Abstract
Background
Dual antiplatelet therapy is a standard protocol for secondary prevention after acute coronary syndrome, but despite a variety of new dual antithrombotic strategies, there is a dearth of studies evaluating the effects and safety of some popular therapies. This study used a network meta-analysis to compare the efficacy and safety of all available antithrombotic therapies.
Methods
PubMed, MEDLINE, and Cochrane library databases were searched for randomized controlled trials, published up to July 1, 2017, that evaluated the efficacy of antithrombotic therapy in acute coronary syndrome treatment. The primary endpoints were clinically significant bleeding and major bleeding and secondary endpoints were major cardiovascular events, all-cause deaths, cardiac deaths, and myocardial infarction.
Results
Compared with treatment with aspirin + new P2Y12 inhibitor, treatment with aspirin + new P2Y12 inhibitor converted to clopidogrel clinically reduced the risk of major cardiovascular events or significant bleeding (OR: 0.30, 95% credibility interval: 0.12–0.75). Both myocardial infarction risk (OR: 0.82, 95% credibility interval: 0.62–1.09) and major bleeding risk (OR: 0.18, 95% credibility interval: 0.01–1.68) were not significantly different between treatment regimens. There were no significant differences in major cardiovascular events, all-cause deaths, cardiac deaths, myocardial infarction, clinically significant bleeding, and major bleeding risk with rivaroxaban + new P2Y12 inhibitor therapy when compared with aspirin + new P2Y12 inhibitor. Compared with aspirin + clopidogrel, the conversion therapy further reduced the risk of myocardial infarction (OR: 1.81, 95%, credibility interval: 1.01–1.34) without an increased clinical risk of significant bleeding (OR: 0.41, 95%, credibility interval: 0.15–1.07). Treatment with aspirin + new P2Y12 inhibitors reduced all-cause deaths (OR: 0.91, 95% credibility interval: 0.84–0.98) and cardiac death risk (OR: 0.86, 95% credibility interval: 0.79–0.93).
Conclusion
We concluded the following from our study: 1) an aspirin + new P2Y12 inhibitor/ clopidogrel conversion treatment strategy was not inferior to aspirin + new P2Y12 inhibitor; 2) compared with aspirin + clopidogrel, the conversion strategy may further reduce the risk of myocardial infarction without increasing the risk of bleeding; and 3) compared with aspirin + clopidogrel, treatment with aspirin + new P2Y12 inhibitors may result in reduced risk of death.
Background
Compared with aspirin combined with clopidogrel, treatment with aspirin combined with new P2Y12 inhibitors, such as prasugrel and ticagrelor, may effectively reduce the incidence of major cardiovascular events (MACE).Citation1,Citation2 Consequently, the 2017 European guidelines for revascularization has recommended that aspirin combined with new P2Y12 receptor antagonist is the preferred secondary prevention therapy for acute coronary syndrome (ACS).Citation3 However, this treatment program has been associated with an increased risk of bleeding.Citation1,Citation4
The TOPIC study divided ACS patients into two groups – the first group was given aspirin orally along with a new P2Y12 receptor antagonist and the second group, after 1 month of oral administration of aspirin, was given clopidogrel instead of new P2Y12 receptor antagonist.Citation5 The results showed that this conversion program reduced the incidence of bleeding complications, without an increase in ischemic events, compared with non-conversion. In addition, the ATLAS-ACS 2-TIMI 51 study showed that rivaroxaban, combined with dual antiplatelet therapy, as a triple antiplatelet therapy, reduced MACEs, but with an increased risk of bleeding, compared with conventional dual antiplatelet therapy.Citation6 In the GEMINI-ACS-1 study, aspirin was removed from the triple antiplatelet therapy to assess the risk of bleeding for the combination of rivaroxaban with P2Y12 receptor antagonists, resulting in no significant differences in the clinical risk of bleeding, compared with the dual antiplatelet therapy.Citation7
Despite the current popularity of a variety of new anti-thrombotic therapies for the secondary prevention of ACS, there are few clinical randomized controlled trials that compare the safety and efficacy of most of the antithrom-botic treatment regimens. Network meta-analysis, as a new methodology, may help to solve this problem. This method indirectly compares two interventions by using a common comparison. For example, if one study compares A and B, and other study compares A and C, then B and C can be indirectly compared through A.Citation8 Finally, our network meta-analysis was based on the combination of direct and indirect comparisons, thereby comparing agents not directly analyzed within an individual trial.Citation8,Citation9 In this study, we used the network meta-analysis method to compare the safety and efficacy of the available antithrombotic strategies.
Materials and methods
Research strategy
“Acute coronary syndromes”, “rivaroxaban”, “clopidogrel”, “prasugrel”, “ticagrelor”, “aspirin”, “dual antiplatelet”, “new P2Y12 receptor antagonist”, “P2Y12 receptor antagonist”, and “randomized controlled trial” were used as search terms. We conducted an electronic search of PubMed, MEDLINE, the Cochrane library, and other databases for articles published up to July 1, 2017. Where necessary, a further search of the United States Clinical Trials Database (https://clinicaltrials.gov/)Citation29 was performed for specific information on clinical trials and appropriate data were collected.
Inclusion criteria
Screening of retrieved articles was carried out according to the following inclusion criteria: 1) studies with a clinical randomized controlled trial study design; 2) studies included patients with ACS; 3) studies that analyzed the efficacy of antithrombotic therapies such as aspirin, aspirin + clopidogrel, aspirin + a new P2Y12 receptor antagonist, aspirin + rivaroxaban, aspirin + clopidogrel + rivaroxaban, rivaroxaban + clopidogrel, rivaroxaban + a new P2Y12 receptor antagonist, aspirin + a new P2Y12 receptor antagonist/clopidogrel, or other studies that compared two or more treatment regimens; and 4) studies that reported on outcomes such as MACEs, all-cause deaths, cardiac deaths, myocardial infarction (MI), major bleeding, and clinically significant bleeding events.
Exclusion criteria
Study reports that met the following criteria were excluded:
1) observational research, conference literature, and communication articles; 2) studies carried out on patients without ACS; 3) studies that compared the efficacy of different doses of intervention drugs, but without a control group; 4) studies that did not include a proposed treatment program; and 5) studies that did not set any outcome events.
Outcomes
Primary outcomes were major bleeding and clinically signifi-cant bleeding events, which were defined according to TIMI and GUSTO criteria, respectively. The secondary outcomes were all-cause deaths, cardiac deaths, MI, and stroke.
Data extraction and quality evaluation
The data were extracted by two researchers independently. If a study reported results of the same test at different times, or had other forms of repeated reporting, only one test report was included. For controversial decisions on data extraction, the opinion of a third researcher was sought. The data extraction form included the name of the study, the treatment program, the number of events in each group, the total number of subjects, and study characteristics, for example, age, sex, outcomes, and the presence of conditions such as diabetes and hypertension.
For quality assessment of chosen studies, we used the risk assessment tool recommended by the Cochrane Handbook,Citation10 with the following guidelines: 1) randomization and its correct application; 2) mention of hidden or covert allocation methods and their correct application; 3) use of blinding and its correct application; 4) evidence of complete results; 5) evidence of selective reporting; 6) evidence of other biases, such as funding sources, early termination of research, or other deceptive behaviors.
Statistical methods
For the traditional meta-analysis, the estimate of effect used for the binary data was OR with a 95% CI, with the DerSimonian– Laird method for statistical analysis.Citation11 I2 and Cochran Q tests were used to analyze heterogeneity.Citation12 ICitation2 <25% indicated a low degree of heterogeneity; ICitation2 >25% but <50% indicated moderate heterogeneity; and ICitation2 >50% indicated substantial heterogeneity. STATA 12 software (Stata Corp LP, College Station, TX, USA) was used for meta-analysis.
For the network meta-analysis, the effect was expressed by selection ratio (OR) and 95% prediction interval (credibility interval; CrI). Due to the unexplained heterogeneity between studies, we used a random effect model for statistical analysis.Citation13 We used deviance information criterion (DIC) value to judge model convergence; the smaller the DIC value, the greater the degree of convergence.Citation13 The analysis was performed by the Markov Chain Monte Carlo method.Citation13 I2 was calculated to judge heterogeneity.Citation14 A consistency analysis was based on the node-splitting model and was judged by the Bayesian P-value.Citation14 A P-value <0.05 indicated that the direct and indirect comparison results were inconsistent; P>0.05 indicated consistency. R software (version 3.2.0), gemtc package, was used for the network meta-analysis.Citation15
Results
Electronic search results and basic study characteristics
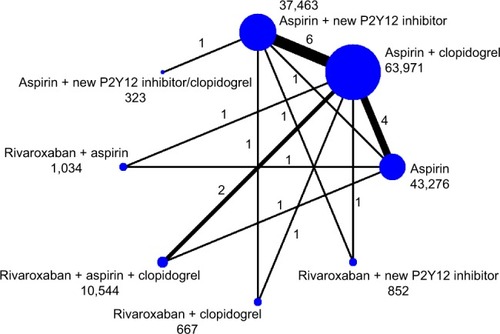
A total of 4,962 articles were retrieved from electronic searches. After screening, 15 articles were included.Citation1,Citation2,Citation5–Citation7,Citation16–Citation25 The literature screening process is shown in . A network diagram of all the research scenarios is shown in . Basic study characteristics are shown in .
Figure 1 The flowchart of search results.
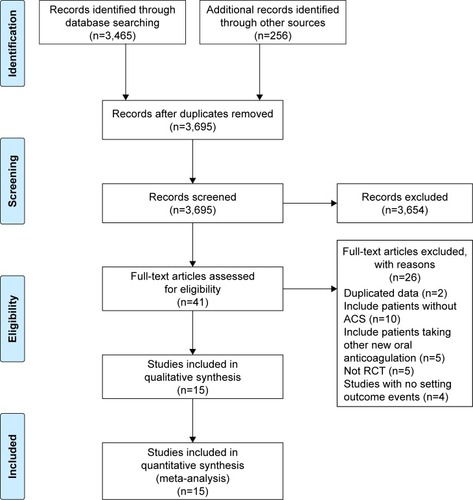
Table 1 Study characteristics
A total of 158,130 patients were included in the analysis. The definition of bleeding was defined according to criteria from TIMI, GUSTO, BARC, PLATO, and clinical practice. The definition of MACE was different among studies. For most studies, MACE was defined as a composite of cardiovascular death, MI, and stroke. The proportion of males ranged from 61% to 80%, in all studies. The mean age ranged from 57 to 67 years. The proportion of patients with DM ranged from 16% to 38%. The proportion of patients with hypertension ranged from 43% to 81%. The proportion of patients with previous MI ranged from 5% to 43%. The follow-up duration ranged from 1 month to 33 months.
Methodological evaluation of included studies
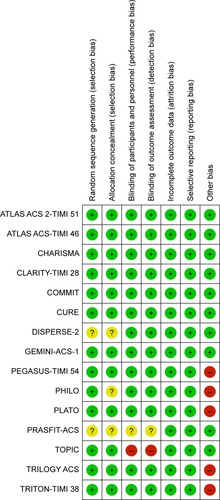
All chosen studies were randomized controlled trials and the results of methodological evaluations are shown in . Random sequence generation was unclear in two studies.Citation21,Citation24 Allocation was unclear in three studies.Citation21,Citation22,Citation24 The blinding of participants and personnel was unclear in one study and high risk in another study.Citation5,Citation24 Similarly, the blinding of outcome assessment was unclear in one study and high risk in another study.Citation5,Citation24 Five studies had other biases. We provided this information in the results section.Citation1,Citation2,Citation20,Citation22,Citation23 Overall, the chosen studies were classified as low risk for bias.
Results of clinical outcomes
Clinically significant bleeding
Thirteen studies with 147,629 patients were included to evaluate the risk of clinically significant bleeding.Citation1,Citation2,Citation5–Citation7,Citation16–Citation20,Citation22–Citation24 The results of network meta-analysis are shown in .
Table 2 Estimated relative treatment effects of clinical significant bleeding events as ORs and its corresponding 95% CIs based on the network meta-analysis
Compared with aspirin + new P2Y12 receptor antagonist, treatment with aspirin (OR: 0.46, 95% CrI: 0.31–0.65), aspirin + P2Y12 receptor antagonist (OR: 0.73, 95% CrI: 0.57–0.93), and aspirin + new P2Y12 receptor antagonist/ clopidogrel (OR: 0.30, 95% CrI: 0.12–0.75) significantly reduced the risk of clinically significant bleeding. However, there was no significant difference between rivar-oxaban + novel P2Y12 receptor antagonist and aspirin + novel P2Y12 receptor antagonist (OR: 1.28, 95% CrI: 0.69–2.4).
Compared with aspirin + new P2Y12 receptor antagonist/ clopidogrel, rivaroxaban + aspirin (OR: 3.74, 95% CrI: 1.19–12.51), rivaroxaban + aspirin + clopidogrel (OR: 9.20, 95% CrI: 3.17–28), and rivaroxaban + new P2Y12 receptor antagonists (OR: 4.23, 95% CrI: 1.40–13.21) increased the risk of clinically significant bleeding.
Compared with triple programs, dual programs had a reduced risk of clinically significant bleeding. Of these, aspirin + new P2Y12 receptor antagonist reduced the risk of bleeding (OR: 0.46, 95% CrI: 0.21–1.02) but the results were not statistically significant.
Compared with rivaroxaban + clopidogrel, treatment with rivaroxaban + new P2Y12 receptor antagonist resulted in a higher risk of bleeding (OR: 2.16, 95% CrI: 1–4.76).
Major bleeding
Fifteen studies with 158,130 patients were included to evaluate the risk of major bleeding.Citation1,Citation2,Citation5–Citation7,Citation16–Citation25 The results of network meta-analysis are shown in .
Table 3 Estimated relative treatment effects of major bleeding risk as ORs and its corresponding 95% CIs based on the network meta-analysis
Compared with aspirin + new P2Y12 inhibitor, therapy with aspirin (OR: 0.64, 95% CrI: 0.42–0.91) and aspirin + clopidogrel (OR: 0.77, 95% CrI: 0.57–1.02) reduced the risk of major bleeding.
Rivaroxaban + aspirin (OR: 2.63, 95% CrI: 1–7.02) and rivaroxaban + aspirin + clopidogrel (OR: 4.08, 95% CrI: 2.03–9.36) increased the risk of major bleeding, while aspirin + new P2Y12 receptor antagonist/clopidogrel (OR: 0.18, 95% CrI: 0.01–1.68), rivaroxaban + new P2Y12 receptor antagonist (OR: 1.02, 95% CrI: 0.27–3.64), and rivaroxaban + clopidogrel (OR: 1.33, 95% CrI: 0.35–4.74) did not increase the risk of major bleeding.
Compared with aspirin + new P2Y12 receptor antagonist/ clopidogrel, rivaroxaban + aspirin (OR: 14.61, 95% CrI: 1.27–461.53) and rivaroxaban + aspirin + clopidogrel (OR: 22.9, 95% CrI: 2.2–09.15) showed an increased risk of bleeding.
Compared with aspirin + clopidogrel, rivaroxaban + aspirin (OR: 3.40, 95% CrI: 1.33–8.89) and rivaroxaban + aspirin + clopidogrel (OR: 5.27, 95% CrI: 2.77–11.66) also increased the risk of bleeding.
Major cardiovascular events
Fourteen studies with 157,816 patients were included to evaluate the risk of MACE.Citation1,Citation2,Citation5–Citation7,Citation16–Citation20,Citation22–Citation25 The results of net work meta-analysis are shown in .
Table 4 Estimated relative treatment effects of major cardiovascular events as ORs and its corresponding 95% CIs based on the network meta-analysis
Compared with new P2Y12 receptor antagonist/ clopidogrel therapy, all antithrombotic therapies increased the incidence of MACE except for rivaroxaban + aspirin + clopidogrel therapy (OR: 1.88, 95% CrI: 0.91–3.75).
There was no significant difference in MACE between rivaroxaban + novel P2Y12 receptor antagonist and aspirin + novel P2Y12 receptor antagonist (OR: 1.02, 95% CrI: 0.59–1.77).
All-cause deaths
Twelve studies with 140,294 patients were included to evaluate the risk of all-cause mortality.Citation1,Citation2,Citation7,Citation17–Citation25 The results of network meta-analysis are shown in .
Table 5 Estimated relative treatment effects of death as ORs and its corresponding 95% CIs based on the network meta-analysis
Compared with aspirin, aspirin + clopidogrel (OR: 0.94, 95% CrI: 0.89–1) and aspirin + new P2Y12 receptor antagonist (OR: 0.86, 95% CrI: 0.79–0.93) reduced the risk of death from all causes.
Aspirin + new P2Y12 receptor antagonist, in contrast to aspirin + clopidogrel, further reduced the risk of death (OR: 0.91, 95% CrI: 0.84–0.98). There was no significant difference in all-cause mortality risks between rivaroxaban + novel P2Y12 receptor antagonist and aspirin + novel P2Y12 receptor antagonist (OR: 1.09, 95% CrI: 0.44–2.68).
Cardiac deaths
Twelve studies with 95,088 patients were included to evaluate the risk of cardiac deaths.Citation1,Citation2,Citation5,Citation7,Citation17,Citation19–Citation25 The results of network meta-analysis are shown in . Compared with aspirin, aspirin + clopidogrel (OR: 0.92, 95% CrI: 0.81–1.03) and aspirin + new P2Y12 receptor antagonist (OR: 0.79, 95% CrI: 0.68–0.91) reduced cardiac deaths.
Table 6 Estimated relative treatment effects of cardiac death as ORs and its corresponding 95% CIs based on the network meta-analysis
Compared with aspirin + clopidogrel, aspirin + new P2Y12 receptor antagonist (OR: 0.86, 95% CrI: 0.79–0.93) further reduced the risk of cardiac death.
There was also no significant difference in the risk of cardiac death between rivaroxaban + novel P2Y12 receptor antagonist and aspirin + novel P2Y12 receptor antagonist (OR: 1.34, 95% CrI: 0.47–3.85).
Myocardial infarction
Twelve studies with 131,554 patients were included to evaluate the risk of MI.Citation1,Citation2,Citation5,Citation7,Citation17–Citation19,Citation21–Citation25 The results of network meta-analysis are shown in .
Table 7 Estimated relative treatment effects of myocardial infarction risk as ORs and its corresponding 95% CIs based on the network meta-analysis
Compared with aspirin + new P2Y12 receptor antagonist, aspirin (OR: 1.48, 95% CrI: 1.20–0.83) and aspirin + clopidogrel (OR: 1.81, 95% CrI: 1.01–1.34) increased MI risk, whereas aspirin + new P2Y12 receptor antagonist/ clopidogrel (OR: 0.82, 95% CrI: 0.62–1.09) and rivaroxa-ban + novel P2Y12 receptor antagonist (OR: 1.72, 95% CrI: 0.93–3.17) did not. Compared with aspirin + new P2Y12 receptor antagonist/clopidogrel, treatment with aspirin + clopidogrel (OR: 1.44, 95% CrI: 1.03–1.94), aspirin (OR: 1.8, 95% CrI: 1.26–2.55), and rivaroxaban + new P2Y12 receptor antagonist (OR: 2.09, 95% CrI: 1–4.05) increased MI risk.
Other results
The convergence degree of each clinical outcome event was better. The node-splitting model demonstrated that the traditional and network meta-analyses results were consistent. For clinically significant bleeding and major bleeding events, I2 was 20% and 60%, respectively, suggesting a lack of substantial heterogeneity. Heterogeneity was 31.2% for MACE, 23.1% for all-cause deaths, 35.6% for cardiac deaths, and 40% for MI and other events, suggesting low to moderate heterogeneity.
Discussion
In this study, we used network meta-analysis to evaluate the safety and efficacy of antithrombotic therapies in the treatment of ACS. First, we observed that neither an aspirin + new P2Y12 receptor antagonist/clopidogrel conversion strategy nor treatment with aspirin + new P2Y12 receptor antagonist as a standard dual antiplatelet strategy significantly affected MI incidence or the risk of major bleeding, but the former resulted in reduced MACE incidence and reduced clinically significant bleeding risk.
Second, our results demonstrated that, compared with aspirin + clopidogrel, the conversion strategy could further reduce the risk of MI, without increasing the risk of bleeding.
Third, we found that compared with aspirin + new P2Y12 receptor antagonist, there were no clinically significant benefits from rivaroxaban + new P2Y12 receptor antagonists in terms of MACE events, all-cause deaths, cardiac deaths, MI, clinically significant bleeding, major bleeding, or any other aspects.
Finally, compared with aspirin + clopidogrel, aspirin + new P2Y12 receptor antagonist reduced the risk of all-cause deaths and cardiac deaths.
ACS patients have unstable plaques that rupture easily, leading to thrombosis, thereby requiring oral antiplatelet agents to reduce the risk of ischemia. The TRITON-TIMI study showed that aspirin combined with prasugrel resulted in significantly reduced incidences of ischemic events compared with aspirin, combined with clopidogrel.Citation2 The PLATO trial also demonstrated a net benefit of aspirin combined with ticagrelor in ACS patients.Citation1 Subsequently, the ESC/EACTS and ACCF/AHA guidelines recommended aspirin + new P2Y12 receptor antagonist as the preferred antithrombotic therapy in ACS patients.Citation3,Citation26 However, in the PLATO study, the ischemic benefit of aspirin + ticagrelor predominantly occurred within 1 month of oral administration and thereafter ischemic benefit reduced and bleeding risk increased.Citation27 Thus, whether ticagrelor conversion to clopidogrel after 1 month of ticagrelor increases the risk of bleeding and reduces the risk of ischemia remains inconclusive. The TOPIC study was the only clinical study to address this issue; it included 645 patients and compared the efficacy and safety of conversion and non-conversion strategies. The results showed that the conversion strategy did not increase the risk of ischemia or bleeding compared with the non-conversion strategy.Citation5
No studies in our meta-analysis directly compared the conversion strategy with aspirin + clopidogrel therapy. However, we used the network meta-analysis methodology to indirectly compare the efficacy of the two therapies. The results showed that compared with aspirin + clopidogrel, the conversion strategy could further reduce the risk of MI, without increasing the risk of bleeding.
The ATLAS ACS2-TIMI 51 study evaluated the efficacy and safety of rivaroxaban in the treatment of ACS.Citation6 The results showed that, compared with dual antiplatelet therapy, patients with ACS treated with a dual platelet therapy plus rivaroxaban had a reduced risk of cardiac death, MI, or stroke, but an increased risk of major bleeding and intrac-ranial hemorrhage.
In the GEMINI-ACS-1 study, for patients receiving the P2Y12 receptor antagonist, the addition of rivaroxaban was not significantly different in terms of clinical bleeding when compared with aspirin.Citation7 This conclusion was consistent with our study results. In addition, our study indirectly compared rivaroxaban + new P2Y12 receptor antagonist and aspirin + new P2Y12 receptor antagonist. These results demonstrated no difference in risk for ischemia and bleeding. Because the GEMINI-ACS-1 trial sample size was small and the statistical power insufficient, the effectiveness of rivaroxaban + new P2Y12 receptor antagonists has to be further evaluated.
The CURE study confirmed the important role of aspirin combined with clopidogrel in the treatment of ACS.Citation17 Compared with aspirin alone, dual antiplatelet therapy was associated with a further reduction in the composite end points of cardiac death, MI, and stroke. However, when decomposing the composite into individual endpoints, the dual antiplatelet therapy did not reduce the risk of cardiac death. Our study combined direct and indirect evidence to improve statistical efficacy, and the results showed that dual antiplatelet therapy could further reduce the risk of cardiac death compared with aspirin.
The PLATO study showed that aspirin + ticagrelor reduced the risk of cardiac death compared with aspirin + clopidogrel and that the duration continued with the prolongation of medication.Citation1 The results of the second PLATO study showed no difference in the therapeutic effect of ticagrelor in genetically resistant and nonresistant populations and that ticagrelor was superior to clopidogrel in gene resistant populations.Citation28 Therefore, the survival benefit of ticagrelor, compared with clopidogrel, might be related to the genetic resistance of clopidogrel.
Limitations
The definitions of MACE differed between studies; therefore, the results for MACE were less credible than the overall efficacy of the dual antiplatelet therapies. A MACE event, as defined by TOPIC, is a composite endpoint of cardiac death, stroke, and bleeding, and the MACE event reduction of the conversion strategy is mainly driven by a reduction in bleeding risk.Citation5 The composite endpoint of the PLATO study did not include bleeding events, and oral aspirin + ticagrelor significantly reduced the risk of ischemia compared with aspirin + clopidogrel.Citation2 Therefore, to validate whether the conversion strategy significantly reduced ischemia, a bigger sample size and a more rigorous composite endpoint definition are required.
The results from the comparison between aspirin + newer P2Y12 blockers/clopidogrel and aspirin + new P2Y12 blockers were based mainly on the TOPIC trial, which was the smallest trial included, thanks to the broad CIs. Therefore, further evidence is warranted to evaluate the effectiveness of the conversion therapy.
Some studies did not report death events, for example, the TOPIC trial, and therefore, the conversion strategy could not be compared with other treatment programs for differences in survival.Citation5
Our study was based on the study level and individualized data could not be extracted for analysis. Therefore, the best antithrombotic scheme could not be established according to population characteristics.
Stent thrombosis or urgent revascularization events were not reported in many studies. Therefore, we did not evaluate stent thrombosis or urgent revascularization risks in patients taking antithrombotic therapies.
Finally, our study did not consider cost benefits.
Conclusion
We conclude that 1) the dual antiplatelet therapy of aspirin + new P2Y12 receptor antagonist was not inferior to that of aspirin + new P2Y12 receptor antagonist/clopidogrel; 2) compared with aspirin + clopidogrel, the conversion strategy may further reduce the risk of MI, without increasing the risk of bleeding; and 3) compared with aspirin + clopidogrel, therapy with aspirin + new P2Y12 receptor antagonists may reduce the risk of death.
Disclosure
The authors report no conflicts of interest in this work.
References
- WallentinLBeckerRCBudajATicagrelor versus clopidogrel in patients with acute coronary syndromesN Engl J Med2009361111045105719717846
- WiviottSDBraunwaldEMcCabeCHPrasugrel versus clopi-dogrel in patients with acute coronary syndromesN Engl J Med2007357202001201517982182
- ValgimigliMBuenoHByrneRAESC focused update on dual anti-platelet therapy in coronary artery disease developed in collaboration with EACTS: The task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS)Eur Heart J201839321326028886622
- BaberUChandrasekharJSartoriSAssociations Between Chronic Kidney Disease and Outcomes With Use of Prasugrel Versus Clopidogrel in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention: A Report From the PROMETHEUS StudyJACC Cardiovasc Interv201710202017202528780028
- CuissetTDeharoPQuiliciJBenefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized studyEur Heart J201738413070307828510646
- MegaJLBraunwaldEWiviottSDRivaroxaban in patients with a recent acute coronary syndromeN Engl J Med2012366191922077192
- OhmanEMRoeMTStegPGClinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEMINI-ACS-1): a double-blind, multicentre, randomised trialLancet2017389100811799180828325638
- GlennyAMAltmanDGSongFIndirect comparisons of competing interventionsHealth Technol Assess20059261134
- JansenJPCrawfordBBergmanGStamWBayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisonsValue Health200811595696418489499
- HigginsJPAltmanDGGøtzschePCThe Cochrane Collaboration’s tool for assessing risk of bias in randomised trialsBMJ2011343d592822008217
- DersimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- DiasSSuttonAJAdesAEWeltonNJEvidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trialsMed Decis Making201333560761723104435
- DiasSWeltonNJCaldwellDMAdesAEChecking consistency in mixed treatment comparison meta-analysisStat Med2010297–893294420213715
- GertvanVJoelKGemtc: Network meta-analysis using Bayesian methods. R package version 0.8-2 Available from: http://github.com/gertvv/gemtcAccessed September 28, 2018
- MegaJLBraunwaldEMohanaveluSRivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trialLancet20093749683293819539361
- YusufSZhaoFMehtaSRClopidogrel in Unstable Angina to Prevent Recurrent Events Trial InvestigatorsEffects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevationN Engl J Med2001345749450211519503
- ChenZMJiangLXChenYPAddition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trialLancet200536694971607162116271642
- SabatineMSCannonCPGibsonCMAddition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevationN Engl J Med2005352121179118915758000
- BonacaMPBhattDLCohenMLong-term use of ticagrelor in patients with prior myocardial infarctionN Engl J Med2015372191791180025773268
- CannonCPHustedSHarringtonRASafety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphos-phate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trialJ Am Coll Cardiol200750191844185117980250
- GotoSHuangCHParkSJEmanuelssonHKimuraTTicagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome – randomized, double-blind, phase III PHILO studyCirc J201579112452246026376600
- RoeMTArmstrongPWFoxKAPrasugrel versus clopidogrel for acute coronary syndromes without revascularizationN Engl J Med2012367141297130922920930
- SaitoSIsshikiTKimuraTEfficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS studyCirc J20147871684169224759796
- BhattDLFlatherMDHackeWPatients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trialJ Am Coll Cardiol200749191982198817498584
- LevineGNBatesERBittlJAACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery (vol 68, pg 1082, 2016)J Am Coll Cardiol201668101150115127585519
- BeckerRCBassandJPBudajABleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trialEur Heart J201132232933294422090660
- WallentinLJamesSStoreyRFEffect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trialLancet201037697491320132820801498
- US National Library of Medicine Available from: https://clinicaltrials.gov/AccessedAugust 18, 2017