Abstract
A major cell defense mechanism against oxidative and xenobiotic stress is mediated by the Nrf2/Keap1 signaling pathway. The Nrf2/Keap1 pathway regulates gene expression of many cytoprotective and detoxifying enzymes, thus playing a pivotal role in maintaining redox cellular homeostasis. Many diseases including cancer have been closely related to impaired Nrf2 activity. Targeting Nrf2 and modulating its activity represents a novel modern strategy for cancer chemoprevention and therapy. In this review, different design strategies used for the development of Nrf2 modulators are described in detail. Moreover, the main focus is on important and recently developed Nrf2 activators and inhibitors, their in vitro and in vivo studies, and their potential use as chemopreventive agents and/or cancer therapeutics.
Introduction
Cancer presents a major public health problem around the world, and it is currently the second leading cause of death in developed countries.Citation1 Tens of millions of people are diagnosed with different types of cancer each year, and more than half eventually die because of cancer progression.Citation2 Uncontrolled proliferation, resistance to apoptosis, metastasis, and escape from immune surveillance are the main features of tumor cells.Citation3 Furthermore, a high level of oxidative stress, which is exerted by ROS and/or RNS, is also one of the main characteristics of cancer cells.Citation4 In humans, complex defensive machinery acts to defend against the attacks of ROS and RNS, which are regularly generated in the human body from cellular metabolism and environmental exposure.Citation5,Citation6 One of the main pathways responsible for cell defense against oxidative and xenobiotic stress is the Nrf2/Keap1 signaling pathway.Citation7
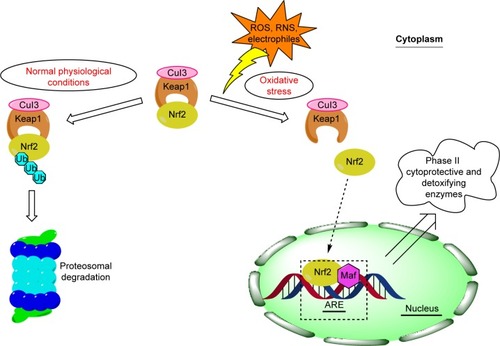
The Nrf2/Keap1 pathway is an inherited defense system offering protection against a wide spectrum of stressors, including oxidative and chemical stress.Citation8 Nrf2 belongs to the cap “n” collar subfamily of basic region leucine zipper transcription factors and plays an important role in the transcriptional stimulation of β-globin genes.Citation9 Under normal physiological conditions, Nrf2 is bound to cysteine-rich protein Keap1, a repressor protein that binds to Nrf2, and promotes its ubiquitination via Cul3-based E3 ligase and degradation by the ubiquitin-proteasome pathway,Citation10,Citation11 whereas upon exposure to oxidative and xenobiotic stress, reactive cysteine residues of Keap1 become modified. Nrf2 is thus released from Keap1 and further on translocated into nucleus where it triggers cytoprotection of the cell by binding together with small Maf proteins to the ARE in the regulatory regions of target genes ().Citation10,Citation11 Nrf2, which was first identified in humans in 1994,Citation12 is a 605-amino-acid-long protein composed of seven highly conserved regions, which were named as Neh domains. Four years later, a negative regulator of Nrf2 was discovered and named as Keap1 due to structural similarity to a Drosophila actin-binding protein called Kelch.Citation13,Citation14 Keap1 is a 624-amino-acid-long cytoplasmic protein which contains five domains: the amino terminal region domain, BTB domain involved in homodimerization of Keap1, the intervening region domain important for functional interaction of Keap 1 with Nrf2, the double-glycin repeat (also called Kelch domain), and C-terminal region domain, both responsible for mediating the binding of Keap1 to the Neh2 domain of Nrf2.Citation10,Citation15–Citation17 Two conserved motifs important for Keap1 binding were identified within the Neh2 domain of Nrf2: the weaker binding DLG motif essential for ubiquitination and degradation of Nrf2, and stronger binding ETGE motif needed for interaction with Keap1.Citation17
Figure 1 Schematic overview of the Nrf2/Keap1 signaling pathway in homeostatic and stress conditions.
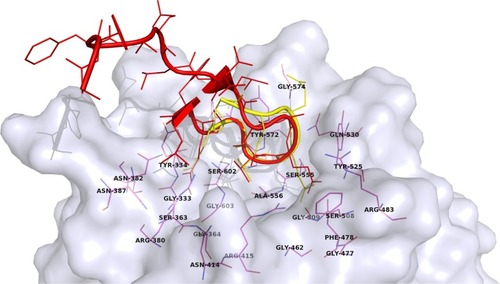
The main interactions between Nrf2 and Keap1 could be seen from two published crystal structures (): human Kelch domain bound to the 16mer peptide (PDB ID: 2FLU)Citation18 and Nrf2-derived peptide consisting of residues 77–82 of human Nrf2 cyclized by an additional glycine residue (cycloGD77 EETGE82, PDB ID: 3ZGC)Citation19. The Nrf2-derived peptides adopt the β-turn conformation that binds into the shallow pocket on the top face of the Kelch domain with a buried surface area of 420 ÅCitation2.Citation18 The entrance of the pocket is surrounded by hydrophobic residues Tyr572, Tyr525, Phe478, and Tyr334, which forms hydrogen bond with NH of Glu82.Citation18 The most important contacts are between arginine residues from the Kelch domain of Keap1 and glutamate residues of the Nrf2-derived peptide. Keap1 residues Arg415, Arg483, and Ser508 form hydrogen bonds with Glu79 from the ETGE motif of Nrf2, whereas Keap1 residues Ser363, Arg380, and Asn382 are bound to Glu82 of Nrf2 with four additional hydrogen bonds. Keap1 residues Gln530 and Ser602 also form hydrogen bonds with peptide residues Glu78 and Thr80, respectively.Citation18,Citation20
Figure 2 A surface presentation of the Kelch domain (gray) with two bound peptides from crystal structures: 2FLU (red)Citation18 and 3ZGC (yellow).Citation19
The Nrf2/Keap1 pathway regulates the gene expression of many cytoprotective and detoxifying enzymes, thus playing an important role in maintaining redox cellular homeostasis.Citation21 Indeed, the target genes of Nrf2 have been implicated in the antioxidant activity via activation and production of different proteins and enzymes, the synthesis and conjugation of GSH, metabolizing enzymes of xenobiotics, drug transport, and the activity of metabolic genes and transcription factors.Citation22,Citation23 Therefore, it is evident that modulating the activity of Nrf2 could represent a novel modern therapeutic strategy. Moreover, designed Nrf2 activators and also inhibitors could be of use for the treatment of many diseases, especially those that involve oxidative stress, such as cancer. A number of reviewsCitation16,Citation22–Citation34 have covered the Keap1/Nrf2/ARE pathway as a potential preventive and therapeutic anticancer strategy.
Herein, we present the role of Nrf2 in cancer and different strategies that have been used for the design and development of Nrf2 modulators. Moreover, we provide an update on the most important and recently developed Nrf2 modulators (activators and inhibitors), their in vitro and in vivo studies, and their potential use as chemopreventive agents and/or cancer therapeutics.
The connection between cancer and the Nrf2 pathway
Three main distinct stages of the carcinogenesis process have been defined: initiation, promotion, and progression.Citation35,Citation36 Several in-depth reviewsCitation4,Citation36–Citation38 have covered the connection between oxidative stress and cancer explaining the critical role of oxidative stress during the initiation and progression of cancer. It is now evident that the level and duration of the oxidative stress exposure may contribute to tumor formation (at low-to-moderate levels of ROS by inducing the mutations of genomic DNA or stimulation of cell proliferation) or severe cellular damage and cell death (at high levels of ROS by excessive DNA damage).Citation4,Citation39 Since the Nrf2/Keap1 signaling pathway is the key player in regulating oxidative stress,Citation25,Citation40 the connection between Nrf2 and cancer is inevitable. However, the dual role of Nrf2 in cancerCitation26,Citation27 has to be considered when designing novel Nrf2 modulators. Since the discovery of Nrf2, many studies have shown its protective role against cancer. Because of its importance in maintaining redox cellular homeostasis, Nrf2 has been generally considered as a cytoprotective transcription factor, and furthermore, a tumor suppressor. Indeed, at lower homeostatic levels, Nrf2 is able to eliminate ROS, carcinogens, and many other DNA-damaging agents, which lead to the inhibition of tumor initiation and cancer metastasis.Citation23 On the other hand, there are many reviews in the last few years suggesting that the activation of Nrf2/Keap1 signaling pathway is not favorable in all cancer types and stages.Citation16,Citation23,Citation27 Indeed, the increased Nrf2 activity in many cancer types assists malignant cells in defending against excessive oxidative stress, chemotherapeutic agents, and radiotherapy. The Nrf2 activation in cancer cells can occur due to disrupted binding of Keap1 to Nrf2 as a consequence of somatic mutations within the Keap1 and Nrf2 genes, epigenetic silencing of Keap1 gene, accumulation of Keap1-interacting proteins, and cysteine modification of Keap1 by metabolites ().Citation10,Citation16,Citation34,Citation41–Citation56 In addition, Nrf2 assists in avoiding apoptosis via activation of cytoprotective genes that contribute to enhanced cell proliferation.Citation23,Citation27 Therefore, when deciding to design modulators of the Nrf2/Keap1 signaling pathway, both aspects have to be considered to achieve the desired positive effect in cancer cells. However, due to the oncogenic role of Nrf2 at the early stages of cancer development, the design of inhibitors of Nrf2/Keap1 signaling pathway seems to be the right strategy at the moment.
Table 1 The origin and mechanisms of the Nrf2 activation in different cancer types
Design of Nrf2 modulators
Electrophilic modifiers
Different strategies have been described for the design of Nrf2/Keap1 signaling pathway modulators. Since the Nrf2/Keap1/ARE pathway is activated in the presence of xenobiotics, especially different reactive species (ROS, RNS, and different electrophiles), the first logical approach was to design compounds with reactive functional groups, which are usually electrophiles, or that contain groups that can be metabolically transformed to electrophilic species. These so-called electrophilic modifiers activate the Nrf2/Keap1/ARE pathway by covalently binding to cysteine residues of the target Nrf2 or Keap1 protein. Nrf2 contains seven highly conserved cysteine residues which were demonstrated to be critically involved in the regulation of oxidant/electrophile-sensing, Keap1-dependent ubiquitinationproteasomal degradation and transcription activation.Citation57 On the other hand, Keap1 contains about 25 cysteine residues required for either Nrf2 activation (Cys151) or suppression (Cys273 and Cys288).Citation57 All these residues could be affected by electrophilic species leading to indirect inhibition of Keap1-Nrf2 interaction.
Many structurally diverse electrophiles have been reported to date and are described in detail by some excellent reviews.Citation22,Citation28,Citation58 Magesh et al provided a detailed description (with representative examples) of ten chemically distinct classes of indirect small-molecule Keap1-Nrf2 interaction inhibitors, namely Michael acceptors (caffeic acid phenethyl ester (1) (), curcumin (2), chalcones), oxidizable diphenols and quinones (quercetin [4]), isothiocyanates and sulfoxythiocarbamates (sulforaphane [4]), dithiolethiones and diallyl sulfides (3H-1,2-dithiole-3-thione (5), diallyl sulfide [6]), vicinal dimercaptans ([R]-lipoic acid [7]), trivalent arsenicals, selenium-based compounds, polyenes, hydroperoxides, and heavy metals and metal complexes.Citation28 Although there are large number of “reactive” indirect Nrf2 modulators, their therapeutic potential is low and very limited due to “off-target” side effects caused by the attack on cysteine residues of other important cellular proteins.
Figure 3 Different strategies used for the design of Nrf2/Keap1 signaling pathway modulators.
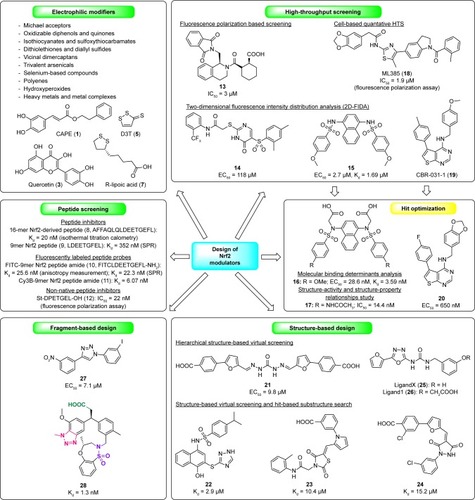
Therefore, in the past few years, attention has been paid to develop direct Nrf2 modulators targeting Keap1-Nrf2 PPI.Citation59 Different design strategies and techniques () have been used so far to identify the agents that can disrupt Keap1-Nrf2 PPI, such as peptide screening,Citation18,Citation60–Citation65 HTS,Citation66–Citation69 structure-based design,Citation70–Citation72 and fragment-based approach (in silicoCitation73 or experiment-basedCitation74,Citation75 fragment screening).
Peptide screening
Initially, the design of Nrf2 modulators started with the synthesis and screening of different peptides. In 2006, the crystal structure of the human Kelch domain bound to a 16mer peptide (8) derived from its substrate Nrf2 was determined by X-ray crystallography.Citation18 The application of ITC enabled the determination of a Kd value of 20 nM for 8 bound to the Kelch domain of Keap1. According to structural and functional studies, the DxETGE motif was suggested as the principal Keap1-binding site in Nrf2. Later, a series of Nrf2 peptides with the ETGE motif were synthesized to determine the minimal Nrf2 sequence that is required for binding to Keap1 protein. According to binding affinities obtained by SPR-based competition assay, the minimal Nrf2 peptide sequence required for Keap1 binding is the 9mer sequence of LDEETGEFL (9) with a Kdsolution of 352 nM.Citation60 A year later, a fluorescent probe based on the DxETGE motif was developed (FITC-9mer Nrf2 peptide amide [10]) and optimized to cyanine-labeled 9mer Nrf2 peptide amide (11) and found to be useful as a probe in a HTS assay for the discovery of novel small-molecule inhibitors of Keap1-Nrf2 interactions.Citation61 Many other probes and peptide inhibitors have been developed and are summarized in a review by Zhuang et al.Citation62 A phage display library approach was also used for the identification of putative peptide ligands with a nonnative sequence motif.Citation63 The same group used the standard solid-phase synthesis for the preparation of peptides with modified C- and N-termini and reduced overall charge.Citation64 The most potent peptide containing the C18 fatty acid stearic acid (St-DPETGEL-OH [12]), with an IC50 of 22 nM as determined in a cell-free fluorescence polarization assay, induced the expression of Nrf2-dependent gene products in cells and thus might be used as a starting molecule for the development of peptidomimetic inhibitors.Citation64
High-throughput screening
Due to pharmacological weaknesses of peptides such as limited stability and poor oral bioavailability,Citation76 small-molecule inhibitors of Keap1-Nrf2 interaction are more promising for drug development. The small-molecule first-in-class direct inhibitor of Keap1-Nrf2 PPI (13) with an IC50 value of 3 µM was discovered in 2013 by HTS of the NIH Molecular Libraries Probe Production Centers Network library in a homogenous fluorescence polarization assay.Citation66 A year later, an X-ray crystal structure of Keap1 was co-crystallized with the pure stereoisomer (S,R,S) of hit compound 13Citation77 and the binding pose was identified which has paved the way for more efficient development of novel small-molecule Nrf2 modulators. Another HTS approach using a confocal two-dimensional fluorescence intensity distribution analysis (2D-FIDA) Nrf2-Keap1 competition assay, led to the discovery of compound 14 (EC50 of 118 µM) and sulfonamide 15 (EC50 of 2.7 µM),Citation67 which was further optimized on the basis of molecular binding determinants analysis of Keap1 and by using structure-based design to compound 16 with improved ligand affinity (Kd value of 3.59 nM).Citation78 Moreover, the effective inhibition of Nrf2-Keap1 interaction (EC50 of 28.6 nM) and activation of the Nrf2 transcription in a dose-dependent manner were also confirmed for compound 16 by using a cell-based ARE-luciferase reporter assay and a quantitative reverse transcription PCR. This research clearly demonstrated that small molecules could achieve direct and highly efficient interruption of the Keap1-Nrf2 PPI and that this strategy could be successful for the prevention of cancer and other chronic diseases in the future. Further chemical optimization of compound 16 to 17, with para-acetamido substituents on the side chain of phenyl rings, provided the optimal balance between inhibition of Nrf2-Keap1 PPI, physicochemical properties, and cellular Nrf2 activity. In addition, in vivo experiments performed in a lipopolysaccharide-challenged mice model also indicated the anti-inflammatory effects of compound 17.Citation79
Two other HTS approaches rendered small-molecule inhibitors of Nrf2. A cell-based quantitative HTS of small-molecule library of approximately 400,000 compounds using a novel Nrf2 reporter gene assay multiplexed with a cytotoxicity readout was used to identify ML385 (18) as a specific small-molecule inhibitor of Nrf2.Citation68 Compound 18 was obtained after basic SAR studies which revealed the importance of an ortho-substituent on the benzoyl group. To evaluate the interaction of 18 with Nrf2 and its influence on DNA-binding activity of the Nrf2-MAFG protein complex, a fluorescence polarization assay using fluorescein-labeled ARE-DNA duplex was developed. In the presence of ML385, anisotropy was decreased in a dose-dependent manner (IC50 of 1.9 µM), indicating the dissociation of the Nrf2-MAFG protein complex from fluorescein-labeled ARE-DNA.Citation68 Another HTS was conducted to identify small molecules inhibiting deregulated Nrf2 transcriptional activity.Citation69 Thienopyrimidine-containing compound CBR-031-1 (19) was selected as a promising hit due to chemical tractability and reproducible broad inhibition of Nrf2-controlled gene expression. An additional SAR study around the thienopyrimidine scaffold with approximately 100 commercially available structural analogs furnished AEM1 (20) with improved potency and metabolic stability. Compound 20 significantly decreased the ARE-luciferase signal and inhibited HO-1 suppression at both transcript and protein levels with an EC50 of approximately 650 nM.Citation69
Structure-based design
A successful application of structure-based design was also reported for the discovery of small-molecule Nrf2 modulators. Based on the receptor–ligand binding model of Keap1-Nrf2, hierarchical structure-based virtual screening was performed by Sun et al.Citation70 Compound 21 was discovered as a potent Nrf2-Keap1 PPI (EC50 of 9.80 µM) at both target-based and cell-based level. In this study, it was also demonstrated that long-acting and less toxic effect could be achieved by Nrf2 activation via direct inhibitors of Keap1-Nrf2 PPI in comparison to the covalent binding-based Nrf2 activators. Three classes of novel inhibitors (the most potent representative examples are 22–24) disrupting Keap1-Nrf2 PPI were successfully identified by rapid structure-based virtual screening and hit-based substructure search.Citation71 The obtained Kd values were in the low micromolar range from 2.9 (compound 22) to 77.8 µM, and furthermore, additional cell-based mechanism studies supported the disruption of Keap1-Nrf2 PPI. Another in silico screening of in-house and commercially available compounds performed on the crystal structure of the Kelch domain of human Keap1 in complex with the ETGE peptide of Nrf2 yielded 65 compounds.Citation72 After binding ability evaluation by SPR, 27 compounds were active and the most promising one was ligand X (25). After further structural improvement, where the phenol moiety of 25 was replaced by the oxyacetic to mimic the side chain of the glutamic acid residue in the ETGE peptide, ligand 1 (26) was synthesized and later crystallized in two crystal structures at 2.1 Å resolution.Citation72
Fragment-based design
The available crystal structuresCitation18,Citation80 of the human Keap1 Kelch domain were also used for an in silico fragment-based approach to discover 1,4-diaryl-1,2,3-triazole inhibitors of the Keap1-Nrf2 PPI.Citation73 The most active compound 27 showed reversible binding to Keap1, similar cell-based activity to indirect Keap1-Nrf2 inhibitor sulforaphane (4) but without any cytotoxic activity over a wide concentration range. Another use of a fragment-based approach to directly target the Keap1 Kelch-Nrf2 interaction was reported by Davies et al.Citation74 Three distinct “hot-spots” were identified within the Nrf2-binding pocket of Keap1 by X-ray crystallographic screen of approximately 330 fragments. Further optimization of weak fragment hits led to a promising lead compound 28 that implemented the three-point pharmacophore (acid, aromatic acceptor, and sulfonamide) from fragment screening and exhibited very high affinity for the Keap1 Kelch domain (ITC Kd = 1.3 nM).Citation74 Furthermore, the activation of the Nrf2 antioxidant response in cellular and in vivo models was also observed.
Inhibitors of the Keap1-Nrf2 interactions
Most of the Nrf2 modulators are the inhibitors of the Keap1-Nrf2 interactions, and some of them have been already crystallized in a complex with Keap1.Citation67,Citation74,Citation77,Citation81–Citation83 The majority of PPI inhibitors share similar interface, which could be seen in . Some mimic the β-turn of Nrf2-derived peptide (ETGE motif) interacting with some of the previously mentioned Keap1 residues Ser363, Arg380, Asn382, Arg415, Arg483, Ser508, Gln530, and Ser602 (), whereas the others bind deeper within the pocket defined by residues Ala556, Gly603, Gly364, Gly462, and Gly509. According to available crystal structures, there is still some space left for further optimization, perhaps with the introduction of fragments that interact with additional residues in the extension of the pocket, screening of other turn mimetics, or maybe even the introduction of reactive functional groups to design covalent inhibitors.
Figure 4 A surface presentation of the Kelch domain (gray) in complex with inhibitors of the Keap1-Nrf2 interactions from crystal structures: (1S,2R)-2-{[(1S)-1-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]-3,4-dihydroisoquinolin-2(1H)-yl]carbonyl}cyclohexanecarboxylic acid (13)Citation77 (green, PDB ID: 4L7B), 2-({5-[(2,4-dimethylphenyl) sulfonyl]-6-oxo-1,6-dihydropyrimidin-2-yl}sulfanyl)-N-[2-(trifluoromethyl)phenyl]acetamide (14)Citation67 (cyan, PDB ID: 4IN4), 2,2′-(naphthalene-1,4-diylbis(((4-methoxyphenyl) sulfonyl)azanediyl))diacetamideCitation81 (yellow, PDB ID: 4XMB), N,N′-[2-(2-oxopropyl)naphthalene-1,4-diyl]bis(4-ethoxybenzenesulfonamide) (51)Citation82 (light blue, PDB ID: 4ZY3), (3S)-1-(4-{[(2,3,5,6-tetramethylphenyl)sulfonyl]amino}naphthalen-1-yl)pyrrolidine-3-carboxylic acidCitation83 (orange, PDB ID: 5CGJ), and 3-(4-chlorophenyl)propanoic acidCitation74 (blue, PDB ID: 5FNQ).
![Figure 4 A surface presentation of the Kelch domain (gray) in complex with inhibitors of the Keap1-Nrf2 interactions from crystal structures: (1S,2R)-2-{[(1S)-1-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]-3,4-dihydroisoquinolin-2(1H)-yl]carbonyl}cyclohexanecarboxylic acid (13)Citation77 (green, PDB ID: 4L7B), 2-({5-[(2,4-dimethylphenyl) sulfonyl]-6-oxo-1,6-dihydropyrimidin-2-yl}sulfanyl)-N-[2-(trifluoromethyl)phenyl]acetamide (14)Citation67 (cyan, PDB ID: 4IN4), 2,2′-(naphthalene-1,4-diylbis(((4-methoxyphenyl) sulfonyl)azanediyl))diacetamideCitation81 (yellow, PDB ID: 4XMB), N,N′-[2-(2-oxopropyl)naphthalene-1,4-diyl]bis(4-ethoxybenzenesulfonamide) (51)Citation82 (light blue, PDB ID: 4ZY3), (3S)-1-(4-{[(2,3,5,6-tetramethylphenyl)sulfonyl]amino}naphthalen-1-yl)pyrrolidine-3-carboxylic acidCitation83 (orange, PDB ID: 5CGJ), and 3-(4-chlorophenyl)propanoic acidCitation74 (blue, PDB ID: 5FNQ).](/cms/asset/6c930dab-5770-4334-8ded-221bdc1aaab3/dddt_a_12181827_f0004_c.jpg)
Nrf2 modulators for cancer prevention and therapy
Cancer chemoprevention is associated with the application of natural or synthetic chemical agents to minimize the risk of cancer development.Citation29,Citation30 One of the possible and the most commonly described approaches to achieve effective cancer chemoprevention involves the induction of cytoprotective and detoxifying enzymes. The expression of these inducible genes is regulated by the Nrf2/Keap1 signaling pathway. Nrf2 is substantially involved in the upregulation of these genes in response to oxidative stress and the application of natural or synthetic compounds. There have been a few recent reviews about natural compounds which have the ability to induce genes involved in antioxidant defense through activating the Nrf2/Keap1 signaling pathway.Citation31
Natural Nrf2 activators
Two major classes of natural compounds acting as promising chemopreventive agents are phenolic and sulfur-containing compounds.Citation29 Phenolic compounds are well known for multiple biological activities including anticarcinogenic activity.Citation84,Citation85 Induction of phase II detoxifying and antioxidant defense enzymes via Nrf2/Keap1 signaling pathway has been recognized as one of the most important molecular mechanisms for cancer chemopreventive effects of polyphenols.Citation85 Curcumin (2)Citation86 and resveratrol (29)Citation87,Citation88 () have been extensively studied as natural compounds with chemopreventive properties in several types of cancer. The inhibition of proliferation, invasion, angiogenesis, and metastasis of different cancers through interaction with multiple cell signaling proteins is discussed in a review by Kunnumakkara et al.Citation89 In a recent study, it was suggested that curcumin’s inhibition of the proliferation of breast cancer cells is achieved via Nrf2-mediated downregulation of Fen1 expression.Citation90 However, safety (especially long-term toxicity) and poor pharmacokinetic properties (in particular, low systemic bioavailability) have to be considered for 2 to be used as a dietary supplement.Citation91,Citation92 Even though no major toxicity has been found in short-term studies in humans, a long-term toxicity study conducted in rats and mice does raise some concern about safety of 2.Citation93 Despite the high oral doses of 2, the plasma concentrations still remained very low, typically in the nanomolar range,Citation91 which limits the therapeutic potential of orally administered 2. On the other hand, resveratrol (29) was able to inhibit estrogen-induced breast carcinogenesis via induction of the Nrf2-mediated protective pathway.Citation94 Induction of Nrf2 signaling by 29 leads to trans-activation of antioxidant and phase II detoxifying enzymes, and enables protection against phase I enzyme-activated carcinogens and associated carcinogenicity.Citation95 However, due to low bioavailability and rapid clearance of 29, to be used therapeutically as a chemopreventive agent, it is necessary to develop analogs with improved bioavailability and more potent Nrf2 activation.
Figure 5 Natural and synthetic Nrf2 modulators as potential cancer chemopreventive and therapeutic agents.
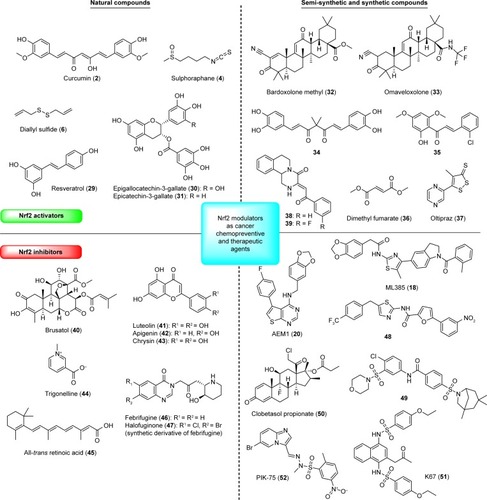
The suppression of tumorigenesis in several chemical-induced animal carcinogenesis models was also demonstrated for green tea polyphenols. Epigallocatechin-3-gallate (30) and epicatechin-3-gallate (31) are known Nrf2 activators which showed potent induction of ARE-mediated luciferase activity. It was suggested that the induction of the ARE reporter gene is structurally related to the 3-gallate group.Citation96 Isothiocyanates (such as sulforaphane [4]), which are present in cruciferous vegetables, and diallyl sulfide (6), a flavor compound derived from garlic, are sulfur-containing compounds with chemopreventive properties mediated by the Nrf2/ARE pathway.Citation30 Sulforaphane (4) has been recognized as a chemo-preventive agent due to its ability to induce phase II detoxifying enzymes and to inhibit phase I enzymes involved in the activation of carcinogens.Citation30 In comparison with curcumin (2) and resveratrol (29), 4 exhibited more potent activation of Nrf2 to induce the expression of a battery of cytoprotective genes. Furthermore, the lipophilic nature and low molecular weight of 4 offer significantly better bioavailability than the polyphenol-based Nrf2 activators.Citation97 The absolute bioavailability of dietary doses of 4 following oral and intravenous administration was determined in rats. After oral administration, 4 was rapidly absorbed and achieved high absolute bioavailability at low dietary doses; however, dose-dependent pharmacokinetics was observed with bioavailability decreasing with increasing dose.Citation98 In another study with 65 human volunteers, orally administrated 4 effectively induced mucosal phase II enzyme expression in the upper airway without significant adverse events.Citation99 Two different studies with single dose of a broccoli sprout preparation containing about 200 µmol of 4 were conducted in human volunteers.Citation100,Citation101 Rapid absorption of isothiocyanates with peak concentrations from 0.943 to 2.27 µmol/L in plasma, serum, and erythrocytes was observed after 1 hour, and declined following first-order kinetics with a half-life of 1.77 hours.Citation100 When 4 was given to eight healthy women undergoing reduction mammoplasty, dithiocarbamate metabolites were readily measurable in breast tissue at concentrations of 1.45–2.00 pmol/mg tissue.Citation101 In addition, NQO1 and HO-1 transcripts, as well as NQO1 enzymatic activity, were measured in human breast tissue. All clinical studies with broccoli preparation are extensively summarized in a review by Dinkova-Kostova et al.Citation102 Efficacy, safety, and distribution in selected tissues suggest 4 has a great potential for the chemoprevention of different types of cancer. Sulforaphane (4) as a pleiotropic natural compound acts on several targets and pathways directly or indirectly connected to cancer (summarized in a recent review by Russo et al),Citation103 and this multi-target activity is definitely an advantage in case of a complex disease, such as cancer. However, due to recent findings about the oncogenic role of Nrf2 in cancer cells, the therapeutic or other use of 4 should be considered with caution due to its ability of potent activation of Nrf2. More in vivo studies are still needed to confirm the beneficial and safe use of 4 as a chemopreventive agent or adjuvant without the risk of developing serious side effects.
Numerous other natural compounds that are able to induce detoxifying enzymes through Nrf2-signaling pathway have been reported as potential cancer chemopreventive agents.Citation31,Citation32 The list of the so-called natural inducers has been increasing each day; however, more studies, including the elucidation of the detailed molecular mechanisms, are needed for their possible application in cancer chemoprevention.
Semisynthetic and synthetic Nrf2 activators
Semisynthetic and synthetic analogs are more likely to be used in clinical practice. Some have been already investigated in clinical trials.Citation22,Citation104 For example, bardoxolone methyl (CDDO-Me, 32), a semisynthetic triterpenoid originated from the natural product oleanolic acid, potently induces Nrf2 and has been investigated in different types of cancer (including leukemia and solid tumors).Citation22,Citation105,Citation106 In a Phase I first-in-human trial of 32 in patients with lymphomas and advanced solid tumors, objective tumor responses and pharmacodynamic effects were observed. However, the Phase III trial of 32 was discontinued in 2012 due to severe cardiovascular side effects. Nevertheless, synthetic triterpenoids offer great potential for further development as anticancer agents. Many patents involving 32 analogs have been filed in the recent years.Citation104 Omaveloxolone (33) was reported as one of the most potent synthetic triterpenoid analogs inducing ARE activity over 16-fold at 62.5 nM in an Nrf2-GST-ARE luciferase reporter assay. Early clinical trials for the oral treatment of melanoma and non-small-cell lung cancer have been in progress. Recently, the first-in-human Phase I clinical trial of patients with advanced solid tumors was conducted and showed favorable tolerability at biologically active doses.Citation107 No dose-limiting toxicities were detected for 33 at four dose levels up to 15 mg given orally once daily. Furthermore, rapid absorption and proportional increases in exposure across dose levels were observed for 33. General tendency toward time- and dose-dependent activation of Nrf2 antioxidant genes was observed in this study. Further investigations of 33 in cancer are supported and will determine its efficacy and potential therapeutic use for the treatment of cancer. Recently, a Phase Ib/II study of omaveloxolone in combination with checkpoint inhibitors in patients with unresectable or metastatic melanoma was conducted.Citation108 No dose-limiting toxicities were observed for 33, and it was well tolerated at doses up to 150 mg. Initial efficacy data showed that checkpoint inhibitors resistance may be overcome in the presence of 33. According to current clinical data, 33 definitely shows a high potential and further clinical studies will hopefully confirm its beneficial effects, not only for the treatment of cancer but also for the treatment of other pathologies, such as neurological disorders.Citation109
In another study, natural curcumin-directed Nrf2 activators were synthesized and evaluated for their cytoprotective effects.Citation110 The catechol-type curcumin analog (compound 34) was selected as one of the most promising compounds due to high stability and cytoprotective activity against the tert-butyl hydroperoxide-induced death of HepG2 cells. According to mechanistic studies, this activity is mediated by the activation of the Nrf2 signaling pathway in the Michael acceptor- and catechol-dependent manners.Citation75 Many other synthetic curcumin analogsCitation111 have been prepared up to date; however, their chemical and metabolic instability, poor membrane permeability, and extremely low oral bioavailability are just some of the issues that have to be considered in the development of synthetic curcumin analogs as preventive and therapeutic agents. A synthetic chalcone, 2-chloro-4′,6′-dimethoxy-2′-hydroxychalcone (35), was also found to be a potent activator of Nrf2 transcriptional activity with the ability to modulate intracellular levels of GSH.Citation112 The study was carried out in a human breast cancer cell line expressing a luciferase reporter gene driven by ARE. Furthermore, it was also observed that chalcones posttranslationally enhance formation of glutamate cysteine ligase holoenzyme.Citation112
Among other synthetic Nrf2 activators, much attention has been focused on DMF (36) and oltipraz (37). DMF (Tecfidera®; Biogen Inc., Cambridge, MA, USA) is a well-known Nrf2 activator, which has been used in clinics for the treatment of multiple sclerosis.Citation113,Citation114 Recently, 36 was also identified as a promising Nrf2-DJ-1 axis inhibitor in cancer cells.Citation115 At concentrations lower than 25 µmol/L, a cytoprotective role due to activation of the Nrf2 antioxidant pathway was observed, whereas at higher concentrations 36 decreased the expression of the Nrf2 protein stabilizer DJ-1 and caused cytotoxicity in several cancer cell lines due to oxidative stress.Citation115 Recently, the same research group also discovered that 36 is highly and selectively cytotoxic against patient-derived cancer cell lines harboring a G12V KRAS mutation.Citation116 It has been known for a decade that 36 has antiproliferative and proapoptotic properties and can reduce melanoma growth and metastasis in animal models.Citation117 Moreover, in two mice models of colon cancer, an antitumoral effect of 36 (administered daily, 20 mg/kg) was also observed.Citation115 All of these results indicate that 36 has the potential for therapeutic intervention in cancer; however, additional in vivo studies are needed. Another well-studied synthetic Nrf2 activator, which has also entered clinical trials, is 4-methyl-5-[2-pyrazinyl]-1,2-dithiole-3-thione (oltipraz, 37).Citation27 Compound 37 is best known for its activity against aflatoxin-induced liver cancer;Citation33 however, for potential therapeutic application, further clinical trials are needed to clearly demonstrate the effectiveness of 37 for the prevention of human hepatocarcinomas.
Screening of an in-house database using the luciferase reporter assay led to the discovery of compound 38, which was determined to be a potent activator of the Nrf2/ARE signaling pathway in a human cancer cell-based and azoxymethane/dextran sodium sulfate mouse model.Citation118 Moreover, 38 also significantly inhibited the development of inflammation-associated colorectal adenomas. In addition, structural modifications suggested from the SAR study led to compound 39 with a higher potency to enhance ARE levels. However, further optimization and studies are still needed to develop suitable compounds for therapeutic use. Lastly, in recent years, two interesting patents were published describing the functionalized heteroaryl enonesCitation119 and pyridopyrazine compoundsCitation120 as Nrf2 activators and their potential use in the treatment of cancer.Citation104
Natural Nrf2 inhibitors
It has also been shown that prolonged expression of Nrf2 offers protection to cancer cells which can be achieved by inducing the metabolism and efflux of chemotherapeutics. Consequently, both intrinsic and acquired chemoresistance to cancer drugs occur.Citation121 Therefore, inhibition of the Nrf2 signaling pathway has also gained more attention in the past few years.Citation122 There have been several reports about small-molecule inhibitors of Nrf2 that display in vivo antitumor activity.Citation104 Among natural products, several studies have been performed on brusatol (40), a quassinoid from Brucea javanica.Citation123,Citation124 Compound 40 was identified as one of the most potent inhibitors of the Nrf2 signaling pathway which decreases the protein level of Nrf2. The reduction in Nrf2 was observed in many cancer cell lines regardless of the status of Keap1 or Nrf2 being wild type or mutated.Citation123 Accordingly, 40 could enhance the cytotoxic effect of chemotherapeutic agents. The recently reported brusatol’s mode of action is via inhibition of cap-dependent and cap-independent protein translation having an impact on a great number of other proteins, especially those with a short half-life. Therefore, 40 is not specific to Nrf2 and is more of a global protein synthesis inhibitor.Citation125,Citation126 Hall et alCitation127 also reported that 40 significantly inhibited DNA, RNA, and protein synthesis in p-388 lymphocytic leukemia cells; however, micromolar concentrations of 40 were used in this study. The nonspecificity of 40 seems to be related to its concentration, which was later showed in the study by Ren et al,Citation128 where 20–80 nM of 40 specifically inhibited the protein level of Nrf2 in a dose-dependent manner. Nevertheless, most of the results were obtained only in cancer cell lines and selected animal models. The lack of results (especially in humans), pleiotropic effects, and potential toxicity of 40 limits its possible use. A series of brusatol analogs, which were up to 100-fold and more less toxic than 40, have been already synthesized recently.Citation129 The development of more specific Nrf2 inhibitors with limited toxicity and off-target effects seems to be the best strategy for the future.
Several other natural small-molecule Nrf2 inhibitors have also been reported, such as the flavonoids luteolin (41),Citation130 apigenin (42),Citation131 and chrysin (43),Citation132 the coffee-derived alkaloid trigonelline (44),Citation133 and ATRA (45).Citation28 In the presence of ATRA, Nrf2 forms a complex with the alpha retinoic acid receptor, which can no longer bind to ARE sequences, thereby blocking the activation of the signaling pathway.Citation27,Citation28 In fact, the Nrf2 inhibitory activity of 45 was observed in human breast cancer (MCF-7) cells transfected with an ARE-luciferase reporter construct.Citation134 Lastly, inhibition of the Nrf2 transcription factor was also observed by the alkaloid trigonelline (44) which has the potential to be used in combination therapy of highly resistant tumors such as pancreatic cancer. Compound 44 is capable of changing pancreatic cancer cells such that they become more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity.Citation135
Another plant alkaloid febrifugine (46), found in the roots of the blue evergreen hydrange, was observed to inhibit Nrf2 activity, as shown by repressed luciferase activity in A549-ARE-Luc cells.Citation136 Further studies focused on the less toxic febrifugine derivative halofuginone (47) which rapidly suppressed the accumulation of Nrf2 protein in Nrf2-addicted cancer cells. Furthermore, 47 repressed protein synthesis via an amino acid starvation response elicited by inhibition of prolyl-tRNA synthetase and possess the ability to enhance the sensitivity of Nrf2-addicted cancer cells to anticancer drugs both in vitro and in vivo. Thus, 47 could be used as a chemosensitizer by inhibiting Nrf2 accumulation in various Nrf2-addicted cancers.Citation136 However, it should be noted that 47 is a multifaceted molecule with many biological activities (reviewed by Pines and Spector)Citation137 and could act on several different targets and pathways, such as inhibition of Smad3 phosphorylation downstream of the TGFβ signaling pathway. This promiscuity could be problematic in the further development of 47 for a therapeutic use.
Semisynthetic and synthetic Nrf2 inhibitors
In addition to several natural Nrf2 inhibitors, a few synthetic small-molecule Nrf2 inhibitors have been described. HTS was used to identify small molecules which decrease Nrf2 transcriptional activity at ARE sites.Citation69 An additional SAR study of thienopyrimidine-containing compounds to identify analogs with improved potency and metabolic stability showed AEM1 (20) as the most promising compound. Broad suppression of Nrf2-driven genes was observed for 20 when treating A549 cells, the lung adenocarcinoma cell line with an inactivating mutation in Keap1 (G333C) that leads to constitutive Nrf2. Furthermore, 20 was also able to sensitize A549 cells to various chemotherapeutic agents and oxidative stress and led to inhibition of cell growth in vitro and in vivo. The pharmacokinetic profile of orally administered 20 was also determined in mice at a dose of 20 mg/kg (t1/2 2 hours; Cmax 176 ng/mL; AUC(0–24) 453 hours⋅ng/mL). Treatment with a twice-daily oral 50 mg/kg dose of 20 resulted in a modest reduction in A549 tumor volume without affecting body weight over the treatment period. However, the potency and pharmacokinetic properties still need to be improved. Small-molecule Nrf2 inhibitors like 20 represent an important starting point and a great potential for the design of alternative anticancer treatments.Citation69
Another specific small-molecule Nrf2 inhibitor ML385 (18), discovered by quantitative HTS,Citation68 blocked Nrf2 transcriptional activity and showed significant antitumor activity in combination with carboplatin. The ability of 18 to enhance the efficacy of carboplatin and other chemotherapeutic drugs in lung cancer cells renders 18 a great potential for the treatment of advanced non-small-cell lung cancer. Along with ML385 (18), two other compounds (48 and 49) were also extensively described in a patent.Citation138 All three compounds were able to suppress the growth of osteosarcoma cells and two different pancreatic cell lines (Panel one and MiaPaCa) as single agents as well as in combination with chemotherapeutic drugs (doxorubicin, gemcitabine). According to in vivo pharmacokinetic studies, all three compounds have a fairly good retention time in the blood and thus possess very good drug-like properties. The pharmacokinetic plasma profiles of compounds 18 and 48 in CD1 mice indicated that in vivo exposure and pharmacokinetics were appropriate for in vivo use.Citation138 In a recent studyCitation139 investigating the protective effects of isoliquiritigenin on acute pancreatitis, the inhibitory effect of 18 on Nrf2 activation was also examined. The results obtained confirmed that 18 actually inhibited nuclear translocation of Nrf2 in pancreatic tissue. Moreover, 18 also showed protective effects in acute pancreatitis via an unknown mechanism. Very little has been known about other biological activities of 18, so it might be interesting to evaluate its possible effects on the activity of other transcription factors or signaling pathways.
In order to develop potent NRF2 inhibitors for therapeutic use, Choi et alCitation140 screened around 4,000 clinical compounds. Clobetasol propionate (50) was identified as the most potent Nrf2 inhibitor. The induction of oxidative stress and potent suppression of the anchorage-independent growth of tumors with Keap1 mutation was observed for 50. Furthermore, potent in vitro and in vivo inhibition of tumor harboring mutations in Keap1 that are frequently observed in a lung cancer was determined for clobetasol propionate alone or in combination with rapamycin. The authors of this study suggested that 50 could be used as a repurposed therapeutic agent for cancers with high Nrf2 activity.Citation140
Phosphorylated p62 is able to bind with high affinity to Keap1, thus inhibiting Keap1-driven ubiquitination of Nrf2 which results in stabilization of Nrf2 and its translocation into nucleus.Citation82 It is known that dysregulation of the p62-Keap1-Nrf2 axis has been implicated in the development of cancer. Indeed, Nrf2 activation by phosphorylated p62 contributes to tumor growth which was demonstrated by a significant reduction of tumor size in liver-specific autophagy-deficient mice by simultaneous deletion of p62 or Nrf2. Therefore, a specific inhibitor of phosphorylated p62-Keap1 interaction would facilitate the binding of Keap1 and subsequent degradation of Nrf2, and thus could be used as a potential anticancer drug.Citation34 In a chemical screen, Saito et alCitation82 identified N-[2-acetonyl-4-(4-ethoxybenzenesulfonylamino) naphthalene-1-yl]-4-ethoxybenzene-sulfonamide (K67, 51) as a small-molecule inhibitor of the interaction between phosphorylated p62 and Keap1. Their results clearly indicated that treatment of human hepatocellular carcinoma cells with 51 suppressed proliferation and reduced tolerance to an anticancer drug. Therefore, with some further structural improvement of 51 and due to its lower solubility, this type of inhibitor could be used as drugs against cancer cells resistant to anticancer agents in a manner that depends upon p62.
Blocking the Nrf2 signaling pathway by small-molecule inhibitors is also a very promising therapeutic approach for the treatment of pancreatic cancers.Citation141 In a recent study, AsPC-1 cells were transfected with a luciferase reporter gene construct containing the ARE from NQO1 gene and treated with various kinase inhibitors. PIK-75, which is known as a PI3K/DNA-PK inhibitor (52), was identified as a potent inhibitor of Nrf2 which reduced its protein levels and induced its proteasome-mediated degradation in human pancreatic cancer cells. Furthermore, 52 potentiated the antitumor effect of gemcitabine both in vitro and in vivo indicating its potential benefit in treating pancreatic cancer in combination with gemcitabine.Citation141
Conclusion and perspectives
It is now evident that the Nrf2/Keap1 signaling pathway is the key player in regulating oxidative stress which may contribute to tumor formation. Nrf2 induces the expression of a battery of cytoprotective genes, thereby being able to eliminate ROS, carcinogens, and many other DNA-damaging agents. Modulation of the activity of Nrf2 thus represents a novel modern strategy for cancer chemoprevention and therapy. However, many studies showed that Nrf2 plays a dual role in cancer being capable of acting as a tumor suppressor in one way and as a proto-oncogene in another. Many factors including tight control of Nrf2 activity, cell types, and special tumor microenvironment regulate the switch between pro-oncogenic and anticancer role of Nrf2. It is now evident that activation of the Nrf2/Keap1 signaling pathway in cancer cells can lead to the metabolic inactivation of antitumor agents and a decrease of their intracellular concentration. Thus, the question remains: should we develop Nrf2 activators or inhibitors? All of the important features mentioned above have to be considered when designing novel Nrf2 modulators. However, due to the many recent findings about the oncogenic role of Nrf2, the design and development of novel selective inhibitors of Nrf2/Keap1 pathway might present a better strategy for cancer prevention and therapy.
Different design strategies for modulating the Nrf2/Keap1 signaling pathway have been used to date, from electrophilic modifiers, peptide and natural products screening, and HTS to structure-based design and in silico fragment-based approach. Targeting Keap1-Nrf2 PPI has become one of the most popular design strategies in the past 10 years. Many Nrf2 modulators have been discovered by starting from natural compounds and continuing to small-molecule semisynthetic and synthetic compounds. Some of them have already been investigated in clinical trials (eg, DMF, omaveloxolone, oltipraz, ML385), and basic in vivo pharmacokinetic studies have been performed. Nevertheless, inevitable “off-target” side effects for electrophilic modifiers, and chemical and/or metabolic instability, poor membrane permeability and low oral bioavailability for most of natural compounds, give the key advantage and higher potential to synthetic compounds. Additional in vitro and in vivo studies to gain insights and to understand the mechanism of Nrf2 modulation are needed to be successful in the development of Nrf2 modulators for cancer chemoprevention and therapy.
Abbreviations
| ARE | = | antioxidant response element |
| ATRA | = | all-trans retinoic acid |
| BTB | = | Broadcomplex, Tramtrack, and Bric-a-Brac |
| DMF | = | dimethyl fumarate |
| GSH | = | glutathione |
| HTS | = | high-throughput screening |
| ITC | = | isothermal titration calorimetry |
| Keap1 | = | Kelch-like ECH-associated; protein 1 |
| Nrf2 | = | nuclear factor E2-related factor 2 |
| PPI | = | protein–protein interaction |
| RNS | = | reactive nitrogen species |
| ROS | = | reactive oxygen species |
| SAR | = | structure–activity relationship |
| SPR | = | surface plasmon resonance |
Acknowledgments
The authors thank Professor Frank M Scalzo for critical reading of the manuscript and Doctor Marko Jukič for preparation of and .
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2015CA Cancer J Clin201565152925559415
- MaXYuHGlobal burden of cancerYale J Biol Med2006793–4594
- HuangBZhaoJUnkelessJCFengZHXiongHTLR signaling by tumor and immune cells: a double-edged swordOncogene200827221822418176603
- GorriniCHarrisISMakTWModulation of oxidative stress as an anticancer strategyNat Rev Drug Discov2013121293194724287781
- MaQTranscriptional responses to oxidative stress: Pathological and toxicological implicationsPharmacol Ther2010125337639319945483
- ValkoMRhodesCJMoncolJFree radicals, metals and antioxidants in oxidative stress-induced cancerChem Biol Interact2006160114016430879
- StępkowskiTMKruszewskiMKMolecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosisFree Radic Biol Med20115091186119521295136
- FuseYKobayashiMConservation of the Keap1-Nrf2 System: An evolutionary journey through stressful space and timeMolecules2017223436
- MoiPChanKAsunisICaoAKanYWIsolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control regionProc Natl Acad Sci U S A19949121992699307937919
- KansanenEKuosmanenSMLeinonenHLevonenA-LThe Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancerRedox Biol201311454924024136
- TaguchiKMotohashiHYamamotoMMolecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolutionGenes Cells201116212314021251164
- MoiPChanKAsunisICaoAKanYWIsolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control regionProc Natl Acad Sci U S A19949121992699307937919
- ItohKWakabayashiNKatohYKeap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domainGenes Dev199913176869887101
- ItohKMimuraJYamamotoMDiscovery of the Negative Regulator of Nrf2, Keap1: A Historical OverviewAntioxid Redox Signal201013111665167820446768
- ItohKTongKIYamamotoMMolecular mechanism activating nrf2–keap1 pathway in regulation of adaptive response to electrophilesFree Radic Biol Med200436101208121315110385
- PandeyPSinghAKSinghMThe see-saw of Keap1-Nrf2 pathway in cancerCrit Rev Oncol Hematol2017116899828693803
- TongKIKatohYKusunokiHKeap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition modelMol Cell Biol20062682887290016581765
- LoSCLiXHenzlMTBeamerLJHanninkMStructure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signalingEmbo J200625153605361716888629
- HörerSReinertDOstmannKHoevelsYNarHCrystal-contact engineering to obtain a crystal form of the Kelch domain of human Keap1 suitable for ligand-soaking experimentsActa Crystallogr Sect F Struct Biol Cryst Commun2013696592596
- CanningPSorrellFJBullockANStructural basis of Keap1 interactions with Nrf2Free Radic Biol Med201588Pt B10110726057936
- GaoBDoanAHybertsonBMThe clinical potential of influencing Nrf2 signaling in degenerative and immunological disordersClin Pharmacol20146193424520207
- LuMCJiJAJiangZYYouQDThe Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: An UpdateMed Res Rev201636592496327192495
- MilkovicLZarkovicNSasoLControversy about pharmacological modulation of Nrf2 for cancer therapyRedox Biol20171272773228411557
- Krajka-KuźniakVPaluszczakJBaer-DubowskaWThe Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatmentPharmacol Rep201769339340228267640
- MaQRole of Nrf2 in oxidative stress and toxicityAnnu Rev Pharmacol Toxicol201353140142623294312
- LauAVilleneuveNSunZDual roles of Nrf2 in cancerPharmacol Res2008585–626227018838122
- MenegonSColumbanoAGiordanoSThe dual roles of NRF2 in cancerTrends Mol Med201622757859327263465
- MageshSChenYHuLSmall molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agentsMed Res Rev201232468772622549716
- SandersKMoranZShiZNatural products for cancer prevention: Clinical update 2016Semin Oncol Nurs201632321524027539278
- KouXKirbergerMYangYChenNNatural products for cancer prevention associated with Nrf2–ARE pathwayFood Sci Hum Wellness2013212228
- ZhaoCRGaoZHQuXJNrf2–ARE signaling pathway and natural products for cancer chemopreventionCancer Epidemiol201034552353320638930
- CatanzaroECalcabriniCTurriniENrf2: a potential therapeutic target for naturally occurring anticancer drugs?Expert Opin Ther Targets201721878179328675319
- ZhangYGordonGBA strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathwayMol Cancer Ther20043788589315252150
- TaguchiKYamamotoMThe KEAP1–NRF2 System in CancerFront Oncol201778528523248
- PitotHCGoldsworthyTMoranSThe natural history of carcinogenesis: Implications of experimental carcinogenesis in the genesis of human cancerJ Supramol Struct Cell Biochem19811721331467033553
- KlaunigJEKamendulisLMThe role of oxidative stress in carcinogenesisAnnu Rev Pharmacol Toxicol20044423926714744246
- ZiechDFrancoRPappaAPanayiotidisMIReactive Oxygen Species (ROS) – Induced genetic and epigenetic alterations in human carcinogenesisMutat Res Fundam Mol Mech Mutagen20117111–2167173
- ViscontiRGriecoDNew insights on oxidative stress in cancerCurr Opin Drug Discov Devel2009122240245
- van der WijstMGPBrownRRotsMGNrf2, the master redox switch: The Achilles’ heel of ovarian cancer?Biochim Biophys Acta20141846249450925270772
- NguyenTNioiPPickettCBThe Nrf2-antioxidant response element signaling pathway and its activation by oxidative stressJ Biol Chem200928420132911329519182219
- PadmanabhanBTongKIOhtaTStructural basis for defects of Keap1 activity provoked by its point mutations in lung cancerMol Cell200621568970016507366
- OhtaTIijimaKMiyamotoMLoss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growthCancer Res20086851303130918316592
- YooNJKimHRKimYRAnCHLeeSHSomatic mutations of the KEAP1 gene in common solid cancersHistopathology201260694395222348534
- SinghAMisraVThimmulappaRKDysfunctional KEAP1–NRF2 interaction in non-small-cell lung cancerPLoS Med2006310e42017020408
- NioiPNguyenTA mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activityBiochem Biophys Res Commun2007362481682117822677
- ZhangPSinghAYegnasubramanianSLoss of Keap1 function in prostate cancer cells causes chemo- and radio-resistance and promotes tumor growthMol Cancer Ther20109233620124447
- ShibataTKokubuAGotohMGenetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancerGastroenterology200813541358136818692501
- KonstantinopoulosPASpentzosDFountzilasEKeap1 mutations and Nrf2 pathway activation in epithelial ovarian cancerCancer Res201171155081508921676886
- ShibataTOhtaTTongKICancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancyProc Natl Acad Sci U S A200810536135681357318757741
- KimYROhJEKimMSOncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skinJ Pathol2010220444645119967722
- ShibataTKokubuASaitoSNRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancerNeoplasia201113986487321969819
- WangRAnJJiFHypermethylation of the Keap1 gene in human lung cancer cell lines and lung cancer tissuesBiochem Biophys Res Commun2008373115115418555005
- HanadaNTakahataTZhouQMethylation of the KEAP1 gene promoter region in human colorectal cancerBMC Cancer20121216622325485
- IchimuraYWaguriSSouYSPhosphorylation of p62 Activates the Keap1-Nrf2 Pathway during Selective AutophagyMol Cell201351561863124011591
- InamiYWaguriSSakamotoAPersistent activation of Nrf2 through p62 in hepatocellular carcinoma cellsJ Cell Biol2011193227528421482715
- OoiAWongJ-CPetilloDAn antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinomaCancer Cell201120451152322014576
- HeXMaQNRF2 cysteine residues are critical for oxidant/electrophile-sensing, Kelch-Like ECH-associated protein-1-dependent ubiquitination-proteasomal degradation, and transcription activationMol Pharmacol20097661265127819786557
- WilsonAJKernsJKCallahanJFMoodyCJKeap Calm, and Carry on CovalentlyJ Med Chem201356197463747623837912
- AbedDAGoldsteinMAlbanyanHDiscovery of direct inhibitors of Keap1–Nrf2 protein–protein interaction as potential therapeutic and preventive agentsActa Pharm Sin B20155428529926579458
- ChenYInoyamaDKongA-NTKinetic analyses of Keap1-Nrf2 interaction and determination of the minimal Nrf2 peptide sequence required for Keap1 binding using surface plasmon resonanceChem Biol Drug Des20117861014102121920027
- InoyamaDChenYHuangXOptimization of fluorescently labeled Nrf2 peptide probes and the development of a fluorescence polarization assay for the discovery of inhibitors of Keap1-Nrf2 interactionJ Biomol Screen201217443544722156223
- ZhuangCMiaoZShengCUpdated research and applications of small molecule inhibitors of Keap1-Nrf2 protein-protein interaction: a reviewCurr Med Chem201421161861187024533814
- HancockRBertrandHCTsujitaTPeptide inhibitors of the Keap1–Nrf2 protein–protein interactionFree Radic Biol Med201252244445122107959
- HancockR1SchaapMPfisterHWellsGPeptide inhibitors of the Keap1-Nrf2 protein-protein interaction with improved binding and cellular activityOrg Biomol Chem201311213553355723615671
- SteelRCowanJPayerneEAnti-inflammatory effect of a cell-penetrating peptide targeting the Nrf2/Keap1 interactionACS Med Chem Lett20123540741022582137
- HuLMageshSChenLDiscovery of a small-molecule inhibitor and cellular probe of Keap1–Nrf2 protein–protein interactionBioorg Med Chem Lett201323103039304323562243
- MarcotteDZengWHusJ-CSmall molecules inhibit the interaction of Nrf2 and the Keap1 Kelch domain through a non-covalent mechanismBioorg Med Chem201321144011401923647822
- SinghAVenkannagariSOhKHSmall molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumorsACS Chem Biol201611113214322527552339
- BollongMJYunHSherwoodLA small molecule inhibits deregulated NRF2 transcriptional activity in cancerACS Chem Biol201510102193219826270491
- SunH-PJiangZ-YZhangM-YNovel protein–protein interaction inhibitor of Nrf2–Keap1 discovered by structure-based virtual screeningMed Chem Commun2014519398
- ZhuangCNarayanapillaiSZhangWRapid Identification of Keap1–Nrf2 Small-Molecule Inhibitors through Structure-Based Virtual Screening and Hit-Based Substructure SearchJ Med Chem20145731121112624417449
- SatohMSaburiHTanakaTMultiple binding modes of a small molecule to human Keap1 revealed by X-ray crystallography and molecular dynamics simulationFEBS Open Bio201551557570
- BertrandHCSchaapMBairdLDesign, synthesis, and evaluation of triazole derivatives that induce Nrf2 dependent gene products and inhibit the Keap1–Nrf2 protein–protein interactionJ Med Chem201558187186719426348784
- DaviesTGWixtedWECoyleJEMonoacidic Inhibitors of the Kelch-like ECH-Associated Protein 1: Nuclear factor erythroid 2-related factor 2 (KEAP1:NRF2) protein–protein interaction with high cell potency identified by fragment-based discoveryJ Med Chem20165983991400627031670
- ZhongMLynchAJehleSFragment-based drug discovery targeting KEAP1/Nrf2 bindingFaseb J2015291_Suppl Abstract 712.20
- FosgerauKHoffmannTPeptide therapeutics: current status and future directionsDrug Discov Today201520112212825450771
- JnoffEAlbrechtCBarkerJJBinding mode and structure-activity relationships around direct inhibitors of the Nrf2-Keap1 complexChemMedChem20149469970524504667
- JiangZYLuMCXuLLDiscovery of potent Keap1–Nrf2 protein–protein interaction inhibitor based on molecular binding determinants analysisJ Med Chem20145762736274524512214
- JiangZYXuLLLuMCStructure-activity and structure-property relationship and exploratory in vivo evaluation of the nano-molar Keap1–Nrf2 protein–protein interaction inhibitorJ Med Chem201558166410642126258437
- LiXZhangDHanninkMBeamerLJCrystal structure of the Kelch domain of human Keap1Journal of Biological Chemistry200427952547505475815475350
- JainADPottetiHRichardsonBGProbing the structural requirements of non-electrophilic naphthalene-based Nrf2 activatorsEur J Med Chem201510325226826363505
- SaitoTIchimuraYTaguchiKp62/Sqstm1 promotes malignancy of HCV-positive hepatocellular carcinoma through Nrf2-dependent metabolic reprogrammingNat Commun201671203027345495
- WinkelAFEngelCKMargerieDCharacterization of RA839, a noncovalent small molecule binder to Keap1 and selective activator of Nrf2 signalingJ Biol Chem201529047284462845526459563
- WahleKWJBrownIRotondoDHeysSDPlant phenolics in the prevention and treatment of cancerAdv Exp Med Biol2010698365121520702
- HuangMTFerraroTPhenolic compounds in food and cancer preventionHuangMTHoCTLeeCYPhenolic Compounds in Food and their Effects on Health II: Antioxidants and Cancer PreventionWashington, DCAmerican Chemical Society1992834
- HatcherHPlanalpRChoJCurcumin: From ancient medicine to current clinical trialsCell Mol Life Sci200865111631165218324353
- WhitlockNCBaekSJThe anticancer effects of resveratrol: Modulation of transcription factorsNutr Cancer201264449350222482424
- RaufAImranMButtMSResveratrol as an anti-cancer agent: A reviewCrit Rev Food Sci Nutr20185891428144728001084
- KunnumakkaraABAnandPAggarwalBBCurcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteinsCancer Lett2008269219922518479807
- ChenBZhangYWangYCurcumin inhibits proliferation of breast cancer cells through Nrf2-mediated down-regulation of Fen1 expressionJ Steroid Biochem Mol Biol2014143111824486718
- AdiwidjajaJMclachlanAJBoddyAVCurcumin as a clinically-promising anti-cancer agent: pharmacokinetics and drug interactionsExpert Opin Drug Metab Toxicol201713995397228776444
- Burgos-MorónECalderón-MontañoJMSalvadorJRoblesALópez-LázaroMThe dark side of curcuminInt J Cancer201012671771177519830693
- National Toxicology ProgramNTP toxicology and carcinogenesis studies of turmeric oleoresin (CAS No. 8024-37-1) (Major component 79%–85% curcumin, CAS No. 458-37-7) in F344/N rats and B6C3F1 mice (Feed studies)Natl Toxicol Program Tech Rep Ser1993427127512616304
- SinghBShoulsonRChatterjeeAResveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathwaysCarcinogenesis20143581872188024894866
- LiCXuXWangXJPanYImine resveratrol analogues: Molecular design, Nrf2 activation and SAR analysisPLoS One201497e10145525028928
- ChenCYuROwuorEDActivation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and deathArch Pharm Res200023660561211156183
- HoughtonCAFassettRGCoombesJSSulforaphane and other nutrigenomic Nrf2 activators: Can the clinician’s expectation be matched by the reality?Oxid Med Cell Longev201620161, supplement78571861726881038
- HanlonNColdhamNGielbertAAbsolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in ratBr J Nutr2008990355956417868493
- RiedlMASaxonADiaz-SanchezDOral sulforaphane increases Phase II antioxidant enzymes in the human upper airwayClin Immu-nol20091303244251
- YeLDinkova-KostovaATWadeKLQuantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humansClin Chim Acta20023161–2435311750273
- CornblattBSYeLDinkova-KostovaATPreclinical and clinical evaluation of sulforaphane for chemoprevention in the breastCarcinogenesis20072871485149017347138
- Dinkova-KostovaATFaheyJWKostovRVKenslerTWKEAP1 and done? Targeting the NRF2 pathway with sulforaphaneTrends Food Sci Technol201769Pt B25726929242678
- RussoMSpagnuoloCRussoGLNrf2 targeting by sulforaphane: A potential therapy for cancer treatmentCrit Rev Food Sci Nutr20185881391140528001083
- SunHZhuJLinHGuKFengFRecent progress in the development of small molecule Nrf2 modulators: a patent review (2012–2016)Expert Opin Ther Pat201727776378528454500
- WangYYYangYXZheHHeZXZhouSFBardoxolone methyl (CDDO-Me) as a therapeutic agent: an update on its pharmacokinetic and pharmacodynamic propertiesDrug Des Devel Ther2014820752088
- HongDSKurzrockRSupkoJGA phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomasClin Cancer Res201218123396340622634319
- CreelanBCGabrilovichDIGrayJESafety, pharmacokinetics, and pharmacodynamics of oral omaveloxolone (RTA 408), a synthetic triterpenoid, in a first-in-human trial of patients with advanced solid tumorsOnco Targets Ther2017104239425028919776
- PatelSPHodiFSGabrilovichD5O_PRA phase 1b/2 study of omaveloxolone in combination with checkpoint inhibitors in patients with unresectable or metastatic melanomaAnn Oncol201728suppl_11mdx760
- BenarrochEENrf2, cellular redox regulation, and neurologic implicationsNeurology201788201942195028424271
- TuZSWangQSunDDDaiFZhouBDesign, synthesis, and evaluation of curcumin derivatives as Nrf2 activators and cytoprotectors against oxidative deathEur J Med Chem2017134728528399452
- VyasADandawatePPadhyeSAhmadASarkarFPerspectives on new synthetic curcumin analogs and their potential anticancer propertiesCurr Pharm Des201319112047206923116312
- KachadourianRDayBJPugazhentiSA synthetic chalcone as a potent inducer of glutathione biosynthesisJ Med Chem20125531382138822239485
- GoldRKapposLArnoldDLPlacebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosisN Engl J Med2012367121098110722992073
- LinkerRAHaghikiaADimethyl fumarate in multiple sclerosis: latest developments, evidence and place in therapyTher Adv Chronic Dis20167419820727433310
- SaiduNENoéGCerlesODimethyl fumarate controls the NRF2/DJ-1 axis in cancer cells: Therapeutic applicationsMol Cancer Ther201716352953928069874
- Bennett SaiduNEBretagneMMansuetALDimethyl fumarate is highly cytotoxic in KRAS mutated cancer cells but spares non-tumorigenic cellsOncotarget20189109088909929507676
- LoeweRValeroTKremlingSDimethyl fumarate impairs melanoma growth and metastasisCancer Res20066624118881189617178886
- XiMYJiaJMSunHP3-aroylmethylene-2,3,6,7-tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-ones as potent Nrf2/ARE inducers in human cancer cells and AOM-DSS treated miceJ Med Chem201356207925793824053646
- ChildersWEAbou-GharbiaMBiswalSThimmulappaRKinventorsCureveda, LLC, assigneeFunctionalized heteroaryl enones exhibiting Nrf2 activation and their method of useUnited States patent USWO2015187934A1Cureveda, Llc20151210
- YouQYangTSunYinventorsChina Pharmaceutical University, assigneePyridopyrazine compounds as Nrf2 activators and their preparation, pharmaceutical compositions and use in the treatment of inflammation and cancerChinese patentCN105566323A2016511
- HarderBTianWLa ClairJJBrusatol overcomes chemoresistance through inhibition of protein translationMol Carcinog20175651493150028019675
- ZhuJWangHChenFAn overview of chemical inhibitors of the Nrf2-ARE signaling pathway and their potential applications in cancer therapyFree Radic Biol Med20169954455627634172
- RenDVilleneuveNFJiangTBrusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanismProc Natl Acad Sci U S A201110841433143821205897
- JaramilloMCZhangDDThe emerging role of the Nrf2-Keap1 signaling pathway in cancerGenes Dev201327202179219124142871
- VartanianSMaTPLeeJApplication of mass spectrometry profiling to establish brusatol as an inhibitor of global protein synthesisMol Cell Proteomics20161541220123126711467
- HarderBTianWLa ClairJJBrusatol overcomes chemoresistance through inhibition of protein translationMol Carcinog20175651493150028019675
- HallIHLeeKHEigebalySAAntitumor agents. XXXIV: Mechanism of action of bruceoside A and brusatol on nucleic acid metabolism of P-388 lymphocytic leukemia cellsJ Pharm Sci1979687883887458610
- RenDVilleneuveNFJiangTBrusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanismProc Natl Acad Sci U S A201110841433143821205897
- TangWXieJXuSNovel nitric oxide-releasing derivatives of brusatol as anti-inflammatory agents: design, synthesis, biological evaluation, and nitric oxide release studiesJ Med Chem201457187600761225179783
- TangXWangHFanLLuteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugsFree Radic Biol Med201150111599160921402146
- GaoAMKeZPWangJNApigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathwayCarcinogenesis20133481806181423563091
- GaoAMKeZPShiFSunGCChenHChrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathwayChem Biol Interact2013206110010823994249
- LiaoJCLeeKTYouBJRaf/ERK/Nrf2 signaling pathway and MMP-7 expression involvement in the trigonelline-mediated inhibition of hepatocarcinoma cell migrationFood Nutr Res2015592988426699938
- BroekgaardenMWeijerRvan GulikTMHamblinMRHegerMTumor cell survival pathways activated by photodynamic therapy: a molecular basis for pharmacological inhibition strategiesCancer Metastasis Rev201534464369026516076
- ArltASebensSKrebsSInhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activityOncogene201332404825483523108405
- TsuchidaKTsujitaTHayashiMHalofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulationFree Radic Biol Med201710323624728039084
- PinesMSpectorIHalofuginone – the multifaceted moleculeMolecules201520157359425569515
- BiswalSSSinghARastinejadFinventorsNational Institutes of Health (NIH), Johns Hopkins University, Sanford-Burnham Prebys Medical Discovery Institute, assigneeNrf2 small molecule inhibitors for cancer therapyUnited States patentUS20160046616A12016218
- LiuXZhuQZhangMIsoliquiritigenin ameliorates acute pancreatitis in mice via inhibition of oxidative stress and modulation of the Nrf2/HO-1 pathwayOxid Med Cell Longev20182018 Article 7161592
- ChoiEJJungBJLeeSHA clinical drug library screen identifies clobetasol propionate as an NRF2 inhibitor with potential therapeutic efficacy in KEAP1 mutant lung cancerOncogene201736375285529528504720
- DuongHQYiYWKangHJInhibition of NRF2 by PIK-75 augments sensitivity of pancreatic cancer cells to gemcitabineInt J Oncol201444395996924366069
