Abstract
Glutathione S-transferase π (GSTπ) is a Phase II metabolic enzyme that is an important facilitator of cellular detoxification. Traditional dogma asserts that GSTπ functions to catalyze glutathione (GSH)-substrate conjunction to preserve the macromolecule upon exposure to oxidative stress, thus defending cells against various toxic compounds. Over the past 20 years, abnormal GSTπ expression has been linked to the occurrence of tumor resistance to chemotherapy drugs, demonstrating that this enzyme possesses functions beyond metabolism. This revelation reveals exciting possibilities in the realm of drug discovery, as GSTπ inhibitors and its prodrugs offer a feasible strategy in designing anticancer drugs with the primary purpose of reversing tumor resistance. In connection with the authors’ current research, we provide a review on the biological function of GSTπ and current developments in GSTπ-targeting drugs, as well as the prospects of future strategies.
Introduction
In addition to combating a variety of noxious substances from the external environment, cell-detoxification mechanisms are capable of resisting the deleterious effects of certain endogenous substances (eg, ROS, a product generated from normal cellular metabolism), in order to maintain physiological homeostasis. Drug metabolism represents an important component of cellular detoxification and involves two enzymes: Phase I and Phase II drug-metabolism enzymes. The Glutathione S-transferase π (GST) family of enzymes is a group of typical Phase II detoxification enzymes and is found in many prokaryotes and eukaryotes.Citation1 Of these enzymes, GSTπ catalyzes the conjunction between GSH and its electrophilic substrates upon exposure to damaging free radicals. Besides metabolite detoxification, GSTπ also exhibits ligand-binding properties that initiate cellular apoptosis when triggered by cellular stress.Citation2 Further research has also demonstrated that GSTπ is expressed abundantly in tumor cells and associated closely to carcinogenesis, tumor formation, and chemotherapy resistance.Citation3,Citation4 Moreover, experiments involving drug-resistant cell lines have also demonstrated increased GSTπ expression.Citation5,Citation6 In multidrug-resistant HL60/VCR acute myelogenous leukemia cells, GSTπ is found to be expressed at higher levels than HL60.Citation7 GSTπ is involved in facilitating tumor resistance and suppressing apoptosis in tumor cells via two mechanisms. First, GSTπ weakens the efficacy of chemotherapy drugs by promoting their in vitro extrusion. Second, GSTπ also functions as an MAPK-pathway inhibitor to prevent tumor-cell apoptosis.
A variety of anticancer drugs based on these principles have been synthesized in efforts to improve their therapeutic indices and reverse tumor resistance. Drugs that work through the GST system include GSTπ inhibitors and their respective prodrugs. The former work by exerting high GSTπ-inhibitory activity, while the latter comprise inactive compounds designed to target tumor tissue locally by undergoing GSTπ catalysis in the tumor to release cytotoxic metabolites.Citation8 Development of therapies targeting GSTπ is a major field of research. As such, we believe that there is a need for more detailed studies outlining the diverse biological functions of GSTπ, in order to assist drug discovery and unlock more exciting possibilities in the realm of tumor treatments.
Structure
GSTs consist of the following three superfamilies: cytoplasmic (cGSTs), mitochondrial (κGSTs), and microsomal (membrane-associated proteins in eicosanoid and glutathione metabolism [MAPEG]). Among these families, cGSTs are the most complex and most closely linked to the development of human diseases.Citation9,Citation10 The cGSTs are divided into seven subtypes according to similarities in amino-acid sequence, different structure of genes, and immunological cross-reactivity. These subtypes are α, π, μ, θ, ω, σ, and δ.Citation11 Among them, GSTα is highly expressed in many normal cells.Citation12 However, recent studies have shown that GSTα also takes part in promoting multidrug resistance in p53-mutated lung cancer cells.Citation13 GSTμ has been found to be able to act synergistically with MRP1 to decrease the effects of vincristine treatment.Citation14 GSTπ is widespread in tumor cells, and is intricately involved with cellular carcinogenesis, tumor formation, and tumor-drug resistance.Citation15 Current evidence supports the role of cGSTs in facilitating multidrug resistance across different types of tumors.Citation16,Citation17
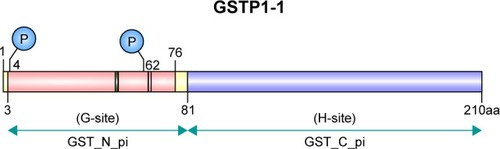
GSTπ is the most frequently and extensively studied of all the GSTs. Its encoding gene is located on chromosome 11 and is composed of seven exons. In humans, GSTπ often consists of two identical dimer subunits, with each subunit consisting of 210 amino acids and two binding sites, the G-site and the H-site (). Different G- and H-site locations in the amino-acid residue of different GSTs exert different functions. GSTπ is able specifically to bind to GSH or GSH analogs via the G-site, which catalyzes the interaction between GST amino-acid residues with GSH sulfhydryl and conventional electrophilic substances at the H-sites to promote catalytic action.Citation18 Therefore, G-site modification often guides the development of specific GSTπ inhibitors.
Biological function
GSTπ in metabolite detoxification and antioxidation
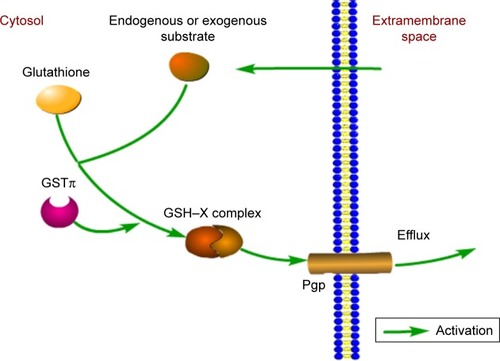
The classical view holds that as a dimeric isoenzyme, GSTπ can conjugate GSH with substrate molecules in efforts to promote clearance of active ionic substances.Citation19 However, tumor cells also utilize GSTπ to form a GSH–X complex between antitumor drugs and GSH, proceeding to excrete the complex out of the cell by Pgp and MRP. Pgp, encoded by MDR1, is often found to be highly expressed in tumor cells.Citation6,Citation20 What is more, GSTπ and Pgp or MRPs (eg, MRP2) are synergistic in driving the development of multidrug resistance in tumor cellsCitation21 (). Studies have documented high GSTπ expression in various tumor cells, such as cancers of the gastrointestinal tract,Citation6 pancreas,Citation22 breast,Citation23 liver,Citation24 lymphoma,Citation25 as well as melanoma.Citation26
Figure 2 Involvement of GSTπ in the detoxification of exogenous and endogenous substrates.
Abbreviation: GST, glutathione S-transferase.
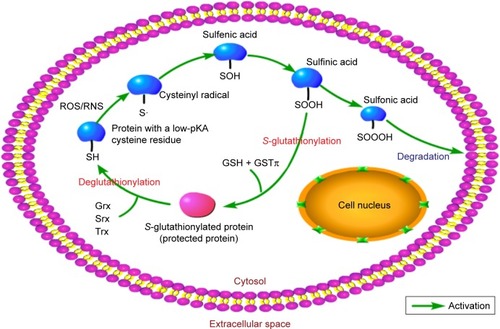
Recent literature has characterized GSH and other related metabolic enzymes as vital in protecting cells from ROSCitation27 through oxidation and reduction (redox) mechanisms.Citation28 GSH carries a cysteine residue with an active thiol group and is responsible for maintaining thiol equilibrium. Meanwhile, it can also modulate the activities of many signaling molecules and redox-sensitive transcription factors through S-glutathionylation, a form of posttranslational modification that combines cysteine residues with GSH.Citation29 GSTπ serves as a general S-glutathionylase enzyme and promotes S-glutathionylation. Its enzymatic function is based on two aspects: its catalytic activity and the auto-S-glutathionylation of GSTπ by itself on Cys47 and Cys10, both of which disturb the subsequent interaction with c-Jun NH2-terminal kinase (JNK), resulting in the formation of a GSTπ multimerCitation30 (). Besides GSTπ, other members of the GSH-redox system, such as glutamate cysteine ligase,Citation31 glutathione peroxidase, and glutathione reductase, also play significant roles in this process.Citation32
Figure 3 The process of S-glutathionylation.
Abbreviation: GST, glutathione S-transferase.
GSTπ in regulation of MAPK pathway
Besides metabolite detoxification, GSTπ also exhibits ligand-binding properties that allow the enzyme to interact covalently and noncovalently with compounds, resulting in inhibition of conjugation activity.Citation18 GSTπ can induce cellular apoptosis in the setting of cellular stress by activating MAPK, MKK4, downstream JNK-signal components, and p38 kinase. Normal cells have low basal JNK activity to maintain optimal cellular growth conditions. However, in the presence of oxidative or nitrosative stress, GSTπ can form homodimers that alter the reduced states of cysteine residues in its structure, resulting in JNK dissociation from the GSTπ–JNK heterocomplex and causing subsequent activation of the c-Jun protein. Ultimately, these series of reactions will activate the apoptotic pathwaysCitation33,Citation34 ().
Figure 4 Ligand-binding properties of JNK and TRAF2.
Abbreviation: GST, glutathione S-transferase.
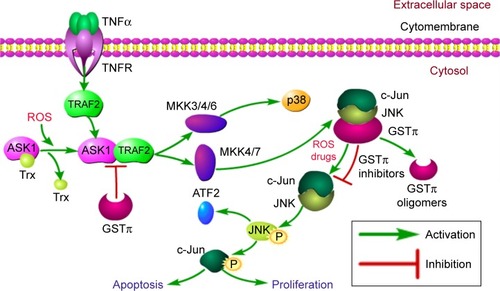
Further research indicates that GSTπ can influence the MAPK pathway both through JNK and TRAF2 modulation. In-depth analysis of biological information tools has revealed that the TRAF family is strongly linked to GSTπ. Of all the TRAF members, TRAF2 is expressed most abundantly and has been subjected to intense research.Citation35 Following the activation of TNFR though TNFα binding, TRAF2 is recruited to the plasma membrane, resulting in the production of ROS. ROS generation leads to oxidation of the ASK1 inhibitor thioredoxin, separating and activating ASK1 from the inactive ASK1–thioredoxin complex. ASK1 goes on to bind to TRAF2, which in turn activates downstream-signaling cascades, including the MKK3/4/6–p38 and MKK4/7–JNK signaling pathwaysCitation36,Citation37 (). Further evidence from steady-state fluorescence analysis confirms that direct binding between TRAF2 and GSTπ also exists.Citation38–Citation42 In tumor cells, GSTπ can suppress JNK activity and block the interaction between TRAF2 and ASK1 to inhibit tumor-cell apoptosis. Consequently, regarding the formation of multidrug resistance in tumor cells, besides acting as a detoxification enzyme though excretion of drugs to decrease pharmacological efficacy, GSTπ can also act as an MAPK-pathway inhibitor to improve tumor-cell survival. GSTπ also represents a scaffold protein to unite different members across signaling pathways.
Other functions
GSTπ also functions as a chaperone protein that regulates common but significant cellular functions. It interacts with several key cellular proteins, including TGM2Citation43 and FANCC.Citation44 Research has found STAT3 to be an active factor in signal transduction and transcription. STAT3 overexpression is a key molecule that drives the progression of hepatocellular carcinoma, and its activation may be critical in initiating oncogenesis.Citation33,Citation45,Citation46 There are studies illustrating that GSTπ interaction with STAT3 can inhibit the STAT3-signaling pathway, curb aberrant cell-cycle progression, and decrease cell proliferation.Citation36 Furthermore, GSTπ can participate in nonhomologous end-joining DNA repair by inhibiting DNA-dependent protein kinase.Citation47 In conclusion, these findings mentioned show that seemingly disparate functions of GSTπ in fact work on several aspects of tumor carcinogenesis, making it an ideal molecule for further antitumor therapy research.
Gene variants and polymorphisms
The study of pharmacogenomics in recent decades has proved that genetic polymorphism of drug-metabolizing enzymes is an important mediating factor in determining individual drug responses. Genetic polymorphism is due to a single-nucleotide mutation in the genomic sequence that fundamentally changes how a person responds to chemotherapeutic drugs. Polymorphisms affecting GSTπ have been documented to exert significant modulatory effects on the biological cascade of carcinogenesis and have been discussed vigorously in the literature.Citation48
The human GSTP1 gene exists as two functionally different variants, with both variants having documented nucleotide transitions from isoleucine to valine at codon 105 (A→G) and alanine to Val at 114 (C→T). This results in four alleles of GSTP1: wild-type GSTP1*A (Ile105 + Ala114), GSTP1*B (Val105 + Ala114), GSTP1*C (Val105 + Val114), and GSTP1*D (Ile105 + Val114).Citation49 Besides GSTP1, cytosolic GSTs demonstrate clinically significant gene polymorphism (). These changes occur in active sites, leading to a decrease in encoded protein activity, decreased excretion of foreign substances, and suboptimal catalytic efficiency. On the other hand, reducing the rate of drug excretion has the benefit of improving an individual’s sensitivity to chemotherapeutic drugs, enhancing their curative effects.Citation50–Citation52
Table 1 Genetic polymorphisms of cytosolic GSTs
There are a great number of reviews that have summarized the associations between individual GSTπ variability and the drug sensitivity of malignant tumors.Citation53–Citation55 However, data are scarce regarding the relationship between GSTπ and clinical response to chemotherapy. Khrunin et al showed that 104 patients with ovarian cancer with mutant-type GSTπ possessed longer progression-free survival compared to wild-type GSTπ.Citation56 These results highlight the possibility that genetic variation may have significant effects on susceptibility toward cancer. In future, precise genetic polymorphic screening tests may play a more central role in determining chemotherapeutic treatment regimens for patients.
GSTπ inhibitors
GSTπ inhibitors reverse tumor resistance by means of suppressing GSTπ activity and improving the chemotherapeutic drug sensitivity of tumor cells. Ethacrynic acid (EA) is a classic GSTπ inhibitor.Citation57 However, due its aspecific pharmacological properties in targeting GSTπ, the newer GSTπ inhibitors TLK117/TLK199 and NBDHEX may prove to be more promising. Antitumor agents targeting GSTπ in context are listed in .
Table 2 Antitumor agents targeting GSTπ in context
EA and its analogs
EA represents the first clinical application of GSTπ inhibitors. Previously, it was widely used for decades as a diuretic in clinical research. EA works to halt GSTπ activity through a number of mechanisms. First, it is able to bind directly to substrate-binding sites of isozymes to inhibit GSTπ. Second, it is able to induce the combination of α,β-unsaturated ketones and GSH through the nucleophilic addition reaction, depleting GSH and reducing the amount of GSH available to combine with chemotherapeutic agents, thus producing an overall GSTπ-inhibitory effect by sensitizing a cell to chemotherapeutic agents.Citation57 However, the clinical applications of EA have been limited, due to its diuretic properties and lack of enzyme specificity, with long-term intake possibly risking water and salt imbalance.Citation18
Zhao et al attempted to modify EA using thiazole derivatives of uric acid to strengthen its GSTπ-inhibitory effects. The team demonstrated that these derivatives had higher GSTπ-inhibitory activity in comparison to unmodified EA when administered to acute myeloid leukemia parental cells (HL60).Citation58 In addition, the combination of EA and GSH has also been proven to possess superior inhibitory activity over EA alone and is able functionally to inhibit many GST isoenzymes. However, this compound also possesses limited clinical viability, given its tendency toward dissociation by γ-glutamyltransferase.Citation59 Burg et al synthesized modified peptidomimetic glutathione analogs of these EA–GSH compounds, which were hypothesized to be stabler against peptidase-mediated dissolution. Unfortunately, these analogs instead reduced its GSTπ-inhibitory activity, despite demonstrating increased resilience toward γ-glutamyltransferase compared to the unmodified EA–GSH compounds.Citation57 Taken together, EA and its analogs still represent novel avenues of research in the search for more efficacious antitumor drugs.
TLK117 and TLK199
Telintra (ezatiostat hydrochloride, TER199, TLK199) is a small-peptide, glutathione-analog molecule and was developed by Telik. Upon entering the body, TLK199 undergoes esterase hydrolysis, which releases TLK117, its activated form that has anti-GSTπ activity. TLK199 is able to enhance the potency of various antineoplastic agents against various tumor cell lines. The agent is also able to inhibit MRAP1 and prevent the combination of GSTπ and JNK, resulting in high JNK production that triggers tumor-cell apoptosis.Citation60
Furthermore, clinical studies have found TLK199 to be able to promote the maturation of hematopoietic progenitor cells, induce cancer-cell death, and inhibit myeloproliferative diseases.Citation61–Citation63 In 2013, TLK199 successfully passed a US Food and Drug Administration audit and was approved to treat low-to intermediate-risk myelodysplastic syndrome. Long-term observation studies have highlighted the ability of TLK199 to enhance bone-marrow maturation and cellularity.Citation64
NBDHEX and its analogs
NBDHEX (6-[7-nitro-2,1,3-benzoxadiazol-4-ylthio] hexanol) is a recently developed compound designed as a “mechanism-based inhibitor” that exerts potent effects on GSTπ. Since its first reports by the Tor Vergata University of Rome,Citation65 numerous preclinical studies have shown that NBDHEX exerts high GSTπ-inhibitory activity across a wide range of tumor types. Pasello et al reported that this agent effectively reversed cisplatin resistance in osteosarcoma, alluding toward potentially improved clinical outcomes when using a combination of NBDHEX and cisplatin.Citation66 NBDHEX GSTπ-inhibitory effects have also been observed in HL60 cells and their chemotherapy-resistant phenotype HL60/DNR.Citation67 Other cell lines that have demonstrated NBDHEX sensitivity include Ewing sarcoma,Citation68 the human mesothelioma cell lines MPP89, MMB1, MSTO211H, and Mero48a,Citation69 melanoma cell lines Me501 and A375,Citation42 and the non-small-cell lung cancer cell line H69AR.Citation70 Further research indicates that aside from inherent antimelanoma activity, NBDHEX also has the ability to enhance the function of temozolomide, with the two able to work synergistically to suppress tumor growth.Citation71
NBDHEX employs several methods in combating malignant cells. First, it is able to accumulate specifically in tumor cells, remaining unaffected by MRP while maintaining good cell-membrane permeability. Second, this agent can decompose the GSTπ–JNK complex and promote activation of the apoptosis pathway.Citation42,Citation65,Citation67,Citation70–Citation72 Further research has also suggested that TRAF2 plays a key role in facilitating NBDHEX-mediated apoptosis. NBDHEX simultaneously activates JNK and TRAF2, interfering with the effect of GSTπ via two different pathways, leading to cell-cycle arrest and cell death.Citation34 Intriguingly, researchers have provided further evidence to demonstrate that NBDHEX can also act as an autophagy inhibitor in tumor cells.Citation40
Despite NBDHEX’s promising anticancer activity, consideration should be given to the relatively low GSTπ-target selectivity and poor water-solubility.Citation73 In efforts to counter this limitation, a study group designed, synthesized, and screened 40 new NBDHEX analogs.Citation74 The group added one or two oxygen atoms on the hydroxyl chain of the NBD bone, forming two NBDHEX analogs, MC3181 and MC3165, that were able to display higher selectivity and better external activity by forming a stable σ-complex with the active site of GSTπ. After extensive experiments, MC3181 was deemed the more promising compound of the two, due to it having a 50-fold rise in aqueous solubility and higher selectivity toward GSTπ. This novel compound yielded positive results when administered to several distinct human melanoma cell lines, particularly when used in BRAFV600E-mutation melanoma cells.Citation75 Moreover, both intravenous and oral treatment of MC3181 in animals with different types of human melanoma xenografts resulted in astonishing curative effects and a satisfactory safety profile.Citation75 In summary, we conclude that NBDHEX and its analogs may serve as potential treatment strategies in the management of patients with melanoma.
Prodrugs of GSTπ
Both conventional chemotherapeutic and targeted agents have well-established toxicity profiles, with a wide range of adverse effects, eg, bone-marrow suppression, gastrointestinal toxicity, immunosuppression, gastrointestinal toxicity, hepatotoxicity, and cardiotoxicity.Citation76 GSTπ prodrugs appear to have a more favorable adverse-event profile, given that they are ingested as an inactive compound and undergo breakdown to release cytotoxic metabolites only in the presence of high concentrations of enzymes that occur in the proximity of tumor cells, thereby reducing collateral damage to healthy cells.Citation2 Additionally, tumor cells generally have heightened expression of GSTπ, providing ideal conditions for GSTπ-activated prodrugs. There are two primary classes of GSTπ prodrugs. The first of these are GSH or GSH derivatives, such as canfosfamide (Telcyta, TER286, TLK286), which have cytotoxic drug segments. When catalyzed by GSTπ, the prodrug releases cytotoxic compounds. The other type, such as JS-K, consists of a similar structure, but without GST analogs. Upon being catalyzed by GSTπ, it forms an intermediate with GSH and releases its cytotoxic drug segments. Currently, prodrugs under research comprise TLK286, purine analogs,Citation77 sulfonamides,Citation78 and brostallicin.
TLK286
l-γ-Glutamyl-3-(bis[bis(2-chloroethyl)amino-phosphinyl] oxy)ethylsulfonyl-l-alanyl-2-phenyl-[2R]-glycine hydrochloride salt (TLK286) represents the most promising GSTπ-prodrug candidate. It can generate a GSH analog and a phosphorodiamidate, the latter an active, alkylating agent.Citation53 Following activation, the former competitively inhibits molecules that stimulate drug resistance, while the nitrogen-mustard segment induces apoptotic activity by influencing the activities of MAPK, p38 kinase, JNK, MKK4, and caspase 3.Citation53,Citation79–Citation81
In vitro studies have revealed that the GSTP1-null cell lines show a different degree of resistance in response to TLK286 compared with GSTP1+/+ cells. These differences were abrogated by cotransfecting these cells with GSTπ. Similar findings were reflected in in vivo analyses with nude mice. These data support the rationale that tumors with elevated GSTπ expression are more sensitive to the cytotoxic effects of TLK286.Citation82
The promising mechanism of action of TLK286 and its positive preclinical data have sparked a series of clinical trials where the prodrugs have been applied alone or in combination with other standard chemotherapeutic agents. Completed Phase II and III clinical trials have indicated that the agent has a nonoverlapping toxicity profile and synergistic effects with carboplatin, paclitaxel, and anthracycline, has no cross-drug resistance and is well tolerated, with patients mostly reporting fatigue and nausea.Citation83–Citation88
A completed Phase I/IIA multicenter dose-ranging clinical trial that sought to assess the safety and efficacy of TLK286 found that the compound was highly efficacious. Patients who underwent TLK286 maintenance treatment experienced prolonged median survival of 16.8 months compared to the 8.8 months experienced by those who did not receive the agent.Citation89 These clinical trials provide sound scientific evidence that supports the therapeutic efficacy of TLK286 in managing different types of malignancies.
Nitric oxide (NO) prodrugs
Another class of prodrugs are the NO prodrugs. These medications work by binding to intracellular GSTπ and undergoing GSTπ-mediated catalysis. This process releases NO molecules that go on to exert antitumor activity. NO is an ephemeral but pleiotropic molecule. It has been shown to have the capacity to affect several vital functions of the body.Citation90 As such, this molecule has been investigated keenly for its role in carcinogenesis, tumor progression, invasion, angiogenesis, and other key biological processes.Citation91 However, available experimental evidence suggests that NO is a double-edged sword when used to manage tumor diseases. NO itself is a source of cytotoxic molecules, and its deleterious effects are enhanced by its ability to concentrate locally around tumor cells and the tumor microenvironment.Citation92 At modest concentrations, NO exerts a protumorigenic response that may benefit tumor growth and survival. Nevertheless, at fairly high concentrations, NO takes on antitumor-agent properties to accelerate tumor-cell death and to inhibit tumor-cell angiogenesis.Citation93 Based on these observations, it is clear that NO has a role to play in combating tumor-cell resistance.
An example of an NO prodrug is O2-(2,4-dinitrophenyl)1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (JS-K), devised by Keefer et al from the National Cancer Institute.Citation94 The antineoplastic properties of JS-K rest on two modes of action: first, it can combine with GSTπ and then be activated to release NO at a high concentration to directly kill tumor cells; second, it binds to cellular GST/GSH, depleting its intracellular content to weaken the efflux of chemotherapy drugs in tumor cells.Citation95 Furthermore, other literature suggests that JS-K inhibits angiogenesis,Citation96 induces cell apoptosis (a process related to PARP, caspase 8 and 9 cleavage, and cell differentiation),Citation95,Citation97 destroys double-stranded DNA,Citation95,Citation97–Citation99 and is also able to interfere with the cell cycle and its respective signaling pathways.Citation92,Citation100–Citation102 These mechanisms are highly codependent, and function in an integrated manner to exert antitumor effects.
Remarkably, flow-cytometry findings have shown that JS-K can improve the formation of acidic vesicle organelles, underscoring its ability to induce autophagy.Citation102 Furthermore, electron-microscopy observations have indicated that JS-K induces autophagic death in cells.Citation102 Nevertheless, JS-K was able to spare surrounding healthy mammary epithelial cells. JS-K has been shown to be effective in several types of cancers, of which leukemia and myeloma appear to be the most susceptible.Citation92,Citation96,Citation100,Citation103 In addition, it is also efficacious in the treatment of solid tumors, such as breast cancer,Citation102,Citation104 lung cancer,Citation97,Citation103,Citation105 glioma,Citation103,Citation106 prostate cancer,Citation107 kidney cancer,Citation108 bladder cancer,Citation109 colon cancer,Citation110 and hepatocellular carcinoma.Citation111 Furthermore, JS-K is tolerated well by healthy tissue. These data indicate that further investigation into JS-K as an alternative chemotherapeutic agent is much needed.Citation102–Citation104,Citation109
It is worth noting that JS-K works synergistically with chemotherapy drugs, such as cytarabine,Citation98 bortezomib,Citation95 cisplatin, and arsenic.Citation112 JS-K acts as a dose-sparing agent when used with typical chemotherapeutic agents and is able to alleviate the severity of adverse effects as a consequence. While JS-K has been shown to be advantageous in treating cancer, its clinical use has been hindered with reports of poor solubility. Structural modification of JS-K is able to prolong its half-life, and combining JS-K with special nanoparticles can greatly improve its solubility and stability,Citation103,Citation113 further improving its prospects for clinical applications. JS-K and many other NO prodrugs represent an innovative biological approach in the development of anticancer therapeutics.
Conclusion
Multidrug resistance to chemotherapy drugs is one of the main obstacles in human cancer chemotherapy and has prompted intense research into discovering novel and innovative mechanisms that can overcome this barrier.Citation6,Citation7,Citation20 There is an intimate connection between abnormal GST expression and multidrug resistance, unequivocally implicating GSTπ, a member of the GST family in tumor-drug resistance.Citation4 GSTπ inhibitors and prodrugs are crucial agents that can help reverse multidrug resistance in tumors and increase the therapeutic index of anticancer drugs, which collectively decreases the physical and economic burden of cancer patients. Nevertheless, while the rapid growth of research on development of medication based on GSTπ inhibition has resulted in clinical studies on several compounds, the drugs that make it to commercial consumption are few. Realistically, fundamental issues that stand in the way of large-scale drug production include the vast number of biological GST family members, with each subtype having their own structural and functional differences, in addition to the existence of several genetic modifiers. The situation is further compounded by the presence of posttranslational modifying factors, such as kinase activities and S-glutathionylation. More in-depth research that clarifies the roles of these components of the GST-detoxification system are much needed, in order to produce compounds that have minimal side effects and high GSTπ selectivity. While modulation of the GSH-antioxidant system has provided promising preclinical results, some of these compounds demonstrate unacceptable toxicity profiles (eg, buthionine sulfoximine). Having said that, GSH-based medication has also been successfully employed to protect against cisplatin induced nephrotoxicity.Citation114,Citation115 The ongoing development of chemical genomics, computer-aided drug design, and more extensive molecular and cellular biology research will serve to be extremely useful in contributing toward the preclinical and clinical development of more efficient GSTπ-targeting drugs.
Acknowledgments
This study was supported by the Jiangsu Provincial Medical Youth Talent (grant number QNRC2016653); the National Nature Science Foundation of Jiangsu Provience (grant number BK20151016); the National Natural Science Foundation of China (grant number 81602441); and the Jiangsu Provincial Key Research Development Program (grant number BE2016794); the Postgraduate Research & Prac-tice Innovation Program of Jiangsu Province (grant number KYCX17_1296).
Disclosure
The authors report no conflicts of interest in this work.
References
- TownsendDMTewKDTapieroHThe importance of glutathione in human diseaseBiomed Pharmacother2003573–414515512818476
- TewKDTownsendDMRegulatory functions of glutathione S-transferase P1-1 unrelated to detoxificationDrug Metab Rev201143217919321351850
- SawersLFergusonMJIhrigBRGlutathione S-transferase P1 (GSTP1) directly influences platinum drug chemosensitivity in ovarian tumour cell linesBr J Cancer201411161150115825010864
- SatoKTsuchidaSTamaiKAnti-cancer drug resistance and glutathione S-transferasesGan To Kagaku Ryoho1989163 Pt 25925982650631
- IjSChengALTsaiTFLayJDRetinoic acid-induced apoptosis and regression of a refractory Epstein-Barr virus-containing T cell lymphoma expressing multidrug-resistance phenotypesBr J Haematol19938548268287918055
- QinFQinXZhangXJiaHWExpression and significance of P-glycoprotein, glutathione S-transferase-pi and Topoisomerase II in gastric carcinomasAi Zheng200221216717012479068
- ZhuXHLiJYXiaXMMultidrug resistance mechanisms in cell line HL-60/VCRAi Zheng200221121310131312520737
- TownsendDMFindlayVLTewKDGlutathione S-transferases as regulators of kinase pathways and anticancer drug targetsMethods Enzymol200540128730716399394
- SchecterRLAlaoui-JamaliMABatistGGlutathione S-transferase in chemotherapy resistance and in carcinogenesisBiochem Cell Biol19927053493531353967
- WuBDongDHuman cytosolic glutathione transferases: structure, function, and drug discoveryTrends Pharmacol Sci2012331265666823121834
- MorelFRauchCPetitEGene and Protein Characterization of the Human Glutathione S-Transferase Kappa and Evidence for a Peroxisomal LocalizationJ Biol Chem200427916162461625314742434
- DengQHeBPanYPolymorphisms of GSTA1 contribute to elevated cancer risk: evidence from 15 studiesJ BUON201520128729525778330
- RussoASaideASmaldoneSFaraonioRRussoGRole of uL3 in Multidrug Resistance in p53-Mutated Lung Cancer CellsInt J Mol Sci2017183547
- DepeillePCuqPMarySGlutathione S-transferase M1 and multidrug resistance protein 1 act in synergy to protect melanoma cells from vincristine effectsMol Pharmacol200465489790515044619
- ChenWYMaoWMZhaoLExpression of P-gp, GST-pi and Topo II alpha in gastric and colorectal cancers and their clinical significanceZhonghua Zhong Liu Za Zhi2005271273874016483485
- AsojoOAHommaKSedlacekMX-ray structures of Na-GST-1 and Na-GST-2 two glutathione S-transferase from the human hookworm Necator americanusBMC Struct Biol2007714217594497
- KelleherAZhanBAsojoOAStructure of monomeric Na-GST-3, a glutathione S-transferase from the major human hookworm parasite Necator americanusActa Crystallogr Sect F Struct Biol Cryst Commun2013698839843
- TownsendDMTewKDThe role of glutathione-S-transferase in anticancer drug resistanceOncogene200322477369737514576844
- VasievaOThe many faces of glutathione transferase piCurr Mol Med201111212913921342130
- LiGDaiJWangYOverexpression and its clinical significance of multi-drug resistance associated genes in lung cancer tissuesZhongguo Fei Ai Za Zhi200251353721315026
- HsuC-HChenC-LHongR-LChenK-LLinJ-FChengA-LPrognostic Value of Multidrug Resistance 1, Glutathione-S-Transferase-π and p53 in Advanced Nasopharyngeal Carcinoma Treated with Systemic ChemotherapyOncology200262430531212138237
- CollierJDBennettMKHallACattanARLendrumRBassendineMFExpression of glutathione S-transferases in normal and malignant pancreas: an immunohistochemical studyGut19943522662698307481
- SuFHuXJiaWGongCSongEHamarPGlutathion s transferase π indicates chemotherapy resistance in breast cancerJ Surg Res2003113110210812943817
- EzzikouriSBenjellounSPineauPHuman genetic variation and the risk of hepatocellular carcinoma developmentHepatol Int20137382083126201919
- Bennaceur-GriscelliABosqJKoscielnySHigh Level of Glutathione-S-Transferase Expression in Mantle Cell LymphomasClin Cancer Res20041093029303415131039
- DepeillePCuqPPassagneIEvrardAVianLCombined effects of GSTP1 and MRP1 in melanoma drug resistanceBr J Cancer200593221622315999103
- LushchakVIGlutathione homeostasis and functions: potential targets for medical interventionsJ Amino Acids20122012573683722500213
- ZwYZhangJTownsendDMTewKDOxidative stress, redox regulation and diseases of cellular differentiationBiochim Biophys Acta18502015816071621
- ChenWSeefeldtTYoungAMicrotubule S-glutathionylation as a potential approach for antimitotic agentsBMC Cancer201212124522703118
- TewKDManevichYGrekCXiongYUysJTownsendDMThe role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancerFree Radic Biol Med2011512z299313
- SeefeldtTZhaoYChenWCharacterization of a novel dithiocarbamate glutathione reductase inhibitor and its use as a tool to modulate intracellular glutathioneJ Biol Chem200928452729273719049979
- LeeH-CKimD-WJungK-YIncreased expression of antioxidant enzymes in radioresistant variant from U251 human glioblastoma cell lineInt J Mol Med200413688388715138630
- GateLMajumdarRSLunkATewKDIncreased Myeloproliferation in Glutathione S-Transferase π-deficient Mice Is Associated with a Deregulation of JNK and Janus Kinase/STAT PathwaysJ Biol Chem2004279108608861614684749
- SauAFilomeniGPezzolaSTargeting GSTP1-1 induces JNK activation and leads to apoptosis in cisplatin-sensitive and -resistant human osteosarcoma cell linesMol Biosyst201284994100622068640
- ZhangLBlackwellKAltaevaAShiZHabelhahHTRAF2 phosphorylation promotes NF-κB–dependent gene expression and inhibits oxidative stress-induced cell deathMol Biol Cell201122112814021119000
- ChenDLiuJRuiBGSTpi protects against angiotensin II-induced proliferation and migration of vascular smooth muscle cells by preventing signal transducer and activator of transcription 3 activationBiochim Biophys Acta20141843245446324321768
- SauAPellizzari TregnoFValentinoFFedericiGCaccuriAMGlutathione transferases and development of new principles to overcome drug resistanceArch Biochem Biophys2010500211612220494652
- de LucaAMeiGRosatoNThe fine-tuning of TRAF2–GSTP1-1 interaction: effect of ligand binding and in situ detection of the complexCell Death Dis201451e101524457959
- CoattiGCMarcariniJCSartoriDFidelisQCFerreiraDTMantovaniMSCytotoxicity, genotoxicity and mechanism of action (via gene expression analysis) of the indole alkaloid aspidospermine (antiparasitic) extracted from Aspidosperma polyneuron in HepG2 cellsCytotechnology20166841161117025894792
- PalumboCde LucaARosatoNForgioneMRotiliDCaccuriAMc-Jun N-terminal kinase activation by nitrobenzoxadiazoles leads to late-stage autophagy inhibitionJ Transl Med20161413726847645
- FulciCRotiliDde LucaAA new nitrobenzoxadiazole-based GSTP1-1 inhibitor with a previously unheard of mechanism of action and high stabilityJ Enzyme Inhib Med Chem201732124024728097896
- Pellizzari TregnoFSauAPezzolaSIn vitro and in vivo efficacy of 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX) on human melanomaEur J Cancer200945142606261719665369
- HwLAli-OsmanFGenetic polymorphism and function of glutathione S-transferases in tumor drug resistanceCurr Opin Pharmacol20077436737417681492
- BartoliniDGalliFThe functional interactome of GSTP: A regulatory biomolecular network at the interface with the Nrf2 adaption response to oxidative stressJ Chromatogr B Analyt Technol Biomed Life Sci201610192944
- KouXChenNFengZLuoLANYinZGSTP1 negatively regulates Stat3 activation in epidermal growth factor signalingOncol Lett2013531053105723426146
- LiFFernandezPPRajendranPHuiKMSethiGDiosgenin, a steroidal saponin, inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cellsCancer Lett2010292219720720053498
- TownsendDMShenHStarosALGateLTewKDEfficacy of a glutathione S-transferase pi-activated prodrug in platinum-resistant ovarian cancer cellsMol Cancer Ther20021121089109512481432
- di PietroGMagnoLAVRios-SantosFGlutathione S-transferases: an overview in cancer researchExpert Opin Drug Metab Toxicol20106215317020078251
- HarrisMJCogganMLangtonLWilsonSRBoardPGPolymorphism of the Pi class glutathione S-transferase in normal populations and cancer patientsPharmacogenetics19988127319511178
- CadoniGBocciaSPetrelliLA review of genetic epidemiology of head and neck cancer related to polymorphisms in metabolic genes, cell cycle control and alcohol metabolismActa Otorhinolaryngol Ital201232111122500060
- HuangWWangWZhouMChenSZhangXAssociation of glutathione S-transferase polymorphisms (GSTM1 and GSTT1) with primary open-angle glaucoma: An evidence-based meta-analysisGene20135262808623747403
- HabdousMSiestGHerbethBVincent-ViryMVisvikisSGlutathione S-transferases genetic polymorphisms and human diseases: overview of epidemiological studiesAnn Biol Clin20046211524
- DouradoDFFernandesPARamosMJMannervikBMechanism of glutathione transferase P1-1-catalyzed activation of the prodrug canfosfamide (TLK286, TELCYTA)Biochemistry201352458069807824066958
- NasrASamiRIbrahimNDarwishDGlutathione S transferase (GSTP 1, GSTM 1, and GSTT 1) gene polymorphisms in Egyptian patients with acute myeloid leukemiaIndian J Cancer201552449049526960454
- SunNSunXChenBMRP2 and GSTP1 polymorphisms and chemotherapy response in advanced non-small cell lung cancerCancer Chemother Pharmacol201065343744619568750
- KhruninAVMoisseevAGorbunovaVLimborskaSGenetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patientsPharmacogenomics J2010101546119786980
- BurgDFilippovDVHermannsRvan der MarelGAvan BoomJHMulderGJPeptidomimetic Glutathione Analogs as Novel γGT Stable GST InhibitorsBioorg Med Chem200210119520511738622
- LiTLiuGLiHYangXJingYZhaoGThe synthesis of ethacrynic acid thiazole derivatives as glutathione S-transferase pi inhibitorsBioorg Med Chem20122072316232222370342
- EnoiuMAberkaneHSalazarJFEvidence for the pro-oxidant effect of gamma-glutamyltranspeptidase-related enzymeFree Radic Biol Med200029982583311063908
- NakajimaTReversal of Multiple Drug Resistance in Cholangiocarcinoma by the Glutathione S-Transferase-pi-Specific Inhibitor O1-Hexadecyl-gamma-glutamyl-S-benzylcysteinyl-D-phenylglycine EthylesterJ Pharmacol Exp Ther2003306386186912805482
- RazaAGalliNCallanderNPhase 1-2a multicenter dose-escalation study of ezatiostat hydrochloride liposomes for injection (Telintra(R), TLK199), a novel glutathione analog prodrug in patients with myelodysplastic syndromeJ Hematol Oncol2009212019439093
- RazaAGaliliNSmithSPhase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndromeBlood2009113266533654019398716
- RazaAGaliliNMulfordDPhase 1 dose-ranging study of ezatiostat hydrochloride in combination with lenalidomide in patients with non-deletion (5q) low to intermediate-1 risk myelodysplastic syndrome (MDS)J Hematol Oncol2012511822546242
- MahadevanDSuttonGREzatiostat hydrochloride for the treatment of myelodysplastic syndromesExpert Opin Investig Drugs2015245725733
- RicciGde MariaFAntoniniG7-Nitro-2,1,3-benzoxadiazole derivatives, a new class of suicide inhibitors for glutathione S-transferases. Mechanism of action of potential anticancer drugsJ Biol Chem200528028263972640515888444
- PaselloMMichelacciFSciontiIOvercoming glutathione S-transferase P1-related cisplatin resistance in osteosarcomaCancer Res200868166661666818701490
- AscioneACianfrigliaMDupuisMLThe glutathione S-transferase inhibitor 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol overcomes the MDR1-P-glycoprotein and MRP1-mediated multi-drug resistance in acute myeloid leukemia cellsCancer Chemother Pharmacol200964241942419288261
- ScotlandiKRemondiniDCastellaniGOvercoming resistance to conventional drugs in Ewing sarcoma and identification of molecular predictors of outcomeJ Clin Oncol200927132209221619307502
- de LucaAPellizzari TregnoFSauAGlutathione S-transferase P1-1 as a target for mesothelioma treatmentCancer Sci2013104222323023121163
- FilomeniGTurellaPDupuisML6-(7-Nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol, a specific glutathione S-transferase inhibitor, overcomes the multidrug resistance (MDR)-associated protein 1-mediated MDR in small cell lung cancerMol Cancer Ther20087237137918281520
- TentoriLDorioASMazzonEThe glutathione transferase inhibitor 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX) increases temozolomide efficacy against malignant melanomaEur J Cancer20114781219123021269821
- de LucaAFedericiLde CanioMStellaLCaccuriAMNew Insights into the Mechanism of JNK1 Inhibition by Glutathione Transferase P1-1Biochemistry201251377304731222920299
- AwasthiYCSharmaRSinghalSSHuman glutathione S-transferasesInt J Biochem19942632953088187927
- RotiliDde LucaATarantinoDSynthesis and structure–activity relationship of new cytotoxic agents targeting human glutathione-S-transferasesEur J Med Chem20158915617125462236
- de LucaARotiliDCarpaneseDA novel orally active water-soluble Inhibitor of human glutathione transferase exerts a potent and selective antitumor activity against human melanoma xenograftsOncotarget2015664126414325595904
- BregniGGalliGGevorgyanAde BraudFdi CosimoSTras-tuzumab cardiac toxicity: a problem we put our heart intoTumori2016102115
- YangA-JLiC-CLuC-YActivation of the cAMP/CREB/Inducible cAMP Early Repressor Pathway Suppresses Andrographolide-Induced Gene Expression of the π Class of Glutathione S-Transferase in Rat Primary HepatocytesJ Agric Food Chem20105831993200020063885
- LinC-YFuR-HChouR-HInhibition of JNK by pi class of glutathione S-transferase through PKA/CREB pathway is associated with carnosic acid protection against 6-hydroxydopamine-induced apoptosisFood Chem Toxicol201710319420228288932
- SatyamAHockerMDKane-MaguireKAMorganASVillarHOLyttleMHDesign, Synthesis, and Evaluation of Latent Alkylating Agents Activated by Glutathione S-TransferaseJ Med Chem1996398173617478648613
- TewKDTLK-286: a novel glutathione S-transferase-activated prodrugExpert Opin Investig Drugs200514810471054
- RamsayEEDildaPJGlutathione S-conjugates as prodrugs to target drug-resistant tumorsFront Pharmacol2014511818125157234
- MorganASSandersonPEBorchRFTumor efficacy and bone marrow-sparing properties of TER286, a cytotoxin activated by glutathione S-transferaseCancer Res19985812256825759635580
- RosenLSBrownJLaxaBPhase I study of TLK286 (glutathione S-transferase P1-1 activated glutathione analog) in advanced refractory solid malignanciesClin Cancer Res2003951628163812738715
- RosenLSLaxaBBoulosLPhase 1 study of TLK286 (Telcyta) administered weekly in advanced malignanciesClin Cancer Res200410113689369815173075
- KavanaghJJGershensonDMChoiHMulti-institutional phase 2 study of TLK286 (TELCYTAtm, a glutathione S-transferase P1-1 activated glutathione analog prodrug) in patients with platinum and paclitaxel refractory or resistant ovarian cancerInt J Gynecol Cancer200515459360016014111
- EdelmanMJNovel cytotoxic agents for non-small cell lung cancerJ Thorac Oncol20061775275517409954
- RaezLELilenbaumRNew developments in chemotherapy for advanced non-small cell lung cancerCurr Opin Oncol200618215616116462185
- KavanaghJJLevenbackCFRamirezPTPhase 2 study of canfosfamide in combination with pegylated liposomal doxorubicin in platinum and paclitaxel refractory or resistant epithelial ovarian cancerJ Hematol Oncol201031920222977
- SequistLVFidiasPMTemelJSPhase 1-2a multicenter dose-ranging study of canfosfamide in combination with carboplatin and paclitaxel as first-line therapy for patients with advanced non-small cell lung cancerJ Thorac Oncol20094111389139619701107
- BogdanCNitric oxide synthase in innate and adaptive immunity: an updateTrends Immunol201536316117825687683
- SinghSGuptaAKNitric oxide: role in tumour biology and iNOS/NO-based anticancer therapiesCancer Chemother Pharmacol20116761211122421544630
- ShamiPJSaavedraJEWangLYJS-K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activityMol Cancer Ther20032440941712700285
- BurkeAJSullivanFJGilesFJGlynnSAThe yin and yang of nitric oxide in cancer progressionCarcinogenesis201334350351223354310
- KeeferLBroad-Spectrum Anti-Cancer Activity of O2-Arylated DiazeniumdiolatesFor Immunopathol Dis Therap201013205218
- KiziltepeTHideshimaTIshitsukaKJS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cellsBlood2007110270971817384201
- KiziltepeTAndersonKCKutokJLJS-K has potent anti- angiogenic activity in vitro and inhibits tumour angiogenesis in a multiple myeloma model in vivoJ Pharm Pharmacol201062114515120723011
- MaciagAEChakrapaniHSaavedraJEThe nitric oxide prodrug JS-K is effective against non-small-cell lung cancer cells in vitro and in vivo: involvement of reactive oxygen speciesJ Pharmacol Exp Ther2011336231332020962031
- ShamiPJMaciagAEEddingtonJKJS-K, an arylating nitric oxide (NO) donor, has synergistic anti-leukemic activity with cytara-bine (ARA-C)Leuk Res200933111525152919193435
- XueRWuJLuoXDesign, Synthesis, and Evaluation of Diazeniumdiolate-Based DNA Cross-Linking Agents Activatable by Glutathione S-TransferaseOrg Lett2016182051965199
- KaczmarekMZHollandRJLavanierSAMechanism of action for the cytotoxic effects of the nitric oxide prodrug JS-K in murine erythroleukemia cellsLeuk Res201438337738224461365
- RenZKarSWangZWangMSaavedraJECarrBIJS-K, a novel non-ionic diazeniumdiolate derivative, inhibits Hep 3B hepatoma cell growth and induces c-Jun phosphorylation via multiple MAP kinase pathwaysJ Cell Physiol2003197342643414566972
- McmurtryVSaavedraJENieves-AliceaRSimeoneAMKeeferLKTariAMJS-K, a nitric oxide-releasing prodrug, induces breast cancer cell death while sparing normal mammary epithelial cellsInt J Oncol201138496397121271218
- KaurITerrazasMKosakKMKernSEBoucherKMShamiPJCellular distribution studies of the nitric oxide-generating antineoplas-tic prodrug O(2)-(2,4-dinitrophenyl)1-((4-ethoxycarbonyl)piperazin-1-yl)diazen-1-ium-1,2-diolate formulated in Pluronic P123 micellesJ Pharm Pharmacol20136591329133623927471
- SimeoneAMMcMurtryVNieves-AliceaRTIMP-2 mediates the anti-invasive effects of the nitric oxide-releasing prodrug JS-K in breast cancer cellsBreast Cancer Res2008103R4418474097
- KitagakiJYangYSaavedraJEColburnNHKeeferLKPerantoniAONitric oxide prodrug JS-K inhibits ubiquitin E1 and kills tumor cells retaining wild-type p53Oncogene200928461962418978812
- WeyerbrockAOsterbergNPsarrasNJS-K, a glutathione S-transferase-activated nitric oxide donor with antineoplastic activity in malignant gliomasNeurosurgery2012702497510 discussion 51021849924
- LaschakMSpindlerKDSchraderAJJS-K, a glutathione/glutathione S-transferase-activated nitric oxide releasing prodrug inhibits androgen receptor and WNT-signaling in prostate cancer cellsBMC Cancer20121213022462810
- ChakrapaniHKalathurRCMaciagAESynthesis, mechanistic studies, and anti-proliferative activity of glutathione/glutathione S-transferase-activated nitric oxide prodrugsBioorg Med Chem200816229764977118930407
- QiuMChenLTanGA reactive oxygen species activation mechanism contributes to JS-K-induced apoptosis in human bladder cancer cellsSci Rep201551510426458509
- EdesKCassidyPShamiPJMoosPJJS-K, a nitric oxide prodrug, has enhanced cytotoxicity in colon cancer cells with knockdown of thioredoxin reductase 1PLoS One201051e878620098717
- LiuLWangDWangJWangSThe Nitric Oxide Prodrug JS-K Induces Ca(2+)-Mediated Apoptosis in Human Hepatocellular Carcinoma HepG2 CellsJ Biochem Mol Toxicol201630419219926616367
- LiuJLiCQuWNitric oxide prodrugs and metallochemo-therapeutics: JS-K and CB-3-100 enhance arsenic and cisplatin cytolethality by increasing cellular accumulationMol Cancer Ther20043670971415210857
- KaurIKosakKMTerrazasMEffect of a Pluronic® P123 formulation on the nitric oxide-generating drug JS-KPharm Res20153241395140625330743
- KuhlmannMKBurkhardtGKöhlerHInsights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical applicationNephrol Dial Transplant19971212247824809430835
- HospersGAEisenhauerEAde VriesEGThe sulfhydryl containing compounds WR-2721 and glutathione as radio- and chemoprotective agents. A review, indications for use and prospectsBr J Cancer1999805–662963810360638
- IorioASpinelliMPolimantiRGSTA1 gene variation associated with gestational hypertension and its involvement in pregnancy-related pathogenic conditionsEur J Obstet Gynecol Reprod Biol2015194343726321410
- BonifaziFStorciGBandiniGGlutathione transferase-A2 S112T polymorphism predicts survival, transplant-related mortality, busulfan and bilirubin blood levels after allogeneic stem cell transplantationHaematologica201499117217924056816
- ZhangWModenOMannervikBDifferences among allelic variants of human glutathione transferase A2-2 in the activation of azathioprineChem Biol Interact2010186211011720434437
- RodriguesDAMartinsJVSilvaKSEGSTM1 polymorphism in patients with clinical manifestations of atherosclerosisGenetics and Molecular Research: GMR2017161
- Abdur RehmanMYKamalATaqiMMMalikRNTracing biomarker of PAH-exposure and susceptibility factor (GSTM-polymorphism) among cancer patients in PakistanChemosphere201717838439028340461
- TanwarRIyengarARNageshKSPatilSSubhashBVGSTM1 null polymorphism prevalence in tobacco users, oral leukoplakia and oral squamous cell carcinoma patients in South Indian population: A polymerase chain reaction studyIndian J Dent Res201627435335827723629
- XuYWangJDongWGSTM3 A/B polymorphism and risk for head and neck cancer: a meta-analysisPLoS One201491e8385124416175
- FengXDongCQShiJJZhouHFHeWZhengBSLack of association of glutathione S-transferase M3 gene polymorphism with the susceptibility of lung cancerAsian Pac J Cancer Prev20121394465446823167362
- BhatAMasoodAWaniKAPromoter methylation and gene polymorphism are two independent events in regulation of GSTP1 gene expressionTumour Biol2017394101042831769756328443466
- HuangXKHuangYHHuangJHLiangJYGlutathione S-transferase P1 Ile105Val Polymorphism and Male Infertility Risk: An Updated Meta-analysisChin Med J (Engl)2017130897998528397729
- SoaresPOMaluf CuryPMendoza LopezRVGTSP1 expression in non-smoker and non-drinker patients with squamous cell carcinoma of the head and neckPLoS One2017128e018260028817620
- BasharatZYasminAEnergy landscape of a GSTP1 polymorph linked with cytological function decay in response to chemical stressorsGene2017609192728153749
- ChoiBKimMGHanNPopulation pharmacokinetics and pharmacodynamics of busulfan with GSTA1 polymorphisms in patients undergoing allogeneic hematopoietic stem cell transplantationPharmacogenomics201516141585159426419450
- SongYShanZLuoCGlutathione S-Transferase T1 (GSTT1) Null Polymorphism, Smoking, and Their Interaction in Coronary Heart Disease: A Comprehensive Meta-AnalysisHeart Lung Circ201726436237027686690
- JangSGKimIJKangHCGSTT2 promoter polymorphisms and colorectal cancer riskBMC Cancer200771617250773
- ChelvanayagamGWilceMCParkerMWTanKLBoardPGHomology model for the human GSTT2 Theta class glutathione transferaseProteins19972711181309037717
- XuYTWangJYinRGenetic polymorphisms in Glutathione S-transferase Omega (GSTO) and cancer risk: a meta-analysis of 20 studiesSci Rep20144657825300926
- PolimantiRGrazianoMELazzarinNVaqueroEManfellottoDFuciarelliMGSTO1 uncommon genetic variants are associated with recurrent miscarriage riskFertil Steril2014101373573924417908
- WangZQuKHuangZGlutathione S-transferase O2 gene rs157077 polymorphism predicts response to transarterial chemoembolization in hepatocellular carcinomaTumour Biol20153686463646925835968
- StamenkovicMRadicTStefanovicIGlutathione S-transferase omega-2 polymorphism Asn142Asp modifies the risk of age-related cataract in smokers and subjects exposed to ultraviolet irradiationClin Exp Ophthalmol201442327728323927022
- LangaeeTYZhongGLiWThe influence of human GSTZ1 gene haplotype variations on GSTZ1 expressionPharmacogenet Genomics201525523924525738370
- ShroadsALCoatsBSMcDonoughCWLangaeeTStacpoolePWHaplotype variations in glutathione transferase zeta 1 influence the kinetics and dynamics of chronic dichloroacetate in childrenJ Clin Pharmacol2015551505525079374
- Karakas-CelikSArasNAtesCGlutathione S-transferase Z1 (GSTZ1) gene polymorphism in gastric cancer: a preliminary study in a Turkish populationLab Med2014451374224719983
- BurgDFilippovDVHermannsRvan der MarelGAvan BoomJHMulderGJPeptidomimetic glutathione analogs as novel gammaGT stable GST inhibitorsBioorg Med Chem200210119520511738622
- LiTLiuGLiHYangXJingYZhaoGThe synthesis of ethacrynic acid thiazole derivatives as glutathione S-transferase pi inhibitorsBioorg Med Chem20122072316232222370342
- MahadevanDSuttonGREzatiostat hydrochloride for the treatment of myelodysplastic syndromesExpert Opin Investig Drugs2015245725733
- FulciCRotiliDDe LucaAA new nitrobenzoxadiazole-based GSTP1-1 inhibitor with a previously unheard of mechanism of action and high stabilityJ Enzyme Inhib Med Chem201732124024728097896
- RotiliDDe LucaATarantinoDSynthesis and structure – activity relationship of new cytotoxic agents targeting human glutathione-S-transferasesEur J Med Chem20158915617125462236
- De LucaAMeiGRosatoNThe fine-tuning of TRAF2-GSTP1-1 interaction: effect of ligand binding and in situ detection of the complexCell Death Dis20145e101524457959
- GrazianiGArtusoSDe LucaAA new water soluble MAPK activator exerts antitumor activity in melanoma cells resistant to the BRAF inhibitor vemurafenibBiochem Pharmacol2015951162725795251
- RamsayEEDildaPJGlutathione S-conjugates as prodrugs to target drug-resistant tumorsFront Pharmacol2014518125157234
- TewKDTLK-286: a novel glutathione S-transferase-activated prod-rugExpert Opin Investig Drugs200514810471054
- VergoteIFinklerNdel CampoJPhase 3 randomised study of canfosfamide (Telcyta, TLK286) versus pegylated liposomal doxorubicin or topotecan as third-line therapy in patients with platinum-refractory or -resistant ovarian cancerEur J Cancer200945132324233219515553
- QiuMKeLZhangSZengXFangZLiuJJS-K, a GST-activated nitric oxide donor prodrug, enhances chemo-sensitivity in renal carcinoma cells and prevents cardiac myocytes toxicity induced by DoxorubicinCancer Chemother Pharmacol201780227528628608259