinetics of Polymerisation of Three Fibrin Sealants and Influence on Timing of Tissue AdhesionThromb Res200098222122810713324
- TredwellSJSawatzkyBThe Use of Fibrin Sealant to Reduce Blood Loss During Cotrel-Dubousset Instrumentation for Idiopathic ScoliosisSpine19901599139152259980
- McgillVKowal-VernALeeMUse of Fibrin Sealant in Thermal InjuryJ Burn Care Rehabil19971854294349313125
- JacksonMRMacpheeMJDrohanWNAlvingBMFibrin sealant: Current and potential clinical applicationsBlood Coagul Fibrinolysis1996787377469034553
- BrigantiESpillerDMirtelliCA composite fibrin-based scaffold for controlled delivery of bioactive pro-angiogenetic growth factorsJ Control Release20101421142119811766
- BarsottiMCMageraAArmaniCFibrin acts as biomimetic niche inducing both differentiation and stem cell marker expression of early human endothelial progenitor cellsCell Prolif2011441334821199008
- de La PuentePLudeñaDLópezMRamosJIglesiasJDifferentiation within autologous fibrin scaffolds of porcine dermal cells with the mes-enchymal stem cell phenotypeExp Cell Res2013319314415223124076
- ZischAHSchenkUSchenseJCSakiyama-ElbertSEHubbellJACovalently conjugated VEGF–fibrin matrices for endothelializationJ Control Release2001721–310111311389989
- de La PuentePLudeñaDCell culture in autologous fibrin scaffolds for applications in tissue engineeringExp Cell Res2014322111124378385
- PradeepARNagpalKKarvekarSPatnaikKNaikSBGuruprasadCNPlatelet-Rich Fibrin With 1% Metformin for the Treatment of Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical TrialJ Periodontol201586672973725762357
- AjwaniHShettySGopalakrishnanDComparative evaluation of platelet-rich fibrin biomaterial and open flap debridement in the treatment of two and three wall intrabony defectsJ Int Oral Health201574323725954068
- WongCInmanESpaetheRHelgersonSFibrin-based biomateri-als to deliver human growth factorsThromb Haemost200389357358212624643
- de La PuentePLudeñaDFernándezAArandaJLVarelaGIglesiasJAutologous fibrin scaffolds cultured dermal fibroblasts and enriched with encapsulated bFGF for tissue engineeringJ Biomed Mater Res A201199A4648654
- LinnesMPRatnerBDGiachelliCMA fibrinogen-based precision microporous scaffold for tissue engineeringBiomaterials200728355298530617765302
- TooleBPHyaluronan: from extracellular glue to pericellular cueNat Rev Cancer20044752853915229478
- El MaradnyEKanayamaNKobayashiHThe role of hyaluronic acid as a mediator and regulator of cervical ripeningHum Reprod1997125108010889194670
- MeyerIKPalmerJWThe polysaccharides of the vitreous humorJ Biol Chem1934107629634
- MoriMYamaguchiMSumitomoSTakaiYHyaluronan-based Biomaterials in Tissue EngineeringActa Histochem Cytochem200437115
- AllisonDDGrande-AllenKJReview G-AKJReview. Hyaluronan: A Powerful Tissue Engineering ToolTissue Eng20061282131214016968154
- PrestwichGDEngineering a clinically-useful matrix for cell therapyOrganogenesis200841424719279714
- BurdickJAPrestwichGDHyaluronic Acid Hydrogels for Biomedical ApplicationsAdv Mater20112312H41H5621394792
- YuANiiyamaHKondoSYamamotoASuzukiRKuroyanagiYWound dressing composed of hyaluronic acid and collagen containing EGF or bFGF: comparative culture studyJ Biomater Sci Polym Ed20132481015102623647255
- NiiyamaHKuroyanagiYDevelopment of novel wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor and vitamin C derivativeJ Artif Organs2014171818724292853
- PrestwichGDHyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicineJ Control Release2011155219319921513749
- HuntDRJovanovicSAWikesjöUMEWozneyJMBernardGWHyaluronan Supports Recombinant Human Bone Morphogenetic Protein-2 Induced Bone Reconstruction of Advanced Alveolar Ridge Defects in Dogs. A Pilot StudyJ Periodontol200172565165811394401
- EppleyBLDadvandBInjectable Soft-Tissue Fillers: Clinical OverviewPlast Reconstr Surg2006118498e106e
- YooHSLeeEAYoonJJParkTGHyaluronic acid modified biodegradable scaffolds for cartilage tissue engineeringBiomaterials200526141925193315576166
- KatoYNakamuraSNishimuraMBeneficial actions of hyaluronan (HA) on arthritic joints: effects of molecular weight of HA on elasticity of cartilage matrixBiorheology2006433–434735416912407
- LepidiSGregoFVindigniVHyaluronan Biodegradable Scaffold for Small-caliber Artery Grafting: Preliminary Results in an Animal ModelEuro J Vasc Endovasc Surg2006324411417
- KumarMNVRMuzzarelliRAAMuzzarelliCSashiwaHDombAJChitosan Chemistry and Pharmaceutical PerspectivesChem Rev2004104126017608415584695
- RaveendranSRochaniAMaekawaTKumarDSmart Carriers and Nanohealers: A Nanomedical Insight on Natural PolymersMaterials2017108929
- MuzzarelliRAAHuman enzymatic activities related to the therapeutic administration of chitin derivativesCell Mol Life Sci19975321311409118001
- KimIYSeoSJMoonHSChitosan and its derivatives for tissue engineering applicationsBiotechnol Adv200826112117884325
- AhsanSMThomasMReddyKKSooraparajuSGAsthanaABhatnagarIChitosan as biomaterial in drug delivery and tissue engineeringInt J Biol Macromol2017
- ChungY-CChenC-YAntibacterial characteristics and activity of acid-soluble chitosanBioresour Technol20089982806281417697776
- AkmanACTigliRSGumusdereliogluMNohutcuRMbFGF-loaded HA-chitosan: a promising scaffold for periodontal tissue engineeringJ Biomed Mater Res A201092395396219291690
- GobinASButlerCEMathurABRepair and Regeneration of the Abdominal Wall Musculofascial Defect Using Silk Fibroin-Chitosan BlendTissue Eng200612123383339417518675
- WuXBlackLSantacana-LaffitteGPatrickCWPreparation and assessment of glutaraldehyde-crosslinked collagen–chitosan hydrogels for adipose tissue engineeringJ Biomed Mater Res A200781A15965
- SunTKhanTHSultanaNFabrication and In Vitro Evaluation of Nanosized Hydroxyapatite/Chitosan-Based Tissue Engineering ScaffoldsJ Nanomater20142014118
- LevengoodSLZhangMChitosan-based scaffolds for bone tissue engineeringJ Mater Chem B20142213161318424999429
- CiccoSRVonaDdeGiglioEChemically Modified Diatoms Biosilica for Bone Cell Growth with Combined Drug-Delivery and Antioxidant PropertiesChempluschem201580711041112
- TamburaciSTihminliogluFDiatomite reinforced chitosan composite membrane as potential scaffold for guided bone regenerationMaterials Sci Eng C201780222231
- di MartinoASittingerMRisbudMVChitosan: A versatile biopo-lymer for orthopaedic tissue-engineeringBiomaterials200526305983599015894370
- DodaneVVilivalamVDPharmaceutical applications of chitosanPharm Sci Technolo Today199816246253
- MadhumathiKSudheesh KumarPTAbhilashSDevelopment of novel chitin/nanosilver composite scaffolds for wound dressing applicationsJ Mater Sci Mater Med201021280781319802687
- KumarPTSAbhilashSManzoorKNairSVTamuraHJayakumarRPreparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applicationsCarbohydr Polym2010803761767
- DevAMohanJCSreejaVNovel carboxymethyl chitin nano-particles for cancer drug delivery applicationsCarbohydr Polym201079410731079
- GuJAl-BayatiKHoEAEaHDevelopment of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytesDrug Deliv Transl Res20177449750628315051
- RamanaLNSharmaSSethuramanSRangaUKrishnanUMEvaluation of chitosan nanoformulations as potent anti-HIV therapeutic systemsBiochim Biophys Acta184020141476484
- DevABinulalNSAnithaAPreparation of poly(lactic acid)/ chitosan nanoparticles for anti-HIV drug delivery applicationsCarbohydrate Polymers2010803833838
- WuJJiangWShenYJiangWTianRSynthesis and characterization of mesoporous magnetic nanocomposites wrapped with chitosan gatekeepers for pH-sensitive controlled release of doxorubicinMater Sci Eng C Mater Biol Appl201770Pt 113214027770872
- diJYuJWangQUltrasound-triggered noninvasive regulation of blood glucose levels using microgels integrated with insulin nanocapsulesNano Research201710413931402
- LiangJYanHPuligundlaPGaoXZhouYWanXApplications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A reviewFood Hydrocolloids201769286292
- JingZ-WMaZ-WLiCChitosan cross-linked with poly (ethylene glycol)dialdehyde via reductive amination as effective controlled release carriers for oral protein drug deliveryBioorg Med Chem Lett20172741003100628087273
- QiaoXPengXQiaoJEvaluation of a photocrosslinkable hydroxyethyl chitosan hydrogel as a potential drug release system for glaucoma surgeryJ Mater Sci Mater Med2017281014928831622
- HillyardIWDocziJKiernanPBAntacid and antiulcer properties of the polysaccharide chitosan in the ratProc Soc Exp Biol Med19641151108111214166571
- SuganoMWatanabeSKishiAIzumeMOhtakaraAHypocholes-terolemic action of chitosans with different viscosity in ratsLipids19882331871912836688
- KozenBGKircherSJHenaoJGodinezFSJohnsonASAn alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClotAcad Emerg Med2008151748118211317
- UenoHMoriTFujinagaTTopical formulations and wound healing applications of chitosanAdvanced Drug Delivery Reviews200152210511511718934
- SashiwaHShigemasaYChemical modification of chitin and chitosan 2: preparation and water soluble property of N-acylated or N-alkylated partially deacetylated chitinsCarbohydrate Polymers1999392127138
- KaurLSinghJLiuQStarch – a potential biomaterial for biomedical applicationsMozafariMRNanomaterials and Nanosystems for Biomedical ApplicationsDordrechtSpringer20078398
- CalvertPThe structure of starchNature1997389338
- BetancurADChelGLCañizaresHEAcetylation and Characterization of Canavalia ensiformis StarchJ Agri Food Chem1997452378382
- Salgado AntónioJCoutinho OlgaPReis RuiLBone Tissue Engineering: State of the Art and Future TrendsMacromol Biosci20044874376515468269
- MahdiehZBagheriREslamiMAmiriMShokrgozarMAMehrjooMThermoplastic starch/ethylene vinyl alcohol/forsterite nanocomposite as a candidate material for bone tissue engineeringMater Sci Eng C201669301310
- Ali Akbari GhavimiSEbrahimzadeh MohammadHSolati-hashjinMAbu Osman NoorAPolycaprolactone/starch composite: Fabrication, structure, properties, and applicationsJ Biomed Mater Res A201410372482249825407786
- AverousLMoroLDolePFringantCProperties of thermoplastic blends: starch–polycaprolactonePolymer2000411141574167
- LabetMThielemansWDufresneAPolymer Grafting onto Starch NanocrystalsBiomacromolecules2007892916292717718501
- WuKJWuCSChangJSBiodegradability and mechanical properties of polycaprolactone composites encapsulating phosphate-solubilizing bacterium Bacillus sp. PG01Process Biochemistry2007424669675
- Carvalho PedroPLeonor IsabelBSmith BrendaJUndifferentiated human adipose-derived stromal/stem cells loaded onto wet-spun starch–polycaprolactone scaffolds enhance bone regeneration: Nude mice calvarial defect in vivo studyJ Biomed Mater Res A201310293102311124123913
- Link DennisPGardel LeandroSCorrelo VitorMGomes ManuelaEReis RuiLOsteogenic properties of starch poly(ε-caprolactone) (SPCL) fiber meshes loaded with osteoblast-like cells in a rat critical-sized cranial defectJ Biomed Mater Res A2013101113059306523505136
- Requicha JoaoFMouraTLeonor IsabelBEvaluation of a starch-based double layer scaffold for bone regeneration in a rat modelJ Orthop Res201432790490924604772
- VmdOCStringhetti Ferreira CuryBEvangelistaRCDaflon GremiãoMPDevelopment and characterization of cross-linked gellan gum and retrograded starch blend hydrogels for drug delivery applicationsJ Mech Behav Biomed Mater20176531733327631170
- DraganESDesign and applications of interpenetrating polymer network hydrogels. A reviewChem Eng J2014243572590
- SaboktakinMRTabatabaieRMMaharramovARamazanovMASynthesis and in vitro evaluation of carboxymethyl starch–chitosan nanoparticles as drug delivery system to the colonInt J Biol Macromolecules2011483381385
- OlivatoJBMüllerCMOCarvalhoGMYamashitaFGrossmannMVEPhysical and structural characterisation of starch/polyester blends with tartaric acidMater Sci Eng C2014393539
- PawarSNEdgarKJAlginate derivatization: A review of chemistry, properties and applicationsBiomaterials201233113279330522281421
- LeeKYMooneyDJAlginate: Properties and biomedical applicationsProgress Polymer Science2012371106126
- ChandikaPKoSCOhGWFish collagen/alginate/chitooligo-saccharides integrated scaffold for skin tissue regeneration applicationInt J Biol Macromol20158150451326306410
- HajialiHHeredia-GuerreroJALiakosIAthanassiouAMeleEAlginate Nanofibrous Mats with Adjustable Degradation Rate for Regenerative MedicineBiomacromolecules201516393694325658494
- RuvinovECohenSAlginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedsideAdv Drug Deliv Rev201696547625962984
- KimHLJungGYYoonJHPreparation and characterization of nano-sized hydroxyapatite/alginate/chitosan composite scaffolds for bone tissue engineeringMater Sci Eng C2015542025
- SharmaCDindaAKPotdarPDChouC-FMishraNCFabrication and characterization of novel nano-biocomposite scaffold of chitosan– gelatin–alginate–hydroxyapatite for bone tissue engineeringMater Sci Eng C201664416427
- DengBShenLWuYDelivery of alginate-chitosan hydrogel promotes endogenous repair and preserves cardiac function in rats with myocardial infarctionJ Biomed Mater Res A2014103390791824827141
- BoatengJCatanzanoOAdvanced Therapeutic Dressings for Effective Wound Healing— A ReviewJ Pharm Sci2015104113653368026308473
- BoatengJBurgos-AmadorROkekeOPawarHComposite alginate and gelatin based bio-polymeric wafers containing silver sulfadiazine for wound healingInte J Biol Macromolecules2015796371
- TellecheaASilvaEAMinJAlginate and DNA Gels Are Suitable Delivery Systems for Diabetic Wound HealingInt J Low Extrem Wounds201514214615326032947
- CatanzanoOD’EspositoVAciernoSAlginate–hyaluronan composite hydrogels accelerate wound healing processCarbohydrate Polymers201513140741426256201
- ContrerasAFayDHanrahanKTrevisoneFReview of article: Ozaki C, Hamdan A, Barshes N, Wyers M, Hevelone N, Belkin M, Nguyen L. Prospective, randomized, multi-institutional clinical trial of a silver alginate dressing to reduce lower-extremity vascular surgery wound complications. Society of vascular surgery 2015;61:419–427J Vasc Nurs2016342596027210453
- VenkatesanJBhatnagarIManivasaganPKangK-HKimS-KAlginate composites for bone tissue engineering: A reviewInt J Biol Macromolecules201572269281
- ÝaMDonatiIStrandBLSkjåk-BrækGMolecular Engineering as an Approach to Design New Functional Properties of AlginateBiomacromolecules2007892809281417696472
- AltmanGHDiazFJakubaCSilk-based biomaterialsBiomaterials200324340141612423595
- CaoT-TZhangY-QProcessing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedi-cinesMater Sci Eng C201661940952
- JaoDMouXHuXTissue Regeneration: A Silk RoadJ Funct Biomaterials20167322
- GiesaTArslanMPugnoNMBuehlerMJNanoconfinement of Spider Silk Fibrils Begets Superior Strength, Extensibility, and ToughnessNano Lett201111115038504621967633
- ChawlaSMidhaSSharmaAGhoshSSilk-Based Bioinks for 3D BioprintingAdv Healthcare Mater2018781701204
- KunduBRajkhowaRKunduSCWangXSilk fibroin biomaterials for tissue regenerationsAdv Drug Deliv Rev201365445747023137786
- ShaoWHeJSangFCoaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite-tussah silk fibroin nanoparticles for bone tissue engineeringMater Sci Eng C Mater Biol Appl20165834235126478319
- TianLPrabhakaranMPHuJChenMBesenbacherFRamakrishnaSCoaxial electrospun poly(lactic acid)/silk fibroin nanofibers incorporated with nerve growth factor support the differentiation of neuronal stem cellsRSC Advances20155624983849848
- BhardwajNSinghYPDeviDKandimallaRKotokyJMandalBBPotential of silk fibroin/chondrocyte constructs of muga silkworm Antheraea assamensis for cartilage tissue engineeringJ Mater Chem B201642136703684
- BhardwajNSowWTDeviDKwNMandalBBChoN-JSilk fibroin-keratin based 3D scaffolds as a dermal substitute for skin tissue engineeringIntegrative Biology201571536325372050
- Font TelladoSChieraSBonaniWHeparin functionalization increases retention of TGF-β2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineeringActa Bio-materialia201872150166
- Gil EunSPanilaitisBBellasEKaplan DavidLFunctionalized Silk Biomaterials for Wound HealingAdv Healthcare Mater201221206217
- MinSGaoXHanCPreparation of a silk fibroin spongy wound dressing and its therapeutic efficiency in skin defectsJ Biomater Sci Polym Ed2012231–49711021176393
- ZhangWChenLChenJSilk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Random-ized Controlled Clinical TrialAdv Healthcare Mater20176101700121
- ZhouYYangHLiuXMaoJGuSXuWElectrospinning of carboxyethyl chitosan/poly(vinyl alcohol)/silk fibroin nanoparticles for wound dressingsInt J Biol Macromol201353889223164753
- ChutipakdeevongJRuktanonchaiUSupapholPHybrid biomimetic electrospun fibrous mats derived from poly(ε-caprolactone) and silk fibroin protein for wound dressing applicationJ Appl Polymer Sci201413211
- UttayaratPJetawattanaSSuwanmalaPEamsiriJTangthongTPongpatSAntimicrobial electrospun silk fibroin mats with silver nanoparticles for wound dressing applicationFibers Polymers20121389991006
- GuZXieHHuangCLiLYuXPreparation of chitosan/silk fibroin blending membrane fixed with alginate dialdehyde for wound dressingInt J Biol Macromol20135812112623562962
- GuangSAnYKeFZhaoDShenYXuHChitosan/silk fibroin composite scaffolds for wound dressingJ Appl Polymer Sci201513235
- WenkEMerkleHPMeinelLSilk fibroin as a vehicle for drug delivery applicationsJ Control Release2011150212814121059377
- MottaghitalabFFarokhiMShokrgozarMAAtyabiFHosseinkhaniHSilk fibroin nanoparticle as a novel drug delivery systemJ Control Release201520616117625797561
- HoferMWinterGMyschikJRecombinant spider silk particles for controlled delivery of protein drugsBiomaterials20123351554156222079006
- PritchardEMValentinTPanilaitisBOmenettoFKaplanDLPritchard EleanorMKaplan DavidLAntibiotic-Releasing Silk Bio-materials for Infection Prevention and TreatmentAdv Funct Mater201323785486123483738
- SeibFPJonesGTRnjak-KovacinaJLinYKaplanDLJones GregoryTKaplan DavidLpH-dependent anticancer drug release from silk nanoparticlesAdv Healthc Mater20132121606161123625825
- OzbolatITBioprinting scale-up tissue and organ constructs for transplantationTrends Biotechnol201533739540025978871
- HolzlKLinSTytgatLvan VlierbergheSGuLOvsianikovABioink properties before, during and after 3D bioprintingBiofabrication20168303200227658612
- SchachtKJungstTSchweinlinMEwaldAGrollJScheibelTBiofabrication of cell-loaded 3D spider silk constructsAngewandte Chemie (International ed. in English)20155492816282025640578
- BillietTGevaertEde SchryverTCornelissenMDubruelPThe 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viabilityBiomaterials2014351496224112804
- HoodEEJilkaJMPlant-based production of xenogenic proteinsCurr Opin Biotechnol199910438238610449314
- LazarisAArcidiaconoSHuangYSpider silk fibers spun from soluble recombinant silk produced in mammalian cellsScience (New York, N. Y.)20022955554472476
- LamboniLGauthierMYangGWangQSilk sericin: A versatile material for tissue engineering and drug deliveryBiotechnology Advances20153381855186726523781
- AramwitPSiritientongTKanokpanontSSrichanaTFormulation and characterization of silk sericin-PVA scaffold crosslinked with genipinInt J Biol Macromol201047566867520804781
- ChenGQA microbial polyhydroxyalkanoates (PHA) based bio- and materials industryChem Soc Rev20093882434244619623359
- LiZLohXJRecent advances of using polyhydroxyalkanoate-based nanovehicles as therapeutic delivery carriersWiley Interdiscip Rev Nanomed Nanobiotechnol201793e1429-n/a:e1429
- ChenGQWuQThe application of polyhydroxyalkanoates as tissue engineering materialsBiomaterials200526336565657815946738
- AndradeAPWitholtBChangDLiZSynthesis and Characterization of Novel Thermoplastic Polyester Containing Blocks of Poly[(R)-3- hydroxyoctanoate] and Poly[(R)-3-hydroxybutyrate]Macromolecules2003362698309835
- LiZYangJLohXJPolyhydroxyalkanoates: opening doors for a sustainable futureNPG Asia Mater20168e265
- HazerBSteinbuchelAIncreased diversification of polyhydroxyal-kanoates by modification reactions for industrial and medical applicationsAppl Microbiol Biotechnol200774111217146652
- SaitoYDoiYMicrobial synthesis and properties of poly(3-hydroxy-butyrate-co-4-hydroxybutyrate) in Comamonas acidovoransInt J Biol Macromol1994162991048011595
- ParkSJAhnWSGreenPRLeeSYBiosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) by metabolically engineered Escherichia coli strainsBiotechnol Bioeng20017418287
- KaiDLiowSSLohXJBiodegradable polymers for electrospinning: towards biomedical applicationsMater Sci Eng C Mater Biol Appl201445Supplement C65967025491875
- LimJYouMLiJLiZEmerging bone tissue engineering via Poly-hydroxyalkanoate (PHA)-based scaffoldsMater Sci Eng C Mater Biol Appl20177991792928629097
- BagdadiAVSafariMDubeyPPoly(3-hydroxyoctanoate), a promising new material for cardiac tissue engineeringJ Tissue Eng Regen Med20181121e495e51227689781
- ShishatskayaEINikolaevaEDVinogradovaONVolovaTGExperimental wound dressings of degradable PHA for skin defect repairJ Mater Sci Mater Med2016271116527655431
- GoonooNBhaw-LuximonAPassanhaPEstevesSRJhurryDThird generation poly(hydroxyacid) composite scaffolds for tissue engineeringJ Biomed Mater Res B Appl Biomater201710561667168427080439
- LohXJWangXLiHLiXLiJCompositional study and cytotoxicity of biodegradable poly(ester urethane)s consisting of poly[(R)-3-hydroxybutyrate] and poly(ethylene glycol)Mater Science Eng C2007272267273
- LohXJGohSHLiJHydrolytic degradation and protein release studies of thermogelling polyurethane copolymers consisting of poly[(R)-3-hydroxybutyrate], poly(ethylene glycol), and poly(propylene glycol)Biomaterials200728284113412317573109
- Y-LWWangHQiuY-KLohXJPLA-based thermogel for the sustained delivery of chemotherapeutics in a mouse model of hepa-tocellular carcinomaRSC Advances20166504450644513
- YumMSTsKKimDWBeta-Hydroxybutyrate increases the pilocarpine-induced seizure threshold in young miceBrain Dev201234318118421723679
- ChengSWuQYangFXuMLeskiMChenGQInfluence of DL-beta-hydroxybutyric acid on cell proliferation and calcium influxBiomacromolecules20056259359715762618
- MaaloufMSullivanPGDavisLKimDYRhoJMKetones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidationNeuroscience2007145125626417240074
- WuQWangYChenG-QMedical Application of Microbial Bio-polyesters PolyhydroxyalkanoatesArtif Cells Blood Substit Immobil Biotechnol200937111219132638
- StrongPJLaycockBMahamudSNThe Opportunity for High-Performance Biomaterials from MethaneMicroorganisms201641
- KollerMMaršálekLde Sousa DiasMMBrauneggGProducing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable mannerNew Biotechnology201737Part A243827184617
- HuangYWangYSunLAgrawalRZhangMSundew adhesive: a naturally occurring hydrogelJ R Soc Interface2015121072015022625948615
- RostKSchauerRPhysical and chemical properties of the mucin secreted by Drosera capensisPhytochemistry197716913651368
- ZhangMLenaghanSCXiaLNanofibers and nanoparticles from the insect-capturing adhesive of the Sundew (Drosera) for cell attachmentJ Nanobiotechnology201082020718990
- SunLHuangYBianZSundew-Inspired Adhesive Hydrogels Combined with Adipose-Derived Stem Cells for Wound HealingACS Appl Mater Interfaces2016832423243426731614
- ZhangMLiuMPrestHFischerSNanoparticles Secreted from Ivy Rootlets for Surface ClimbingNano Lett2008851277128018355053
- LenaghanSCBurrisJNChoureyKIsolation and chemical analysis of nanoparticles from English ivy (Hedera helix L)J R Soc Interface201310872013039223883948
- WuYZhaoXZhangMAdhesion mechanics of ivy nanoparticlesJ Colloid Interface Sci2010344253354020070973
- LiQXiaLZhangZZhangMUltraviolet Extinction and Visible Transparency by Ivy NanoparticlesNanoscale Res Lett2010591487149120730120
- XiaLLenaghanSCZhangMZhangZLiQNaturally occurring nanoparticles from English ivy: an alternative to metal-based nano-particles for UV protectionJ Nanobiotechnology2010811220534157
- SchmidtCEBaierJMAcellular vascular tissues: natural bioma-terials for tissue repair and tissue engineeringBiomaterials200021222215223111026628
- SealBOteroTCPanitchAPolymeric biomaterials for tissue and organ regenerationMater Sci Eng Rep2001344–5147230
- FournierEPassiraniCMontero-MeneiCNBenoitJPBiocompat-ibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibilityBiomaterials200324193311333112763459
- ZhengZYinWZaraJNThe use of BMP-2 coupled – Nanosilver- PLGA composite grafts to induce bone repair in grossly infected segmental defectsBiomaterials201031359293930020864167
- LiuYZhengZZaraJNThe antimicrobial and osteoinductive properties of silver nanoparticle/poly (DL-lactic-co-glycolic acid)-coated stainless steelBiomaterials201233348745875622959466
- BernardiniGChelliniFFredianiBSpreaficoASantucciAHuman platelet releasates combined with polyglycolic acid scaffold promote chondrocyte differentiation and phenotypic maintenanceJ Biosci2015401616925740142
- CameronDJShaverMPAliphatic polyester polymer stars: synthesis, properties and applications in biomedicine and nanotechnologyChem Soc Rev20114031761177621082079
- ManoJFSousaRABoeselLFNevesNMBioinertRRLBiodegradable and injectable polymeric matrix composites for hard tissue replacement: state of the art and recent developmentsComposit Sci Technol2004646789817
- SeyednejadHGhassemiAHvan NostrumCFVermondenTHenninkWEFunctional aliphatic polyesters for biomedical and pharmaceutical applicationsJ Control Release2011152116817621223989
- BuescherJMMargaritisAMicrobial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industriesCrit Rev Biotechnol200727111917364686
- MultaniASLiCOzenMPaclitaxel and water-soluble poly (L-glutamic acid)-paclitaxel, induce direct chromosomal abnormalities and cell death in a murine metastatic melanoma cell lineAnticancer Res1997176D426942749494519
- LiCKeSWuQPQpWTumor irradiation enhances the tumor-specific distribution of poly(L-glutamic acid)-conjugated paclitaxel and its antitumor efficacyClin Cancer Res2000672829283410914731
- IwataHMatsudaSMitsuhashiKItohEIkadaYA novel surgical glue composed of gelatin and N-hydroxysuccinimide activated poly(L-glutamic acid): Part 1. Synthesis of activated poly(L-glutamic acid) and its gelation with gelatinBiomaterials19981920186918769855188
- OtaniYTabataYIkadaYRapidly curable biological glue composed of gelatin and poly(L-glutamic acid)Biomaterials19961714138713918830964
- TakeuchiJSuzukiHMurataMClinical evaluation of application of polyglycolic acid sheet and fibrin glue spray for partial glos-sectomyJ Oral Maxillofac Surg2013712e126e13123164997
- ShinozakiTHayashiREbiharaMMiyazakiMTomiokaTMucosal defect repair with a polyglycolic acid sheetJpn J Clin Oncol2013431333623225906
- RokutandaSYanamotoSYamadaSNaruseTInokuchiSUmedaMApplication of polyglycolic acid sheets and fibrin glue spray to bone surfaces during oral surgery: a case seriesJ Oral Maxillofac Surg20157351017.e1101017.e125883005
- MaurusPBKaedingCCBioabsorbable implant material reviewOper Tech Sports Med2004123158160
- LlorensECalderónSdel ValleLJPuiggalíJPolybiguanidePJPolybiguanide (PHMB) loaded in PLA scaffolds displaying high hydrophobic, biocompatibility and antibacterial propertiesMater Sci Eng C Mater Biol Appl201550748425746248
- DongHDaiWJuHMultifunctional Poly(L-lactide)-Polyethylene Glycol-Grafted Graphene Quantum Dots for Intracellular MicroRNA Imaging and Combined Specific-Gene-Targeting Agents Delivery for Improved TherapeuticsACS Appl Mater Interfaces2015720110151102325942410
- GollwitzerHThomasPDiehlPBiomechanical and allergological characteristics of a biodegradable poly(D,L-lactic acid) coating for orthopaedic implantsJ Orthop Res200523480280916022993
- SchmidmaierGWildemannBStembergerAHaasNPRaschkeMBiodegradable poly(D,L-lactide) coating of implants for continuous release of growth factorsJ Biomed Mater Res200158444945511410904
- Félix LanaoRPJonkerAMWolkeJGJansenJAvan HestJCLeeuwenburghSCPhysicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regenerationTissue Eng Part B Rev201319438039023350707
- PanZDingJPolyDJPoly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicineInterface Focus20122336637723741612
- ShiXWangYRenLGongYWangDAEnhancing alendronate release from a novel PLGA/hydroxyapatite microspheric system for bone repairing applicationsPharm Res200926242243018979188
- LabetMThielemansWSynthesis of polycaprolactone: a reviewChem Soc Rev200938123484350420449064
- Baradaran-RafiiABiazarEHeidari-KeshelSCellular Response of Limbal Stem Cells on Polycaprolactone Nanofibrous Scaffolds for Ocular Epithelial RegenerationCurr Eye Res20164131825849961
- KonopnickiSSharafBResnickCTissue-Engineered Bone With 3-Dimensionally Printed β-Tricalcium Phosphate and Poly-caprolactone Scaffolds and Early Implantation: An In Vivo Pilot Study in a Porcine Mandible ModelJ Oral Maxillofac Surg20157351016.e1101016.e125883004
- McneilSEGriffithsHRPerrieYPolycaprolactone fibres as a potential delivery system for collagen to support bone regenerationCurr Drug Deliv20118444845521235468
- YildirimEDAyanHVasiletsVNFridmanAGuceriSSunWEffect of Dielectric Barrier Discharge Plasma on the Attachment and Proliferation of Osteoblasts Cultured over Poly(ɛ-caprolactone) ScaffoldsPlasma Process Polym2008515866
- SinghRKJinGZMahapatraCPatelKDChrzanowskiWKimHWMesoporous silica-layered biopolymer hybrid nanofibrous scaffold: a novel nanobiomatrix platform for therapeutics delivery and bone regenerationACS Appl Mater Interfaces20157158088809825768431
- SinhaVRBansalKKaushikRKumriaRTrehanAPoly-epsilon-caprolactone microspheres and nanospheres: an overviewInt J Pharm2004278112315158945
- MondrinosMJDembzynskiRLuLPorogen-based solid freeform fabrication of polycaprolactone-calcium phosphate scaffolds for tissue engineeringBiomaterials200627254399440816678255
- ChiariCKollerUDorotkaRA tissue engineering approach to meniscus regeneration in a sheep modelOsteoarthritis Cartilage200614101056106516731009
- JainJPChitkaraDKumarNPolyanhydrides as localized drug delivery carrier: an updateExpert Opin Drug Deliv20085888990718712998
- JainJPModiSDombAJKumarNRole of polyanhydrides as localized drug carriersJ Control Release2005103354156315820403
- KattiDSLakshmiSLangerRLaurencinCTToxicityLCTToxicity, biodegradation and elimination of polyanhydridesAdv Drug Deliv Rev200254793396112384316
- TorresMPVogelBMNarasimhanBMallapragadaSKSynthesis and characterization of novel polyanhydrides with tailored erosion mechanismsJ Biomed Mater Res A200676110211016138330
- LiLCDengJStephensDPolyanhydride implant for antibiotic delivery – from the bench to the clinicAdv Drug Deliv Rev200254796398612384317
- SanterreJPWoodhouseKLarocheGLabowRSUnderstanding the biodegradation of polyurethanes: from classical implants to tissue engineering materialsBiomaterials200526357457747016024077
- GuelcherSABiodegradable polyurethanes: synthesis and applications in regenerative medicineTissue Eng Part B Rev200814131718454631
- ZhangJYDollBABeckmanEJHollingerJOThree-dimensional biocompatible ascorbic acid-containing scaffold for bone tissue engineeringTissue Eng2003961143115714670102
- SaadBHirtTDWeltiMUhlschmidGKNeuenschwanderPSuterUWDevelopment of degradable polyesterurethanes for medical applications: in vitro and in vivo evaluationsJ Biomed Mater Res199736165749212390
- BonzaniICAdhikariRHoushyarSMayadunneRGunatillakePStevensMMSynthesis of two-component injectable polyurethanes for bone tissue engineeringBiomaterials200728342343316979756
- SolankiAThakoreSCellulose crosslinked pH-responsive polyurethanes for drug delivery: α-hydroxy acids as drug release modifiersInt J Biol Macromol20158068369126188306
- NairLSLaurencinCTPolymers as biomaterials for tissue engineering and controlled drug deliveryAdv Biochem Eng Biotechnol2006102479017089786
- LaurencinCTNormanMEElgendyHMUse of polyphospha-zenes for skeletal tissue regenerationJ Biomed Mater Res19932779639738360223
- AmbrosioAMAllcockHRKattiDSLaurencinCTDegradable polyphosphazene/poly(alpha-hydroxyester) blends: degradation studiesBiomaterials20022371667167211924588
- LakshmiSKattiDSLaurencinCTBiodegradable polyphosphazenes for drug delivery applicationsAdv Drug Deliv Rev200355446748212706046
- NairLSLeeDABenderJDSynthesis, characterization, and osteocompatibility evaluation of novel alanine-based polyphospha-zenesJ Biomed Mater Res A200676120621316265637
- KumbarSGBhattacharyyaSNukavarapuSPKhanYMNairLSLaurencinCTIn Vitro and In Vivo Characterization of Biodegradable Poly(organophosphazenes) for Biomedical ApplicationsJ Inorg Organomet Polym Mater2007164365385
- SethuramanSNairLSEl-AminSIn vivo biodegradability and biocompatibility evaluation of novel alanine ester based polyphospha-zenes in a rat modelJ Biomed Mater Res A200677467968716514601
- GreishYEBenderJDLakshmiSBrownPWAllcockHRLaurencinCTLow temperature formation of hydroxyapatite-poly(alkyl oxybenzoate)phosphazene composites for biomedical applicationsBiomaterials20052611915193876
- RothemundSAignerTBIturmendiADegradable glycine-based photo-polymerizable polyphosphazenes for use as scaffolds for tissue regenerationMacromol Biosci201515335136325355036
- KrogmanNRWeikelALKristhartKAThe influence of side group modification in polyphosphazenes on hydrolysis and cell adhesion of blends with PLGABiomaterials200930173035304119345410