?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Currently, microsphere technology plays a major role in the development of many new cancer therapies. In the current study, we proposed a targeted drug-delivery system to improve the treatment efficacy of one of the common conventional chemotherapeutic drugs used to treat lung tumors, 5-fluorouracil (5-FU).
Materials and methods
Following the preparation and optimization of small, solid micro-spheres, ranging in diameter between 5 and 15 µm, the final product 5-fluorouracil gelatin (5-FUG) was formulated using a Buchi Nano Spray Dryer by varying the drug:polymer ratio.
Results
Particle yield was calculated as 65% ± 1.2%, and the drug content in the formulation was recorded as 74% ± 1.6%. Particle surface morphology was examined as shriveled shape (crumpled/folded); particle size distribution displayed a binomial distribution, with a mean diameter of 9.6 µm. In vitro drug release studies revealed that ~36.4% of the 5-FU in 5-FUG was released in the first hour after injection. Clinically, this would lead to initial or burst release, facilitating a quick rise to therapeutic levels. In contrast to the pure 5-FU drug (89.2% of the drug released in the first 30 minutes), 99.1% of the drug in 5-FUG was released from the spray-dried particles for a period of 12 hours. A two-compartment model was used to generate plasma concentration–time curves. 5-FUG injection has a much different distribution in vivo in contrast to intravenous injection of 5-FU. In addition, the half-life after intravenous injection of 5-FUG, t1/2(α) = 1.23 hours and t1/2(β) = 18.3 hours, was considerably longer than that of 5-FU, t1/2(α) = 0.34 hours and t1/2(β) = 8.62 hours. Examination of stained lung tissue sections showed no histopathological tissue changes or evidence of gross pathology. In addition, the optimized formulation demonstrated an increased stability under both long-term and refrigerated storage conditions.
Conclusion
Our goal was to develop similar delivery systems for other chemotherapeutic drugs that are site specific to different disease models/tumor types.
Introduction
According to the National Cancer Institute (NCI) and the Centers for Disease Control and Prevention (CDC) registries, the mortality data project 1,735,350 new cancer diagnoses and 609,640 cancer deaths to occur in the US in the year 2018.Citation1 Worldwide, lung cancer is ranked the third most frequent type among all the cancer cases diagnosed every year.Citation2 The majority of lung cancer patients present with large locally aggressive tumors or an advanced metastatic cancer that has a poor prognosis and is not amenable to conventional therapeutic approaches. Frequently, in these cases, tumors were removed surgically (debulking), and the remaining tumor cells were treated by radiation and chemotherapy.Citation3 In general, both approaches can cause local and/or systemic toxicity, including possible permanent damage to lung tissue, serious enough to discontinue treatment.Citation4 With chemotherapy drugs, the major disadvantage is that they will also damage normal and healthy tissues that can lead to serious adverse effects at therapeutic doses.Citation5 This happens, in part, due to the fact that the conventional drug-delivery method can only deliver a small fraction of the drug dose to the tumor cells in the lung. The majority of the drug then causes systemic toxicity or simply gets eliminated from the body and is wasted.Citation6 Therefore, in order to achieve effective cancer treatment, high doses of the drug are needed. However, chemotherapy drug-induced side effects are often dose dependent; thus, higher doses of chemotherapy drugs are often associated with more severe systemic toxicity.Citation7 Strategies are needed for more efficient drug delivery.
Current efforts to address this disadvantage associated with the delivery and use of conventional chemotherapeutic drugs have focused on providing “targeted” drug delivery and release to the affected area. The ideal solution would be to target the medication solely to tumor cells. Targeted drug-delivery systems, ideally, are engineered for the release of the drug in a controlled manner, dramatically reducing the dosage needed to obtain a desired therapeutic effect. These delivery systems deliver small quantities of chemotherapeutic agents to the target site, reducing the amount of drug traveling freely to undesirable areas, thereby avoiding toxicity and adverse effects.Citation8 However, delivery methods such as nanoparticles are not always consistent with reproducible results to achieve cancer treatment optimization. Microspheres are a well-established method that has been used to deliver, orally or parenterally, various types of therapeutic agents, such as peptides, antibiotics, and proteins.Citation9
We hypothesize that a new formulation utilizing micro-spheres for targeted delivery of chemotherapeutic drugs will decrease toxicity and improve therapeutic efficacy. A major objective in the development of a novel target drug delivery is to minimize toxicity. Consequently, an in vivo animal model was used and lung tissue and other remote organs, eg, liver, kidney, and heart, were monitored by histopathological evaluation for any signs of toxicity.Citation10 Polymeric microspheres can be used to deliver therapeutic agents to a target site in a rate-controlled manner. Gelatin was used in the preparation as it is natural, cost-effective, biodegradable, and biocompatible. These microspheres are small particles with diameters on the order of microns. Microspheres can be trapped by the first capillary system encountered, but to target the drug to the lungs, microspheres must be precisely calibrated to 5–15 µm.Citation11,Citation12 Particles of these sizes have been previously shown to become mechanically entrapped in the lungs after intravenous administration, thereby enhancing their therapeutic action.Citation13
Several types of physical and mechanical methods have been used to formulate microspheres, such as emulsion polymerization, miniemulsion/microemulsion polymerization, interfacial polymerization, ionic gelation, solvent dispersion, and co-crystallization.Citation14 Organic solvents are commonly used in these techniques, and due to physical and chemical barriers, these solvents cannot be removed in the final formulation.Citation15 Furthermore, these techniques produce a broad particle size (PS) distribution with poor reproducibility of microspheres, and the process cannot be used on a commercial scale. In contrast, spray drying is a widely used industrial method to convert a liquid to dry solid powder in one single-step. Compared to conventional methods of preparing microspheres, it is a single-step process, rapid, and relatively easy to reproduce on a commercial scale.Citation16 We have recently reported that, with small changes in the standard preparation technique, we can produce the desired PS, which is a critical step to giving our formulation site specificity.Citation17
Because our approach is based on the results presented earlier by our group and others, we hypothesize that the proposed formulation method is likely to be the most feasible, cost-effective, and successful drug-delivery approach to treat lung tumors. The proposed targeted drug-delivery study will be an addition to an approach already gaining importance in pharmaceutical research and development in comparison to conventional drug delivery due to the desire to minimize the toxicity of current chemotherapeutic regimens.
Materials and methods
Animals
This work was done according to the National Institutes of Health’s (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication, eighth edition, revised 2011). Approval was given by the Protocol Management and Review Committee at the King Faisal University (IAEC/SSP/17/PR-0121). 5-Fluorouracil (5-FU) and a gelatin bloom 200 dialysis bag with a 14,000 MW cutoff were procured from Sigma-Aldrich Co. (St Louis, MO, USA). Phosphate-buffered saline (PBS) and Wistar rats were purchased from the local market; H&E stains (Sigma-Aldrich Co.) and amber high-density polyethylene bottles were also procured from the local market. All reagents were of pharmaceutical grade.
Method of preparation and optimization process of microspheres
5-FU and 5-fluorouracil gelatin (5-FUG) microspheres were dissolved in 250 mL methanol:water (50:50 ratio) and sprayed using a Buchi Nano Spray Drier B-90. Optimization of the microspheres was carried out to achieve the preferred PS of 5–15 µm, as this narrow range of PS is required for site-specific targeting of the microspheres to the lung after intravenous injection. Three optimization parameters were varied: gelatin concentration (0.5%–2.5%), flow rate (25–30 mL/h), and inlet temperature (70°C–110°C). The drug:polymer ratio was 1:2. The fixed parameters were a 7 µm size spray cap and an outlet temperature of 30°C–50°C; relative humidity (RH) was kept at 50%. The solution was filtered earlier to spraying.Citation18,Citation19 The powder collected from the spray-drying glass chamber was stored at room temperature until further use.
Experimental design
A three-level factorial experiment was applied using response surface methodology (RSM), which helps the relationship between the above-selected factors. Statistical test was applied by Stat-Ease, Inc.Citation20
Estimation of the drug in the formulation
A known amount (25 mg) of formulation containing both drug (5-FU) and the polymer was powdered to fine particles with a mortar and pestle, transferred to a beaker containing 100 mL of PBS (pH 7.4):5.5 pH acetate buffer (1:1 ratio) and stirred for 2 hours to extract all the drugs into the solvent. The solution was filtered, and the drug content was estimated using a UV-1601 spectrophotometer (Shimadzu UV-1700 Pharmaspec, Tokyo, Japan) at a wavelength of 266 nm.Citation21,Citation22
Percentage yield of product
The percent yield of the spray-dried product collected from the collecting chamber was calculated as the recovered yield divided by the initial yield multiplied by 100.Citation23
Scanning electron microscopy of microsphere surface morphology
Surface morphology is an analytical technique that is capable of capturing an image at the external surface or near the surface of a product. An FEI Quanta 200 was used to visualize the surface morphology of the specimen. Prior to analysis, the sample was coated with gold, in order to reduce the charge of the sample and improve secondary electron emission.Citation24,Citation25
PS distribution of microspheres
Measurement of PS was carried out using a Malvern Zetasizer (Malvern Zetasizer Nano ZS; Malvern Instruments, Malvern, UK). The reusable glass cuvette was three-quarters filled with an organic solvent, and the sample (ultrasonicated), obtained from the spray dryer, was suspended and measured to determine the PS distribution.Citation26
In vitro release and release kinetics
Drug release studies were performed by placing a known quantity (microspheres equivalent to 100 mg of 5-FU) of microspheres in a dialysis bag and dialyzing with 500 mL of PBS. The test tubes were placed in an incubator shaker in a controlled environment. Sink conditions were maintained during the studies.Citation27 Samples of 5 mL were withdrawn to know the concentration of 5-FU in PBS solution, which was estimated using a UV-1601 spectrophotometer (Shimadzu) at a wavelength of 266 nm. The 5-FU released from the formulation was calculated in terms of cumulative release. Release experiments were performed in triplicate. The data obtained from the in vitro drug release readings were used to extrapolate different kinetic models using version 10 of SigmaPlot Software Inc., San Jose, CA, USA.
International Council for Harmonisation (ICH) stability testing
Spray-dried microspheres were placed in the amber high-density polyethylene bottles and stored at accelerated 25°C ± 2°C and 60% ± 5% RH for a period of 6 months and at long-term 5°C ± 3°C for a period of 12 months.Citation28 The studies were carried out according to International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Q2B guidelines. The PS distribution, external surface morphology, and drug content of these stored samples were evaluated using previously described methods and compared to those obtained directly after synthesis to determine if any changes have occurred and assess the stability of the proposed formulation.
In vivo pharmacokinetics model
In vivo animal studies are a key component of this research because they provide a crucial understanding of how the formulation behaves in a real biological environment. The major objective of this in vivo research is to passively target the microspheres to lungs. Procedures are followed according to our previously published article.Citation8 Concisely, Wistar rats weighing between 20 and 30 g were used for these studies. Animals were divided into six groups (n = 6) in each group. A known amount of (20 mg/kg) formulation was injected into the tail vein of these Wistar rats, and the drug released at predetermined time intervals (10 minutes, 15 minutes, 1 hour, 3 hours, 6 hours, and 12 hours) was recorded. The lyophilized formulation was added to cryoprotectant solution before administration intravenously. The first group was administered the pure drug (5-FU positive control), and the second group was administered with negative control (normal saline 1 mg/mL). The other (experimental) group received the same quantity of 5-FU in the formulation mixture form. Starved rats were deprived of food for 24 hours under standard laboratory conditions but allowed free access to drinking water. At predetermined time intervals, animals were sacrificed by cervical dislocation. The organs (liver, spleen, and lungs) were extracted to determine the amount of drug released at different time intervals; this drug release assay was carried out with a slight modification of the previously reported method.Citation29,Citation30
Histopathological examination
The spray-dried microsphere formulation was tested for any toxic effect on the lung tissue by staining the tissue sections with H&E, which were previously fixed in 10% neutral buffered formalin and embedded in paraffin. Histological examination was carried out using light microscopy.Citation31
Results and discussion
Drug loading and percent yield of particles
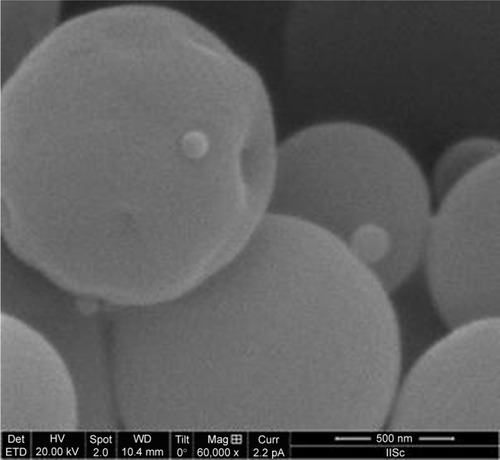
The percent yield of the particles was calculated as 65% ± 1.2%. A portion of the product appeared to stick to the electrostatic particle collector and electrodes during the drying process and therefore was not collected (). This is likely due to the low density of the particles that form after the drying process. We can minimize this problem by increasing the volume of liquid fed into the drying chamber; however, a small amount of particle loss is unavoidable. The percentage of the drug in this 5-FUG formulation was recorded as 74% ± 1.6%.
Surface morphology
The surface morphology of the dried particles was shriveled (crumpled/folded) shape (). The formation of this surface topology was attributed to the high speed of evaporation from the surface of the liquid, accelerated by hot air circulating through the drying chamber. Previous work has shown that use of a highly viscous liquid feed will minimize surface folding.Citation13 However, our attempts to increase the viscosity for >10 centipoise (cps) were unsuccessful due to blockage of the spray nozzle. Through the use of a LVDV-III UCP Ultra Cone Spindle (CPE-40; Brookfield, Middleboro, MA, USA), we maintained the viscosity of the liquid feed at a constant 10 cps.
PS analysis
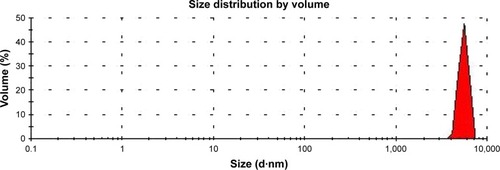
The 5-FUG formulation PS distribution shows a binomial distribution with a mean diameter of 9.6 µm (). In addition, the variance in the particle diameter is relatively small (). Since PS directly affects drug delivery and drug release rates, the variance of the PS needs to be minimized.
Optimization
The statistical model was designed and generated using RSM with the help of Statistical Package Software, Design Expert Version (SigmaPlot version 10, Systat Software, Inc., San Jose, CA, USA). The criteria for the chosen model were the following: independent variables that have statistically significant effects on the response/dependent variable based on an analysis of variance (ANOVA) with P < 0.05, a lack of fit test (P > 0.05) that was not significant, and a correlation coefficient R2 of >0.75 for the least-squares fit to the model. A quadratic model was chosen and optimized based on the following center points: weight of gelatin of 1 g, inlet temperature of 100°C, and flow rate of the liquid feed of 25 mL/h. The desired PS of the 5-FUG formulation was 5–9 µm.
The response of PS generated for various different values of the independent variables (weight of gelatin, inlet temperature, and feed flow rate) around the center point is given . The surface equation and correlation coefficient from the least-squares fit of this data to the quadratic model are given in . The insignificant lack of fit test (P > 0.05) and the value of R2 approaching 1 (0.9954) are consistent with a good fit of the quadratic model to the data. Results from an ANOVA of the model coefficients are given in and show that each of the independent variables individually, weight of gelatin (), inlet temperature (), and feed flow rate (), had significant effects (P < 0.05) on the response/PS (critical Fα= 0.05 statistic < observed F statistic). In addition, specific interactions between the independent variables in this model, namely A2, B2, C2, AC, and BC, had a statistically significant effect on the response as well (). Note that the coefficients for all three independent variables were positive over the range of the response surface, indicating a positive slope, as the independent variables are increased, the PS also increases up to the optimal point.
Figure 4 Response Surface Analysis.
Abbreviation: PS, particle size.
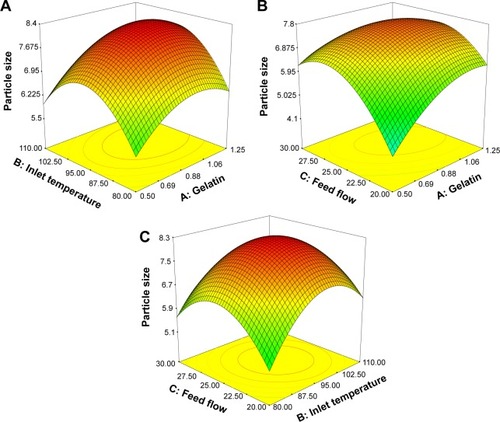
Table 1 Experimental data/runs showing the response (PS) for different values of the independent variables
Table 2 Coefficients of the statistical model fitted to the experimental data
Table 3 ANOVA of coefficients of the quadratic model fitted to the data
The graph of the three-dimensional response surface for the interactions between all three independent variables with the PS is shown in . This surface confirms that PS is increased when any of the independent variables is increased up to the optimum point, as was determined from the positive coefficients calculated for the independent variables. The optimum point was determined by maximizing the value of the PS response (). In summary, our analysis of a quadratic model fitted to the response data depending on three independent variables indicated that the optimum PS is predicted to be 8.1855 µm with the following values of the independent variables: a gelatin of 1 g, an inlet temperature of 100°C, and a flow rate of feed of 25 mL/h.
Table 4 Optimization of PS using CCRD
Drug release testing
Release studies were conducted to determine the release rates for 5-FU (pure drug) and 5-FUG in vitro (). Results showed that ~36.4% of the drug in 5-FUG was released in the first hour after injection, likely due to the fact that a large portion of the drug is confined close to the outer surface of the spray-dried nanoparticles. Clinically, this would lead to initial or burst release, facilitating a quick rise to therapeutic levels. In contrast, unlike the quick release of the pure drug 5-FU (89.2% of the drug released in the first 30 minutes), the drug that was released from the spray-dried 5-FUG particles took ~12 hours. In addition, over those 12 hours, 99.1% of the drug was released. This suggests that 5-FUG may have the capability of performing the sustained release.
Figure 5 In vitro drug release curves for both formulation and pure drug.
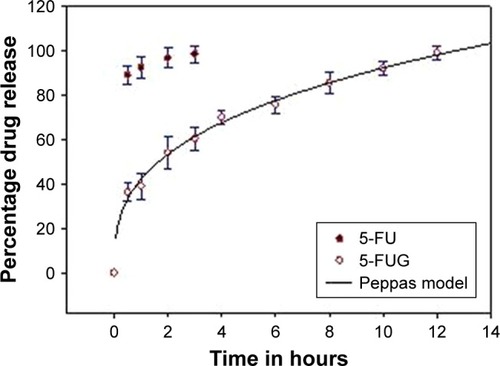
To determine the likely mechanism of action mediating the release of drug from the 5-FUG particles, a range of pharmacokinetic models of drug transport were fitted to the data (ie, Higuchi, R2 = 0.8874; Hixon and Crowell, R2 = 0.8807; Korsmeyer–Peppas, R2 = 0.9923; first order, R2 = 0.9112; and Baker and Lonsdale, R2 = 0.9751). The Peppas model gave the best fit out of this set of models with R2 = 0.9964, suggesting that the polymer structure of the microspheres swells () to form an outer gel layer that obstructs/slows the diffusion of the drug.
Pharmacokinetic studies
Phoenix® WinNonlin version 1.5 (Pharasight Corporation, Mountain View, CA, USA) was used to examine pharmacokinetic parameters of the 5-FUG preparation. A two-compartment model was used to generate a plasma concentration–time profile in plasma of Wistar rats, given an intravenous bolus dose of 50 mg/kg body weight of 5-FU (pure drug) and 5-FUG (). The 5-FUG injection has a much different plasma concentration distribution in vivo in contrast to intravenous injection of 5-FU. The half-life after intravenous injection of 5-FUG, t1/2(α) = 1.10 hours and t1/2(β) = 18.9 hours, was considerably longer than that after intravenous injection of 5-FU, t1/2(α) = 0.46 hours and t1/2(β) = 10.1 hours (). These results confirm that 5-FUG is able to provide a sustained rate of drug delivery. Finally, an analysis of the tissue concentration of the drug showed that the concentration of 5-FU in the lungs (812 µg/g after 15 minutes) was almost twice that after 5-FUG injection (312 µg/g after 15 minutes) than in any other tissue in the body or in the bloodstream. Normal saline injected did not show any allergic reaction and drug stability in serum up to 14 hours.Citation32 In vivo studies also indicated that the prepared 5-FUG microspheres can target the drug to lungs (). These results clearly exhibit that 5-FUG formulation can be used for targeted delivery of 5-FU to lungs.
Figure 7 Plasma concentration profile showing 5-FU (□) and 5-FUG (◊) release.
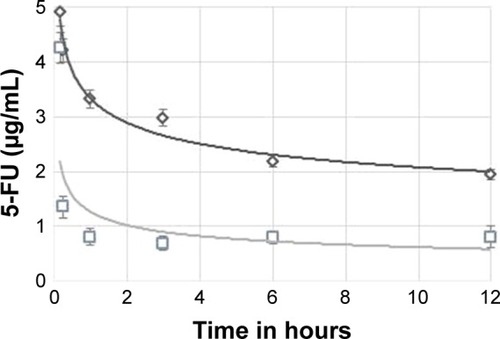
Figure 8 Biodistribution of 5-FU formulation in different organs.
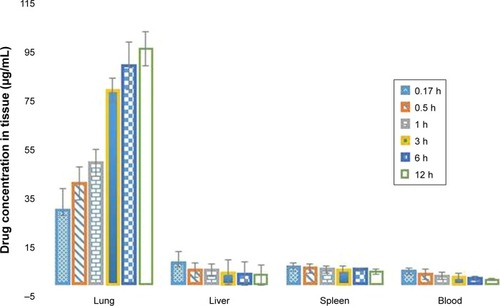
Table 5 Plasma concentration of 5-FUG and 5-FU injection after iv administration
Histopathological studies
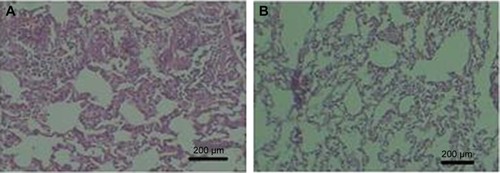
Compatibility studies of the nanoparticle formulation are shown in (intravenous 5-FUG) and (normal saline). The 5-FUG formulation and normal saline were tolerated well when given intravenously with no adverse effects. Examination of stained tissue sections of the lung showed no histopathological tissue changes or evidence of gross pathology.
Figure 9 Histology of lung tissue.
Stability studies
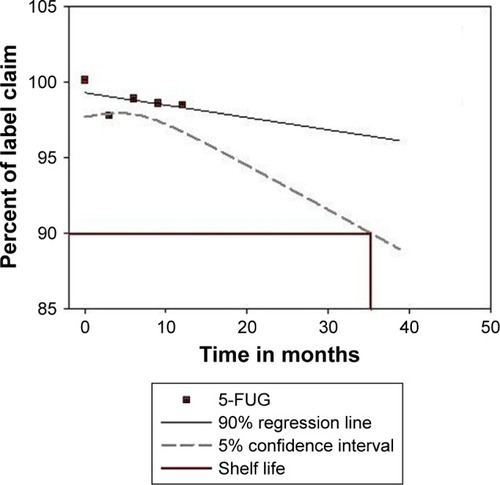
The formulation containing 421 mg (84.2%) of the encapsulated drug was subjected to shelf-life testing and found to be stable, t90 = 35.2 months (). Results showed no marked differences in the amount of drug encapsulated in the microspheres or in the mean PS. The amount of encapsulated drug showed a maximum decrease of 2.8% and 2.1% from the initial amount after long-term ambient storage and refrigerated storage, respectively. This loss probably is due to diffusion of drug molecules through the swollen microsphere matrix.
After storage, there was minimal sedimentation of the microspheres, but they were redispersed easily by simple manual agitation. The mean PS was increased but was consistent with that observed in an earlier study.Citation6 These results demonstrate the stability of the optimized formulation under both long-term and refrigerated storage conditions.
Conclusion
Current first-line treatments for cancer are local radiation therapy, surgery, and chemotherapy. In particular, the major disadvantage of chemotherapeutic drugs is the toxic effect they have on normal, healthy tissue. Our research seeks to minimize the systemic toxicity of the chemotherapeutic drug, 5-FU, by developing a novel site-specific drug-delivery system to the lungs based on microspheres. Specifically, site-specific targeting to the lung is achieved through restriction of the PS to 5–15 µm, allowing the lung to act as a filter. Our results obtained from in vitro and in vivo proves the prepared formulations can target the lung tissue. An in vitro study using the tumor cell line or in vivo study using the lung tumor animal model may be a good system to show the improved therapeutic effect of our formulation (5-FUG) in future studies. If this approach is clinically successful, it has the potential to dramatically reduce the therapeutic dose required, minimizing the systemic toxicity and adverse effects of current chemotherapeutic regimens. This will overcome the major drawbacks of conventional chemotherapy, namely, toxic adverse effects, damage to normal/surrounding healthy tissue, development of drug resistance, and drug–drug interactions.
Acknowledgments
The authors wish to acknowledge the approval and the support of this research study by the grant no. 6976-PHM-2017-1-7-F from the Deanship of Scientific Research in Northern Border University, Arar, Kingdom of Saudi Arabia.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalAStatisticsCCancer statistics, 2016CA Cancer J Clin201666173026742998
- The Global Burden of Cancer 2013JAMA Oncol20151450552726181261
- PurandareNCRangarajanVImaging of lung cancer: implications on staging and managementIndian J Radiol Imaging201525210912025969634
- de SanctisATailladeLVignotSPulmonary toxicity related to systemic treatment of nonsmall cell lung cancerCancer2011117143069308021283982
- HousmanGBylerSHeerbothSDrug resistance in cancer: an overviewCancers (Basel)2014631769179225198391
- SinghRLillardJWNanoparticle-based targeted drug deliveryExp Mol Pathol200986321522319186176
- EdiriwickremaASaltzmanWMNanotherapy for cancer: targeting and multifunctionality in the future of cancer therapiesACS Biomater Sci Eng201512647825984571
- HarshaNSRaniRHDrug targeting to lungs by way of microspheresArch Pharm Res200629759860416903082
- BarrowELWinchesterGAStaasJKQuenelleDCBarrowWWUse of microsphere technology for targeted delivery of rifampin to Mycobacterium tuberculosis-infected macrophagesAntimicrob Agents Chemother19984210268226899756777
- SarfrazMAfzalARazaSMLiposomal co-delivered oleanolic acid attenuates doxorubicin-induced multi-organ toxicity in hepatocellular carcinomaOncotarget2017829471364715328525367
- IliumLDavisSSWilsonCGThomasNWFrierMHardyJGBlood clearance and organ deposition of intravenously administered colloidal particles. The effects of particle size, nature and shapeInt J Pharm1982122–3135146
- DelgadoASorianoISánchezEOlivaMÉvoraCRadiolabelled biodegradable microspheres for lung imagingEur J Pharm Biopharm200050222723610962232
- HarshaSAl-DhubiabBENairABNovel drying technology of microsphere and its evaluation for targeted drug delivery for lungsDrying Technol2015334502512
- BoguslavskyLBaruchSMargelSSynthesis and characterization of polyacrylonitrile nanoparticles by dispersion/emulsion polymerization processJ Colloid Interface Sci20052891718516009219
- GrodowskaKParczewskiAOrganic solvents in the pharmaceutical industryActa Pol Pharm201067131220210074
- I RéMMicroencapsulation by spray dryingDrying Technol199816611951236
- HarshaNAldhubiabBEAlhaiderAIAttimaradMNairACarbopol 934-P loaded with vildagliptin for diabetic delivery: in vitro and in vivo evaluation of nanoparticlesCurr Nanosci201395642647
- HarshaSDual drug delivery system for targeting H. pylori in the stomach: preparation and in vitro characterization of amoxicillin-loaded Carbopol® nanospheresInt J Nanomedicine201274787479622969298
- HarshaSPharmaceutical suspension containing both immediate/sustained-release amoxicillin-loaded gelatin nanoparticles: preparation and in vitro characterizationDrug Des Devel Ther201371027
- MuzaffarKDinkarraoBVKumarPOptimization of spray drying conditions for production of quality pomegranate juice powderCogent Food Agric2016211127583
- OrtizRPradosJMelguizoC5-Fluorouracil-loaded poly(ε-caprolactone) nanoparticles combined with phage E gene therapy as a new strategy against colon cancerInt J Nanomedicine201279510722275826
- KaurSMehraNKJainKJainNKDevelopment and evaluation of targeting ligand-anchored CNTs as prospective targeted drug delivery systemArtif Cells Nanomed Biotechnol201745224225026890213
- HarshaNSIn vitro and in vivo evaluation of nanoparticles prepared by nano spray drying for stomach mucoadhesive drug deliveryDrying Technol2015331011991209
- BürkiKJeonIArpagausCBetzGNew insights into respirable protein powder preparation using a nano spray dryerInt J Pharm20114081–224825621335078
- YoshiiHBucheFTakeuchiNTerrolCOhgawaraMFurutaTEffects of protein on retention of ADH enzyme activity encapsulated in trehalose matrices by spray dryingJ Food Eng20088713439
- García-SaucedoCFieldJAOtero-GonzalezLSierra-ÁlvarezRLow toxicity of HfO2, SiO2, Al2O3 and CeO2 nanoparticles to the yeast, Saccharomyces cerevisiaeJ Hazard Mater201119231572157921782338
- LiuMChenLZhaoYPreparation, characterization and properties of liposome-loaded polycaprolactone microspheres as a drug delivery systemColloids Surf A Physicochem Eng Asp20123950131136
- HarshaSRCRaniSOfloxacin targeting to lungs by way of micro-spheresInt J Pharm20093801–212713219646516
- WangHXuYZhouXDocetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: in vitro and in vivo evaluationInt J Mol Sci20141533519353224577314
- El-SayyadHIIsmailMFShalabyFMHistopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino ratsInt J Biol Sci20095546647319584954
- Abdel-LatifRGMorsyMAEl-MoselhyMAKhalifaMASildenafil protects against nitric oxide deficiency-related nephrotoxicity in cyclosporine A treated ratsEur J Pharmacol20137051–312613423499693
- FrestaMVillariAPuglisiGCavallaroG5-Fluorouracil: various kinds of loaded liposomes: encapsulation efficiency, storage stability and fusogenic propertiesInt J Pharm1993992–3145156