Abstract
Purpose
To evaluate the pharmacokinetics and pharmacodynamics of candesartan and amlodipine in the absence and presence of each other in healthy subjects.
Methods
This study consisted of two parts: part 1, the effect of amlodipine on candesartan; part 2, the effect of candesartan on amlodipine. Each part was designed as a randomized, open-label, two-sequence, two-period, two-intervention crossover study with 20 subjects and performed separately in different populations. Pharmacokinetic assessments were performed over 48 hours for candesartan in part 1 and 72 hours for amlodipine in part 2 after drug administration on Day 10. Safety data included the results of physical examinations, clinical laboratory tests, vital signs, an electrocardiogram, and adverse events.
Results
For both candesartan and amlodipine, the 90% confidence intervals for the geometric mean ratios of area under the concentration-time curve from time zero to the time of dosing interval of 24 hours and maximum concentration after drug administration fell within the bioequivalence acceptance criteria. Although this study was conducted in normotensive subjects, blood pressure lowering effects were observed in all intervention groups and co-administration of candesartan and amlodipine reduced blood pressure more than amlodipine alone, but similar to candesartan alone. No serious adverse event was reported throughout the study, and all treatment emergent adverse events were mild to moderate in severity and were recovered without sequelae.
Conclusion
Co-administration of candesartan and amlodipine did not change the systemic exposure of each drug alone in healthy subjects. The administration of candesartan 32 mg alone, amlodipine 10 mg alone, and co-administration of candesartan and amlodipine were well tolerated during the study.
Introduction
Hypertension is one of the most important risk factors of cardiovascular disease. For the management of hypertension, it is required to reduce blood pressure (BP) to target levels by using antihypertensive drugs. The antihypertensive drugs are divided into several classes such as thiazide-type diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor, and angiotensin receptor blocker according to their action mechanisms.Citation1 Monotherapy with one class drug is usually not adequate for patients with high-risk conditions, and most hypertensive patients will require two or more drugs with different action mechanisms to achieve goal BP.Citation2
Candesartan is one of the angiotensin receptor blockers (ARBs) and amlodipine is the most widely used calcium channel blocker for the treatment of hypertension. Since the action mechanisms of candesartan and amlodipine are quite different, combination therapy with these two drugs was effective for hypertensive patients.Citation3–Citation5 Moreover, candesartan has shown more favorable tolerability in terms of developing peripheral edema compared to amlodipine.Citation6 For patients whose BP is uncontrolled by low dose amlodipine alone, it can be better to use candesartan in combination with amlodipine than to increase the dose of amlodipine. To facilitate this rational prescribing, fixed-dose combinations of candesartan and amlodipine have been available in Japan and Republic of Korea.Citation7,Citation8
A large number of drug interactions are attributable to the inhibition or induction of CYP/CYP450 (CYP) enzymes.Citation9 Since amlodipine is extensively metabolized by the CYP3A4, its systemic exposure may be affected by CYP inhibitors and inducers.Citation10 In contrast, candesartan is mainly excreted unchanged in urine and feces.Citation11 Although they have different disposition and elimination pathways, it is recommended to address the drug interaction potential if they are commonly used in combination.Citation12,Citation13 The clinical efficacy of combination therapy with candesartan and amlodipine in hypertensive patients is well demonstrated; however, there is a lack of study explicitly reporting drug interactions between these drugs. The purpose of this study was to evaluate the pharmacokinetics and pharmacodynamics of candesartan and amlodipine in the absence and presence of each other in healthy subjects.
Materials and methods
Study design and subjects
This study consisted of two parts: part 1, the effect of amlodipine on candesartan; part 2, the effect of candesartan on amlodipine. Each part was designed as a randomized, open-label, two-sequence, two-period, two-intervention (administration of one drug with the absence or presence of the other drug) crossover study with 20 subjects and performed separately in different populations. In each part, subjects were randomized to receive either one drug with absence followed by presence of the other drug, or vice versa. Each intervention was separated by a 14-day washout period except when subjects received candesartan alone first and then candesartan with amlodipine after a washout period of 4 days in part 1 ().
Table 1 Study design and drug administration scheme
Eligible subjects were males between 20–55 years old with body mass index of 18.5–27.0 and were healthy as determined by medical history, physical examination, vital signs, 12-lead electrocardiogram (ECG), and clinical laboratory tests (hematology, blood chemistry, urinalysis, serology) within 28 days prior to enrolment in the study. Subjects were excluded if they had donated blood in the 2 months previous to the study or participated in any clinical trials in the month previous to the study or had received any drug that affects the pharmacokinetics of candesartan or amlodipine in the month previous to the study. During the study, subjects were required to abstain from strenuous physical exercise, smoking, and products, such as over-the-counter medications or beverages containing alcohol or caffeine.
The intra-subject coefficients of variation of the PK exposure of candesartan and amlodipine were assumed to be 18% and 10%, respectively, based on the results of a previous clinical study.Citation21,Citation22 With a sample size of 16, a difference of 20% in the log-transformed PK parameters of candesartan and amlodipine could be detected with >90% test power at a significance level of 0.05. Assuming a drop-out, the total number of subjects for each part was 20 (10 subjects per sequence).
The protocol of this study was approved by the Samsung Medical Center Institutional Review Board and the Ministry of Food and Drug Safety in Republic of Korea before the initiation of the study. The study was conducted in accordance with the Declaration of Helsinki, Korean Good Clinical Practice. All subjects gave written informed consent prior to any study procedures.
Dosing and sampling schedules
Subjects received candesartan alone, amlodipine alone, or both candesartan and amlodipine for 10 days in each part. Candesartan 32 mg (Atacand®, AstraZeneca Korea, Seoul, Korea) and amlodipine 10 mg (Norvasc®, Pfizer Korea, Seoul, Korea) were administered with 240 mL water regardless of meals, while the same were administered under fasted conditions on Day 10. Subjects visited the Samsung Medical Center for drug administration on Days 1, 4, 7, 8, and 9 and documented timing and administration of drugs with dosing diaries on Days 2, 3, 5 and 6. Pharmacokinetic assessments were performed over 48 hours for candesartan in part 1 and 72 hours for amlodipine in part 2 after drug administration on Day 10. For the pharmacokinetic analysis of candesartan, blood samples (8 mL) were collected at predose and at 1, 2, 3, 4, 5, 6, 8, 12, 24, and 48 hours postdose on day 10 in part 1. As for amlodipine, blood samples were collected at predose and at 1, 2, 3, 4, 5, 6, 8, 12, 24, 48, and 72 hours postdose on Day 10 in part 2. Blood samples were centrifuged at 1,800 g for 10 minutes and plasma was collected and frozen at −70°C until analysis.
Determination of plasma concentrations
Plasma samples for PK analysis were determined using ultra performance liquid chromatography – tandem mass spectrometry (UPLC-MS/MS) with a validated methodology to determine plasma concentrations of candesartan and amlodipine.
In the candesartan assay, chromatography (Waters Acquity UPLC™ System; Waters Corporation, Milford, MA, USA) was performed with a Waters Acquity UPLC®BEH C18 column (1.7 µm, 2.1×50 mm; Waters) while the column temperature was maintained at 30°C ± 5°C. The standards for candesartan and the internal standard (IS), candesartan _d5, were provided by Toronto Research Chemicals Inc. (North York, ON, Canada). The mobile phase for candesartan consisted of a mixture of (A) 0.1% (vol/vol) formic acid in distilled water and (B) 0.1% (vol/vol) formic acid in acetonitrile (A:B=6:4, vol/vol) with a flow rate of 0.4 mL/min. Detection of candesartan was conducted by a Waters Micromass Quattro Premier™ XE Mass Spectrometer (Waters Corporation) with a positive electrospray ionization multiple reaction monitoring mode set to transmit at m/z 440.86 → 262.85 and 445.92 → 267.88 for candesartan and the internal standard, respectively. The assay was linear in the concentration ranges of 5–1,000 µg/L and the lower limit of quantification was 5.0 µg/L in the candesartan assay. A mixture of 50 µL aliquot of plasma sample and 10 µL of IS working solution (candesartan-d5 1.50 µg/mL in 70% methanol [vol/vol]) was prepared. The prepared mixture was deproteinized by 150 µL volume of pure acetonitrile. Then, each 100 µL of the supernatant was diluted with 200 µL of 0.1% formic acid. The 5 µL aliquot was injected into the LC/MS/MS system. The intrabatch and interbatch precision (%relative SD) for candesartan in plasma samples was less than 9.8% and 10.4%, respectively. The intrabatch and interbatch accuracy (%deviation of mean from theoretical) for candesartan in plasma samples ranged between 4.7% and 7.5%, and −3.4% and 7.1%, respectively.
In the amlodipine assay, chromatography (Waters Acquity UPLC™ System) was performed with a Waters Acquity UPLC®BEH C18 column (1.7 µm, 2.1×50 mm) while the column temperature was maintained at 30°C ± 5°C. The standards for amlodipine and the IS, amlodipine_d4 maleic acid salt, were provided by Hana Pharam Co., Ltd (Seoul, Korea) and Toronto Research Chemicals Inc., respectively. The mobile phase consisted of: (A) 0.1% (vol/vol) formic acid in distilled water and (B) 0.1% (vol/vol) formic acid in acetonitrile, under gradient condition was changed as follows: (A) 70% at 0.00 and 2.30 minutes; (A) 10% at 2.40 and 3.00 minutes; and (A) 70% at 3.10 and 3.50 minutes. Detection of candesartan was conducted by a Waters Xevo™ TQ-S Mass Spectrometer (Waters Corporation) with a positive electrospray ionization multiple reaction monitoring mode set to transmit at m/z 409.23 → 238.15 and 413.23 → 238.15 for amlodipine and the internal standard, respectively. The assay was linear in the concentration ranges of 0.05–50 µg/L and the lower limit of quantification was 0.05 µg/L in the amlodipine assay. Plasma (100 µL) was added to the IS working solution (amlodipine-d4 maleic acid salt, 10 µg of 20 µg/mL in 50% methanol [vol/vol]). The mixture sample was deproteinized by 250 µL volume of pure acetonitrile. Each 200 µL of the supernatant was diluted with 300 µL of 0.1% formic acid. The 20 µL of mixture was injected into the LC/MS/MS system. The intrabatch and interbatch precision for amlodipine in plasma samples was less than 3.0% and 6.0%, respectively. The intrabatch and interbatch accuracy for amlodipine in plasma samples ranged between −7.2% and −2.0%, and −2.0% and −0.7%, respectively.
Assessments
Noncompartmental analysis was used for the pharmacokinetics of candesartan and amlodipine. Under steady state conditions, the following pharmacokinetic parameters were determined using Phoenix WinNonlin 6.3 (Certara, St Louis, MO, USA): maximum concentration after drug administration (Cmax); area under the concentration-time curve from time zero to the time of dosing interval of 24 hours (AUCτ); time to reach Cmax (tmax); terminal elimination half-life (t1/2); and the concentration at predose on Day 10 (Ctrough). For evaluating the pharmacodynamics, systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse rate (PR) were measured in sitting position at predose and 2, 4, 6, 8, 12, and 24 hours postdose on Day 10 as well as baseline on Day 1. Safety was assessed throughout the study by evaluating treatment-emergent adverse events (TEAEs), clinical laboratory tests, vital signs, physical examinations, and ECG. Particularly, BP decreased by more than expected in healthy subjects following a hypertensive drug administration was documented as a TEAE.
Statistical analysis
The pharmacokinetic parameters except tmax were log-transformed before analysis. The effect of co-administration of candesartan and amlodipine in comparison with each drug alone was analyzed using a linear mixed effects model. The statistical model included sequence, period and intervention as fixed factors, and subjects nested within sequence as a random factor. For Cmax and AUCτ, the geometric means ratios (GMR) and their 90% CI were obtained by back-transformation to the original scale to estimate an interaction effect. Lack of an interaction effect on the pharmacokinetics would be concluded if the 90% CIs of the GMRs were contained within 0.80 and 1.25 for both Cmax and AUCτ. The Wilcoxon signed-rank test was used to analyze the difference between interventions for tmax. As for SBP, DBP, and PR, maximum change from baseline and trend over time were compared using a linear mixed effects model for repeated measurements with first-order autoregressive covariance structure. Demographic characteristics were summarized using descriptive statistics and all pharmacokinetic parameters were summarized by intervention group. All statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA).
Based on the known pharmacokinetic profile of candesartan and amlodipine, the sample size of 20 subjects provided >90% power to discriminate 20% differences in exposures by pharmacokinetic equivalence test using a linear mixed effects model appropriate for a two-way crossover.
Results
Subjects
Twenty subjects in each part were enrolled and completed without any drop-out. The demographic characteristics of subjects are summarized in . Particularly, baseline vital signs were similar between interventions in each part.
Table 2 Demographic and baseline characteristics of subjects
Candesartan pharmacokinetics
Plasma candesartan levels reached a peak at about 5 hours, and then declined with half-lives of about 10 hours (). The pharmacokinetic parameters of candesartan with and without amlodipine are presented in . The 90% CIs of the GMR for Cmax and AUCτ were contained within the predefined limits of 0.80–1.25. There was no statistically significant difference in tmax, t1/2, and Ctrough following co-administration of candesartan and amlodipine compared to candesartan alone ().
Figure 1 Mean (standard deviation) plasma concentration-time profiles of (A) candesartan and (B) amlodipine following multiple doses of the two drugs in the absence and presence of each other in healthy subjects.
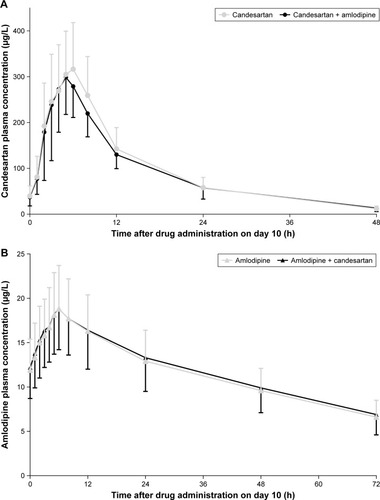
Table 3 Pharmacokinetic parameters following multiple doses of candesartan 32 mg and amlodipine 10 mg in the absence and presence of each other in healthy subjects
Amlodipine pharmacokinetics
Plasma amlodipine levels reached a peak at 6 hours, and then declined with half-lives of about 50 hours (). The pharmacokinetic parameters of amlodipine by intervention group are presented in . The 90% CIs of the GMR for Cmax and AUCτ were contained within the predefined limits of 0.80–1.25. There was no statistically significant difference in tmax, t1/2, and Ctrough following co-administration of candesartan and amlodipine compared to amlodipine alone ().
Blood pressure and pulse rate
Candesartan, alone and in combination with amlodipine, showed similar profiles for the maximum change from baseline, although DBP was more decreased after combination with amlodipine than candesartan alone (). The maximum change in SBP and DBP on Day 10 were −19.5, −15.7 for amlodipine alone and −26.4, −21.3 for co-administration, respectively, and this difference reached statistical significance. Trends over time in vital signs on Day 10 are shown in . As with the maximum change from baseline, there were significant differences in SBP and DBP between amlodipine alone and in combination with candesartan. For PR, however, any differences were not shown between interventions in both parts.
Figure 2 Trends over time in vital signs after co-administration of candesartan 32 mg and amlodipine 10 mg and after administration of each drug alone in healthy subjects (left panel: part 1, right panel: part 2).
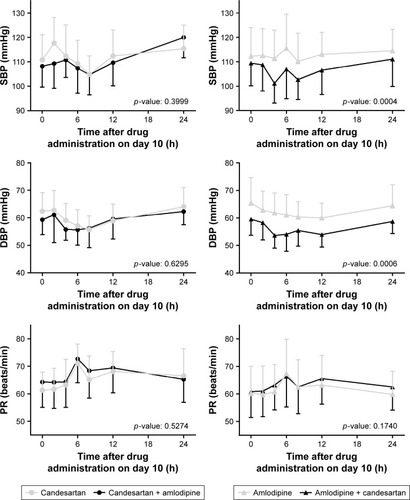
Table 4 The maximum change from baseline (CFB) in vital signs on Day 10 after co-administration of candesartan 32 mg and amlodipine 10 mg and after administration of each drug alone in healthy subjects
Safety
No serious TEAE was reported throughout the study, and all TEAEs were mild to moderate in severity and were recovered without sequelae. None of subjects discontinued the study due to TEAEs. Although more subjects experienced low DBP when administered candesartan in combination with amlodipine, there was no apparent difference in TEAE profiles between interventions in each part ().
Table 5 Treatment-emergent adverse event (TEAE) profiles after co-administration of candesartan and amlodipine and after administration of each drug alone in healthy subjects
Discussion
For the treatment of hypertensive patients, a rational combination of two antihypertensive drugs with different modes of action may be complicated by their drug–drug interactions. This study was performed to determine the potential for a drug interaction between candesartan and amlodipine in healthy subjects.
The present study demonstrated that amlodipine did not influence the rate and extent of candesartan absorption at steady state. In addition, the pharmacokinetics of amlodipine was not affected by multiple oral administrations of candesartan. Several studies have investigated pharmacokinetic interactions between ARBs and amlodipine. Multiple co-administrations of olmesartan 20 mg and amlodipine 5 mg,Citation14 valsartan 320 mg and amlodipine 10 mg,Citation15 and fimasartan 120 mg and amlodipine 10 mgCitation16 did not show any clinical relevant change in the systemic exposure of both ARBs and amlodipine. Similar results were observed when S-amlodipine, an enantiomer of amlodipine, was co-administered with telmisartan.Citation17 It should be noted that candesartan showed the relatively smallest degree of alteration in the pharmacokinetics of amlodipine among ARBs.
The lack of pharmacokinetic interactions between candesartan and amlodipine can be attributed partly to their different disposition pathways. Candesartan is eliminated by renal and biliary excretion,Citation11 while CYP enzymes, mainly CYP3A4, are involved in amlodipine metabolism.Citation10 There are few drugs that affected the systemic exposure of amlodipine, except CYP3A4 inhibitors.Citation10 Candesartan has no effects on CYP enzymes at therapeutic concentrations,Citation11 meaning candesartan is not considered as a CYP3A4 inhibitor. In addition, P-glycoprotein (P-gp) is well known to play a significant role in drug absorption from the gastrointestinal lumen, indicating the modulation of P-gp can mediate drug–drug interactions.Citation18 Candesartan is reported as a P-gp substrate,Citation19 but amlodipine might be devoid of P-gp inhibiting properties.Citation20 Therefore, significant pharmacokinetic alterations both in amlodipine by candesartan and in candesartan by amlodipine are unlikely.
Although this study was conducted in normotensive subjects, BP lowering effects were observed in all intervention groups. Furthermore, co-administration of candesartan and amlodipine reduced BP more than amlodipine alone, but similar to candesartan alone. In patients with essential hypertension, however, it was found that the combination therapy of candesartan and amlodipine for 8-weeks reduced a mean sitting DBP more than candesartan or amlodipine alone.Citation5 It should be noted that the administered dose in the present study was 32 mg candesartan, not 16 mg. Considering the short period of 10 days in normotensive subjects, the interaction effects on lowering BP need to be interpreted with caution.
Conclusion
To our knowledge, this study is the first to investigate the pharmacokinetic interactions between candesartan and amlodipine. Co-administration of candesartan and amlodipine did not change the systemic exposure of each drug alone in healthy subjects. The administration of candesartan 32 mg alone, amlodipine 10 mg alone, and co-administration of candesartan and amlodipine were well tolerated during the study.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was sponsored by CJ Health Care., Ltd, Seoul, Republic of Korea. The sponsor was not involved in the study design or in the analysis and interpretation of data. The sponsor monitored this study during the whole clinical trial period.
Disclosure
The authors report no conflicts of interest in this work.
References
- JamesPAOparilSCarterBL2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8)JAMA2014311550752024352797
- ChobanianAVBakrisGLBlackHRThe Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 reportJAMA2003289192560257212748199
- YamaguchiJHagiwaraNOgawaHEffect of amlodipine + candesartan on cardiovascular events in hypertensive patients with coronary artery disease (from The Heart Institute of Japan Candesartan Randomized Trial for Evaluation in Coronary Artery Disease [HIJ-CREATE] Study)Am J Cardiol2010106681982420816122
- RakugiHOgiharaTMiyataYSasaiKTotsukaNEvaluation of the efficacy and tolerability of combination therapy with candesartan cilexetil and amlodipine besilate compared with candesartan cilexetil monotherapy and amlodipine besilate monotherapy in Japanese patients with mild-to-moderate essential hypertension: a multicenter, 12-week, randomized, double-blind, placebo-controlled, parallel-group studyClin Ther201234483884822440192
- SohnISKimCJAhnTEfficacy and tolerability of combination therapy versus monotherapy with candesartan and/or amlodipine for dose finding in essential hypertension: a phase II multicenter, randomized, double-blind clinical trialClin Ther20173981628163828734660
- KlonerRAWeinbergerMPoolJLComparative effects of candesartan cilexetil and amlodipine in patients with mild systemic hypertension. Comparison of candesartan and amlodipine for safety, tolerability and efficacy (CASTLE) study investigatorsAm J Cardiol200187672773111249891
- YasunoSFujimotoANakagawaYKuwaharaKUeshimaKFixed-dose combination therapy of candesartan cilexetil and amlodipine besilate for the treatment of hypertension in JapanExpert Rev Cardiovasc Ther201210557758322651833
- LeeHSNew antihypertensive combos ready for market entry: Korea Biomedical Review2018 Available from: http://www.koreabiomed.com/news/articleView.html?idxno=2311Accessed July 24, 2018
- LinJHLuAYAyLInhibition and induction of cytochrome P450 and the clinical implicationsClin Pharmacokinet19983553613909839089
- Norvasc® prescribing information Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2013/019787s054lbl.pdfAccessed July 24, 2018
- Atacand® prescribing information Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2015/020838s036lbl.pdfAccessed July 24, 2018
- Food U.S and U.S AdministrationClinical drug interaction studies – study design, data analysis, and clinical implications guidance for industry2017 Available from: https://www.fda.gov/downloads/drugs/guidances/ucm292362.pdfAccessed July 24, 2018
- European Medicines AgencyGuideline on the investigation of drug interactions2012 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdfAccessed July 24, 2018
- BolbrinkerJHuberMScholzeJKreutzRPharmacokinetics and safety of olmesartan medoxomil in combination with either amlodipine or atenolol compared to respective monotherapies in healthy subjectsFundam Clin Pharmacol200923676777419659504
- BhadPAyalasomayajulaSKaranREvaluation of pharmacokinetic interactions between amlodipine, valsartan, and hydrochlorothiazide in patients with hypertensionJ Clin Pharmacol201151693394220852001
- YiSKimTEYoonSHPharmacokinetic interaction of fimasartan, a new angiotensin II receptor antagonist, with amlodipine in healthy volunteersJ Cardiovasc Pharmacol201157668268921394036
- NohYHLimHSKimMJPharmacokinetic interaction of telmisartan with s-amlodipine: an open-label, two-period crossover study in healthy Korean male volunteersClin Ther20123471625163522721873
- LinJHDrug-drug interaction mediated by inhibition and induction of P-glycoproteinAdv Drug Deliv Rev2003551538112535574
- ZhouLChenXGuYLiangJTransport characteristics of candesartan in human intestinal Caco-2 cell lineBiopharm Drug Dispos200930525926419562680
- ZhouSFXueCCYuXQLiCWangGClinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoringTher Drug Monit200729668771018043468
- TjandrawinataRRSetiawatiEYunaidiDASimanjuntakRSantosoIDSusantoLWBioequivalence study of two formulations of candesartan cilexetil tablet in healthy subjects under fasting conditionsDrug Des Devel Ther2013207841847
- LiuYJiaJLiuGPharmacokinetics and bioequivalence evaluation of two formulations of 10-mg amlodipine besylate: an open-label, single-dose, randomized, two-way crossover study in healthy Chinese male volunteersClin Ther200931477778319446150
